Use of mass spectrometric detection as a versatile process monitoring tool: reduction patterns in a milled ZnO/Al mixture†
Anil
Gopala
,
Heinrich
Kipphardt
*,
Ralf
Matschat
and
Ulrich
Panne
BAM Federal Institute for Materials Research and Testing, Richard-Willstätter-Strasse 11, 12489, Berlin, Germany. E-mail: heinrich.kipphardt@bam.de; Fax: +49 30 8104 1117; Tel: +49 30 8104 1116
First published on 22nd April 2010
Abstract
Mass spectrometric detection can play a prominent role in the assessment of different reactions occurring at varied temperatures in a milled ZnO/Al mixture. This is the first time that online mass spectrometric information was used as a tool for monitoring and understanding the chemical reduction process mechanism. We have observed four different types of reaction taking place: (1) distillation of Zn metal, (2) reduction of ZnO by activated Al, (3) melting of Al and finally (4) reduction of ZnO by inactivated Al. The experimental conditions and results observed by QMS were supported with literature data and physical measurement data from X-Ray Diffraction (XRD) which gave us an idea about the complex reaction cascade which occurred during the formation of the zinc metal.
Introduction
Different elemental reactions were monitored at varied temperatures with a milled ZnO/Al mixture in a vacuum system directly interfaced to a gas source quadrupole mass spectrometer (QMS). The information of the intensities of the online measured mass spectrometric intensities of two Zn isotopes allowed us to separate and understand the reaction steps and permitted real time monitoring of the reaction processes during the formation of the product. Isotopic ratios additionally acted as a special chemical marker which helped us to know when a specific reaction proceeded. Isotopic mass spectrometric measurements are used in a wide range of research, which includes geochemistry, forensic science, medicine, food and climatology but rather seldom in chemical material processing.1–3 Unravelling the complexities of a material purification processes can be exceedingly difficult but using mass spectrometric measurements of the stable isotopes helped us in process monitoring. Here we investigated the process mechanism taking place in an incompletely milled ZnO/Al mixture using the information of intensities of the 66Zn and 64Zn isotopes and their ratio. In order to understand the different types of reduction mechanism taking place, the milled powder was subjected under vacuum to varied increase of temperature.Ultra-high-purity metals, often of enriched isotopes, are frequently required for targets in nuclear physics experiments, as reference materials, or as reference material precursors. This work originated in the context of our work to purify ultra-pure metals or isotope spikes to be used as primary calibration materials of highest metrological level in the field of elemental analysis. Isotopically enriched metals are disproportionately expensive compared to their oxide or carbonate form as ultra-pure metals are typically prepared from their corresponding oxides by metallothermic reductions.4 Distillation and reduction are among the oldest known physical and chemical processes which, nevertheless, need to be monitored for their mechanisms. However, surprising deficiency seems to be that there are many intermediate stages involved in this processes. Recently our research has been focused on the online monitoring using gas source mass spectrometry for understanding the melting and volatilisation process of Zn metal in the vacuum distillation system which can be regarded as fundamental steps in a purification procesess.5
Materials and methods
In our investigation the vacuum reduction distillation system consists of a cylindrical shaped vacuum chamber of a volume of 55 L with a base plate mounted on a table. The schematic diagram of the mass spectrometer connected to the vacuum distillation system is shown Fig. 1, this setup was originally developed by EMPA, Switzerland. Via a vacuum valve a turbo pump (PFEIFFER TCP-121) backed by a membrane pump is connected and the vacuum achieved was ∼2.0 × 10−7 mbar measured using a Pirani and cold cathode gauge. A high current/low voltage supply is connected to two copper electrodes mounted side by side and carrying a crucible support made out of tantalum (9.0 mm OD × 7.0 mm ID × 8 mm Ht) which has a high thermal resistance for heating. About ∼0.6 g of a mixture of ZnO (99.9%, Alfa Aesar) and Al (99%, Alfa Aesar) was milled by a ball mill (Retsch MM 301). Aluminium powder, 25% in excess to stoichiometric ratio concerning a total chemical reduction of ZnO, was loaded into the crucible which was closed with a lid made out of glassy carbon SIGRADUR® that had an exit orifice of ∼1 mm diameter. The temperature of the crucible was maintained by varying the current and voltage to the Cu electrodes using a Power Station pe2050 power source and the temperature was monitored by a laser (infra-red) pyrometer, Quantum Logic 3600 through one of the optically transparent quartz windows. The Zn atoms as well as other volatile compounds released during different events by distillation passes through the orifice of the lid into the vacuum chamber and thereby into the hyphenated electron ionisation (thoriated iridium filament) quadrupole mass spectrometer Pfeiffer Prisma—QMS 200, where the Zn atoms were ionised and monitored by Balzers Quadstar—422 V 5.0 software.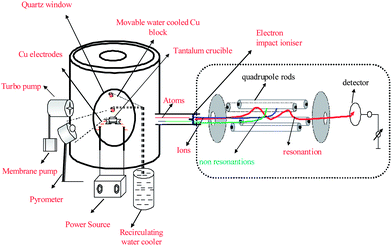 | ||
| Fig. 1 Schematic diagram of mass spectrometer hyphenated vacuum reduction distillation system. | ||
Results and discussions
We have selected a milling duration of 1 h for ball milling of the ZnO/Al powder mixture which led to an incompletely milled material in order to be able to study the formation of all the intermediates and products of the gross reaction:6| 3ZnO + 2Al → 3Zn + Al2O3. |
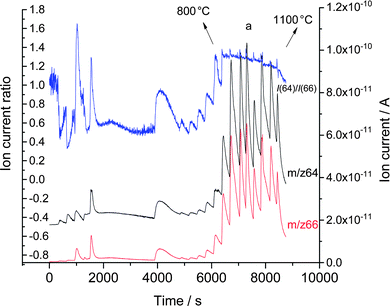
This incomplete state of milling has the advantage that Zn as well as mechanically activated and inactivated Al was contained in the mixture. The milled powder was subjected to a gradual increase of heating temperature by varying the current and voltage of the heating device in the vacuum reduction system while monitoring the change in the intensities m/z 66 (66Zn) and m/z 64 (64Zn) and their ratio. The results are displayed in Fig. 2 with our interpretation of them as an expression of the chemical reactions underwent with the milled mixture with the increase of temperature up to 1100 °C.
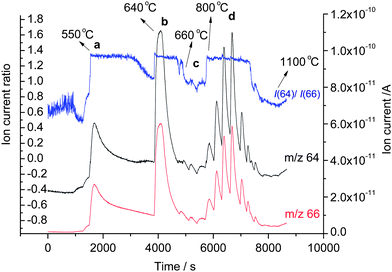 | ||
| Fig. 2 Reactions observed in the milled ZnO and Al powder at varied temperature using gas source QMS: (a) distillation of Zn, (b) reduction of ZnO by activated Al, (c) aluminum melting and (d) reduction of ZnO by inactivated Al. | ||
The figure firstly reveals the presence of Zn metal which gets distilled under vacuum at 550 °C (Zn equilibrium vapour pressure 6.09 × 102 Pa). As there was no Zn metal in the starting material, the Zn metal must have been formed already during the reactive milling. It can be observed that until the temperature reached 550 °C the signal of m/z 66 (66Zn) was overlapping over m/z 64 (64Zn) which can be due to uncharacteristic background signals. The presence of Zn in the vapour phase starting from 550 °C and attributed to the distillation of Zn was indicated by the clear observance of an ion current ratio identical to that of pure metallic zinc at 550 °C as shown in Fig. 3 observed under identical experimental conditions.7 The theoretical isotope ratio of f(64Zn)/f(66Zn) = 48.6%/27.9% = 1.74 for natural material is not matched with the observed value of 1.3, as there was no background correction applied on the individual ion currents and the MS was not specifically calibrated for isotopic ratio measurements. The presence of metallic zinc in the mixture was further confirmed by XRD data.
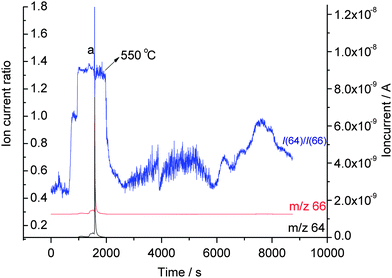 | ||
| Fig. 3 Pure Zn metal distillation observed using gas source QMS: (a) distillation. | ||
After the observed Zn distillation (Fig. 2) the intensities of m/z 66 and m/z 64 almost overlap over each other at 600 °C indicating the absence of Zn signals (only background measured) and hence cessation of distillation. For the heating period starting at about 550 °C the ion current ratio remained constant. The drastic deviation in the ion current ratio from the value before and after this interval of heating was an additional indication that the signals measured at m/z 66 and m/z 64 in those heating periods were no real signals from Zn isotope ions but uncharacteristic mass spectrometric signals. In other words, the Zn isotope ratio could here be used as an additional information to confirm the presence of absence of zinc evaporation. In this way in Fig. 2 all drastic deviations of the Zn isotope ratio from the value for pure zinc metal could helpfully be used to interpret the process of reactions.
During the reactive milling not only the direct formation of Zn occurred, but an additional transformation also took place. The Al abrased by the ZnO during the milling process got activated by the removal of the passivating oxide layer thereby producing a reactive modification of Al, i.e., an activated Al. The normal reduction temperature of Al with ZnO is ∼1000 °C which is a liquid–solid reduction. However, with activated Al the reduction temperature is decreased to ∼640 °C owing to its increased reactivity which is then termed as solid–solid reduction.8 Thermodynamically according to Ellingham diagram the reduction reaction of ZnO with Al proceeds at all temperatures. The activated Al reduction was well observed in Fig 2 at 640 °C (Zn vapour pressure 3.74 × 103 Pa) by the appearance of clear signals of both measured Zn isotopes and by the maintenance of Zn ion current ratio with the value observed for pure zinc metal.
In order to support the above interpretation, activated Al was prepared by milling it not with an oxide but with carbon powder (10% w/w) for 1 h and the activation was observed in the XRD pattern that showed a decrease in particle size of the Al as already mentioned by Streletskii et al.9 The activated Al mixture with carbon was then used to reduce the ZnO, under identical experimental conditions as before and it was observed as shown in Fig. 4 that the ion current pattern of m/z 66 and m/z 64 for zinc was only observed above 640 °C. The absence of Zn below this temperature further strengthens the fact that the presence of Zn in the mixture (Fig. 2) was formed during reactive milling.
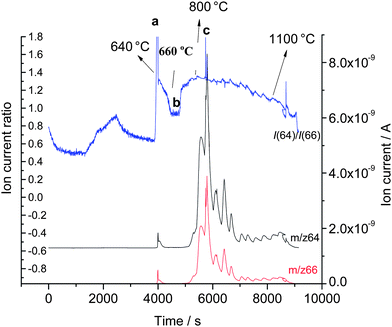 | ||
| Fig. 4 Reduction of ZnO with Al activated by milling with carbon observed using gas source QMS: (a) reduction of ZnO by activated Al, (b) aluminum melting and (c) reduction of ZnO by inactivated Al. | ||
The melting point of Al is 660 °C and with the increase of temperature from 640 °C to 660 °C the activated Al melts thereby being transformed into its inactivated state. This transformation from solid to liquid state of the Al can be clearly observed in Fig. 2 and 4 with the cessation of the signals from m/z 66 and m/z 64 indicating cessation of the reduction. This change in the isotopic patterns confirms two points: (1) the reduction of ZnO to Zn and the oxidation of Al to Al2O3 were due to the activated form of Al and (2) the absence of the natural Zn pattern at 660 °C confirms the melting of the Al with the cessation of the reduction due to the inactivation of the Al.
The inactivated Al reduction with ZnO under normal atmosphere takes place at ∼1000 °C but in our experiment which was performed under vacuum we were able to observe the isotopic pattern of Zn already starting from 800 °C (Fig. 2).8 This variation of temperature can be attributed primarily to the fact that the experiment was performed under vacuum and additionally that the formation of Zn at trace levels can be detected by the mass spectrometer, which otherwise is very difficult to detect under normal atmospheric condition, where the formation of alumina and the collection of Zn can only be observed above the boiling point of Zn at 920 °C at which a substantial vapour pressure is generated to evaporate macroscopic amounts of Zn. Our experiment revealed that the initiation of the reduction of ZnO to Zn and the oxidation of Al to Al2O3 with inactivated Al starts at 800 °C (Zn vapour pressure 4.41 × 104 Pa) and the cessation of the reduction and the absence of Zn are indicated with the decrease of ion current ratio values starting at 1000 °C.
To further strengthen this revelation, a non-milled Al/ZnO mixture was monitored under identical experimental conditions and it was observed that the Zn isotopic ratios were well detectable starting already from 800 °C as shown in Fig. 5. It is important to note that there was no indication of the presence of a detectable Zn isotopic pattern at 640 °C indicating the absence of activated Al.
 | ||
| Fig. 5 Reduction of ZnO with inactivated Al and ZnO observed using gas source QMS: (a) reduction of ZnO by inactivated Al. | ||
Conclusions
Generally we concluded that the observance of mass spectrometric isotopic detection has the potential to study chemical reactions in an efficient and also in a “greener” way by screening and evaluating without the conspicuous release of noxious gasses into environment. Hence, in situ mass spectrometric isotopic measurements can deliver the tools necessary to gather information for a priori design of new or improved reaction systems.Acknowledgements
Anil Gopala is thankful to Adolf Martens association for sponsoring to the Adolf Martens Fellowship. Authors would like to acknowledge the colleagues from BAM, Franziska Emmerling for providing with the measurement data for XRD and Holger Scharf for providing with the ball milled samples.Notes and references
- A. C. Pierson-Wickmann, L. Aquilina and C. Weyer, Geochim. Cosmochim. Acta, 2009, 73(16), 4688 CrossRef CAS; D. De Muynck and F. Vanhaecke, Spectrochim. Acta, Part B, 2009, 64(5), 408 CrossRef; R. A. Kraft, A. H. Jahren and C. D. Saudek, Rapid Commun. Mass Spectrom., 2008, 22(22), 3683 CrossRef CAS; A. L. Cabanero, J. L. Recio and M. Ruperez, Rapid Commun. Mass Spectrom., 2008, 22(20), 3111 CrossRef CAS.
- Handbook of Stable Isotope Analytical Techniques, ed. P. A. de Groot, Elsevier, Amsterdam, 2004, vol. I and II Search PubMed.
- M. Kraiem, K. Mayer, T. Gouder, A. Seibert, T. Wiss, H. Thiele and J. P. Hiernaut, Int. J. Mass Spectrom., 2010, 289(2–3), 108 Search PubMed.
- B. Lommel, W. Hartmann, B. Kindler and J. Steiner, Nucl. Instrum. Methods Phys. Res., Sect. A, 2006, 561, 100 CrossRef CAS.
- A. Gopala, H. Kipphardt, R. Matschat and U. Panne, J. Anal. At. Spectrom., 2009, 24, 887 RSC.
- Y. W. Lao, S. T. Kuo and W. H. Tuan, Ceram. Int., 2009, 35, 1317 CrossRef CAS; F. Karimzadeh, M. H. Enayati and M. Tavoosi, Mater. Sci. Eng., A, 2008, 486, 45 CrossRef; P. Yu, C.-J. Deng, N.-G. Ma and H. L. Ng Dickon, Mater. Lett., 2004, 58, 679 CrossRef CAS.
- A. Gopala, H. Kipphardt, R. Matschat and U. Panne, Mater. Chem. Phys., 2010, 122, 151 CrossRef CAS.
- A. Maleki, M. Meratian, B. Niroumand and M. Gupta, Mater. Sci. Eng., A, 2008, 39, 3034.
- A. N. Streletskii, A. N. Pivkina, I. V. Kolbanev, A. B. Borunova, I. O. Leipunskii, P. A. Pshechenkov, S. F. Lomaeva, I. A. Polunina, Yu. V. Frolov and P. Yu. Butyagin, Colloid J., 2004, 66(6), 819.
Footnote |
| † Electronic supplementary information (ESI) available: XRD supporting data for Fig. 1 and 3. See DOI: 10.1039/c0ay00057d |
| This journal is © The Royal Society of Chemistry 2010 |
