Estimation of the components in titanation mixture used in the synthesis of polyolefin catalysts
Krishna R.
Sarma
*,
Sudhakar
Padmanabhan
* and
Viral
Patel
Reliance Technology Group, Vadodara Manufacturing Division, Reliance Industries Limited, Vadodara, India 391 346. E-mail: Krishna.sarma@ril.com; Sudhakar.padmanabhan@ril.com; Fax: +91 265 669 3934; Tel: +91 265 669 6494
First published on 20th May 2010
Abstract
Traditional Ziegler Natta catalysts used for making polyethylene and polypropylene involve titanation of Mg based supports like magnesium ethoxide using a mixture of TiCl4 and chlorobenzene. Chloroalkoxy titanium species like TiCl3(OEt) are generated in the process which gets mixed with the titanation mixture. This necessitates the need for a quick, reliable and easy method of analysis for arriving at the correct composition of the residual titanation mixture so that the same can be reused after adjusting the TiCl4 and chlorobenzene contents. Estimation of TiCl3(OEt) and TiCl4 separately and also in synthetic mixtures of both has been carried out through a simple acid–base titration. The method is based on the concept that TiCl3(OEt) and TiCl4 liberate stoichiometric amounts of HCl on aqueous or alkaline hydrolysis which can be estimated by an acid–base titration. The analysis has been carried out using different synthetic mixtures of TiCl3(OEt) and TiCl4 in chlorobenzene. TiCl3(OEt) and TiCl4 were subsequently estimated by a combination of acid–base titration/UV-vis spectroscopy and gas chromatography (GC). By accounting for the titanium content due to TiCl3(OEt) and subtracting the same from the overall titanium content, the titanium content from TiCl4 was worked out. The concept and the method developed have been validated through a parity plot. A complementary method for estimating the ethoxy content in TiCl3(OEt) was also used wherein the ethoxy group was quantitatively converted into ethylbenzoate (EB) through esterification employing benzoyl chloride. The TiCl4.ethyl benzoate adduct was further hydrolysed to liberate an equivalent amount of ethylbenzoate which was estimated by quantitative GC.
Introduction
The Ziegler Natta type polyolefin catalyst involves the titanation of magnesium based supports like magnesium ethoxide using a mixture of TiCl4 and chlorobenzene. The catalyst synthesized is mainly used for polyethylene production and the same can be used for polypropylene by introducing suitable adjuvants like esters, ethers etc. at appropriate steps in the catalyst synthesis.1 During the titanation process huge quantities of chloroalkoxy titanium containing species are generated which get mixed with the titanation mixture. Reuse of the titanation mixture necessitates a correct understanding of the proper composition of the components. Due to the corrosive nature of TiCl4 routine estimation methods involving chromatography and spectroscopy are limited.2–5 Even recent findings in this subject use highly sophisticated techniques which are far away from practical use in industries.6,7The estimation of TiCl3(OEt) and TiCl4 in the titanation mixtures used during the catalyst preparation using reused and recovered mixed solvent assumes significance since the composition of the mixture is of utmost importance.8–14 TiCl3(OEt) which gets generated as an intermediate gets mixed with TiCl4 and chlorobenzene generating mixed solvent. This mixed solvent goes to the solvent recovery unit and the distillate analysis towards the TiCl3(OEt), TiCl4 and chlorobenzene contents decide how much of TiCl4 or chlorobenzene has to be added to get the desired composition of the titanation mixture. An idea of the composition of such mixed solvent in terms of TiCl4 and TiCl3(OEt) content can be obtained through a combination of UV-vis spectroscopy15,16 and GC from titanium and ethoxy content estimation.17 The present work also describes the estimation of TiCl4 and TiCl3(OEt) using simple acid–base titration along with GC. Results have been compiled on synthetic mixtures made in the laboratory and the same has been compared against results of mixed solvent generated in the lab. The merits and demerits of the technique have also been discussed.
Experimental
General experimental manipulations
All glasswares used were oven dried and cooled under N2 flow before the experiment. Experiments were performed in a well ventilated fume hood, wherever possible and applicable. Safety-wares were used while handling corrosive and toxic materials like TiCl4. All manipulations like handling and transfer of reagents were carried out in a nitrogen glove bag as far as possible.Preparation of synthetic mixtures of TiCl3(OEt) and TiCl4 in chlorobenzene
A known amount of freshly synthesized TiCl3(OEt) was weighed into a dry 50 mL reagent bottle to which a known quantity of freshly distilled chlorobenzene was added to dissolve the TiCl3(OEt). Subsequently a known quantity of TiCl4 was added and the mixture homogenized to get a synthetic mixture of known composition (Table 1).| Sample number | % of TiCl3(OEt) (By weight) | % of TiCl4 (By weight) | % of Chlorobenzene (By weight) |
|---|---|---|---|
| 1 | 4.92 | 27.30 | 67.80 |
| 2 | 8.05 | 37.09 | 54.91 |
| 3 | 14.75 | 40.02 | 45.31 |
| 4 | 19.46 | 41.39 | 39.22 |
Aqueous hydrolysis
In a typical experiment 0.1786 g of TiCl3(OEt) was transferred quickly into an Erlenmeyer flask containing 30 mL of chilled distilled water. The flask was stoppered immediately to prevent the escape of the generated HCl vapor. After swirling the flask to homogenize the contents it was titrated against 0.268 N NaOH using phenolphthalein as the indicator. The end point (appearance of pink color) was not steady since the rate of hydrolysis was sluggish especially near the end point due to incomplete hydrolysis. Further addition of a couple of drops of alkali completed the hydrolysis and gave the correct end point.Alkaline hydrolysis
This experiment was carried out in an analogous manner as described above, except the material was hydrolysed using excess of standard NaOH solution and the excess alkali was determined through back titration. For 0.2 g of sample, 25 to 30 mL of 0.25 N NaOH was enough for the experiment. Here there was no ambiguity on the end point since complete hydrolysis and neutralization had taken place.Estimation of total titanium by UV-vis method
The titanium content was determined by digesting a known quantity (∼0.5 g) of the synthetic mixture with 4 N sulfuric acid, making up to a known volume (250 mL) and then treating an aliquot with hydrogen peroxide in a 100 mL volumetric flask—the dilutions were done with distilled water, wherever needed. The absorbance of the yellow solution was then taken at 410 nm on a UV-vis spectrophotometer as per standard operating procedure.15 The absorbance was then converted to concentration of Ti using the calibration curve already generated. The overall Ti percentage in the synthetic mixture was computed. This procedure was adopted for the four synthetic mixtures.Reaction of TiCl3(OEt) with benzoyl chloride (formation of TiCl4.ethyl benzoate adduct)17
A weighed quantity of TiCl3(OEt) was dissolved in a conical flask using dry chlorobenzene. Benzoyl chloride was then added dropwise under stirring for about 15 min at ambient temperature resulting in a bright yellow precipitate. The entire contents of the flask were then subjected to hydrolysis using 0.5 N sulfuric acid to liberate the ethyl benzoate. The reaction mixture was then subject to extraction (thrice) with n-heptane. The heptane extracts containing ethyl benzoate were made up to a known volume for quantitative GC analysis. The aqueous portion was also made up to a known volume for quantitative GC analysis for ethoxy content, if any (Table 2).| Sample number | TiCl3(OEt) (g) | Benzoyl chloride (mL) | Solvent (mL) |
|---|---|---|---|
| 1 | 0.20 | 0.2 | 25 |
| 2 | 0.50 | 0.4 | 25 |
| 3 | 1.03 | 0.8 | 25 |
| 4 | 1.82 | 1.5 | 25 |
Estimation of ethoxy content by GC
The ethoxy content was determined by hydrolysing a known quantity (∼10 to 20 g) of the synthetic mixture with 0.5 N sulfuric acid, separating the chlorobenzene layer and making up the aqueous portion to a known volume. The aqueous portion containing the liberated ethanol was quantitatively analyzed by GC to get the ethoxy content in the synthetic mixture. Subtracting this value from the overall Ti, the Ti content due to TiCl4 was calculated.Results and Discussion
The typical Ziegler–Natta catalyst synthesis for ethylene or propylene polymerization involving titanation of Mg support by TiCl4 is a well studied area.1 During this process TiCl3(OEt) which gets generated as an intermediate gets mixed with TiCl4 and the solvent used. Thus, if a suitable method could be improvised to accurately weigh these materials which are hygroscopic and further prevent the escape of the liberated HCl during hydrolysis, the concept of a simple acid–base titration in estimating them should work as shown in the chemical eqn (1) and (2) using phenolphthalein as indicator. Depending upon the care taken during weighing, transfer and storage of the sample, results in the range of 99 ± 1% were obtained. Accommodating the slight error margin (on the higher side), the method probably is the easiest classical way of analyzing TiCl3(OEt) and TiCl4.| TiCl4 + 4H2O → TiO2.2H2O + 4HCl | (1) |
| TiCl3(OEt) + 4H2O → TiO2.2H2O + 3HCl + EtOH | (2) |
Replacing water in eqn (1) and (2) with excess of NaOH considerably favors the forward reaction resulting in quick neutralization of the acid generating sodium chloride as shown in eqn (3).
| HCl + NaOH → NaCl + H2O | (3) |
When both TiCl4 and TiCl3(OEt) are present as in the case of a synthetic mixture or in mixed solvent, the estimation of the ethoxy content through quantitative GC gave the amount of TiCl3(OEt) present in the system. This helped in distributing the liberated HCl in the correct proportion based on the TiCl4 and TiCl3(OEt) content so that the composition could be calculated properly using the above equations and information.
Estimation of TiCl3(OEt) by aqueous hydrolysis
Based on the theoretically expected quantity of HCl that will be generated from the amount of TiCl3(OEt) taken and converting the volume of standard NaOH to the equivalent amount of HCl (practically obtained), the assay or purity of TiCl3(OEt) was calculated. Results obtained indicated a value of 2 to 3% on the higher side in most of the cases. The probable reason for this was the extreme hygroscopicity of this material which always resulted in some free HCl present along with the material even during the weighing/transferring process. If utmost care could be taken during weighing/transfer/storage of the sample, the results were in the range of 99 ± 1%. The assay obtained through ethoxy group estimation by quantitative GC was about 99 ± 2%, also complementing the titration method.Estimation of TiCl3(OEt) by alkaline hydrolysis
This experiment was carried out through hydrolysis using excess of standard NaOH solution and the excess alkali was determined by back titration. The results were on the higher side to an extent of 2 to 3% for the reasons discussed under aqueous hydrolysis.Estimation of TiCl4 by aqueous hydrolysis and alkaline hydrolysis
This involved weighing of an extremely hygroscopic and reactive liquid and hence proper care had to be taken during weighing and transfer of TiCl4 to prevent loss of HCl vapour.6,7 If the reagent bottle was not stoppered under nitrogen properly, progressive hydrolysis took place resulting in the material getting contaminated with free HCl thus giving rise to higher values (∼3–5%).Typical results obtained on a random basis are depicted graphically as shown in Fig. 1. It can be clearly seen that there are values below 100% also indicating that sample handling during weighing/transfer/storage was of utmost importance.
 | ||
| Fig. 1 TiCl4 estimation by acid–base titration. | ||
Estimation of TiCl3(OEt) and TiCl4 in synthetic mixtures by alkaline hydrolysis
The synthetic mixtures prepared as per Table 1 were analyzed for validating the concept. 0.54 g of synthetic mixture 1 was neutralized with 40 mL of 0.268 N NaOH and 19.2 mL of 0.2036 N HCl was required for back titration of the excess NaOH. This worked out to 0.249 g of HCl which was liberated during the hydrolysis of TiCl3(OEt) and TiCl4 present in the synthetic mixture. The theoretically expected amount of HCl was 0.2382 g (0.0053 g from TiCl3(OEt) and 0.2329 g from TiCl4). This revealed that the estimated result was slightly on the higher side. A similar trend was also observed while analyzing the other synthetic mixtures. Overall titanium content in the synthetic mixtures was independently determined by standard UV-vis spectroscopic method and the results were in agreement with the titration results.A parity plot correlating the theoretically expected and practically obtained amounts of TiCl3(OEt) and TiCl4 in the synthetic mixtures through acid–base titration are given in Fig. 2 and Fig. 3.
 | ||
| Fig. 2 TiCl3(OEt) estimation parity plot. | ||
 | ||
| Fig. 3 Parity plot-estimation of TiCl4.. | ||
The slope and R2 values from the parity plots justify the agreement between theoretically expected and practically obtained results for the components in the synthetic mixtures.
Conversion of TiCl3(OEt) in the synthetic mixtures into TiCl4(ethyl benzoate) adduct and subsequent estimation of ethyl benzoate
All the synthetic mixtures prepared videTable 2 where the conversion of TiCl3(OEt) into TiCl4 (ethyl benzoate) adduct through benzoyl chloride reaction was accomplished were separately hydrolysed. The heptane extracts and the aqueous portions were analysed for ethyl benzoate and ethanol respectively by quantitative GC (Table 3).| Sample number | TiCl3(OEt) (g) | Ethylbenzoate (Theoretical, g) | Ethylbenzoate (Obtained by GC, g) | Ethanol (Theoretical, g) | Ethanol (Obtained by GC, g) |
|---|---|---|---|---|---|
| 1 | 0.20 | 0.15 | 0.14 | Nil | Not detected |
| 2 | 0.50 | 0.40 | 0.38 | Nil | Not detected |
| 3 | 1.03 | 0.80 | 0.78 | Nil | Not detected |
| 4 | 1.82 | 1.50 | 1.37 | Nil | Not detected |
A parity plot correlating the theoretically expected and practically obtained amount of ethyl benzoate is shown in Fig. 4.
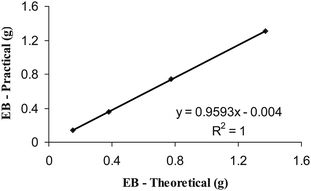 | ||
| Fig. 4 Ethoxy in TiCl3(OEt) to EB through benzoyl chloride—parity plot. | ||
The slope and R2 values reflect the close agreement between theoretically calculated and practically obtained results for ethyl benzoate in the synthetic mixtures. The absence of ethanol in the aqueous portion indicated that the ethoxy group had quantitatively reacted with benzoyl chloride to generate the TiCl4 (ethyl benzoate) adduct. This technique served as an alternate method for estimating TiCl3 (OEt) in isolation or in a mixture.
The developed concept was extended to real time mixed solvent samples generated in the catalyst preparation stage in the laboratory. The ethoxy content was quantitatively determined by GC. This enabled the total HCl liberated to be proportioned between TiCl3(OEt) and TiCl4 present in the mixture. This composition of the used titanation mixture could be adjusted accordingly for further reuse in subsequent catalyst synthesis.
Conclusions
TiCl4 and TiCl3(OEt) could be estimated through simple and elegant ways either separately or in a mixture. The determination is based on the simple concept of acid–base titration which could be carried out easily in a laboratory. The parity plots reflect the excellent agreement between the theoretical and experimental values, thus validating the concept. The efficiency of the method should improve if the technique of weighing TiCl4 and mixed solvent could be perfected.Acknowledgements
The authors sincerely thank Dr. R. V. Jasra and Dr. A. B. Mathur for their continuous encouragement to carry out this work. The authors also thank Dr. Pradip Munshi and Dr. Sumit Bhaduri for their valuable suggestions during the course of this work.References
- For synthesis of catalyst recipes from Mg(OEt)2 + TiCl4 see: J. Berthold, B. Diedrich, R. Franke, J. Hartlapp, W. Schafer and W. Strobel, US 4447587, 1984; US 4448944, 1984.
- R. F. Rolsten and H. H. Sisler, J. Am. Chem. Soc., 1957, 79(8), 1819–1821 CrossRef CAS.
- R. E. Sievers, WheelerJr, G. Ross and W.O., Anal. Chem., 1966, 38(2), 306–309 CrossRef CAS.
- R. S. JuvetWachi and F. M, Anal. Chem., 1960, 32(2), 290–291 CrossRef.
- E. Rytter and S. Kvisle, Inorg. Chem., 1985, 24(5), 639–640 CrossRef CAS.
- Y. K. Agrawal and S. Sudhakar, Talanta, 2002, 57(1), 97–104 CrossRef CAS.
- T. Kapias and R. F. Griffiths, J. Hazard. Mater., 2005, 119(1–3), 41–52 CrossRef CAS.
- J. H. Haslam, Chemistry and Uses of Titanium Organic Compounds, Metal–Organic Compounds, Chapter 25, 1959, 272–281 Search PubMed.
- J. E. McMurry, Acc. Chem. Res., 1974, 7, 281–286 CrossRef CAS.
- I. Shiihara, W. T. SchwartzJr. and H. W. Post, Chem. Rev., 1961, 61, 1–30 CrossRef CAS.
- O. Babaiah, C. K. Rao, T. S. Reddy and V. K. Reddy, Talanta, 1996, 43(4), 551–558 CrossRef CAS.
- P. J. Elving and E. C. Olson, Anal. Chem., 1955, 27(11), 1817–1820 CrossRef CAS.
- M. K. Akhtar, S. Vemury and S. E. Pratsinis, AIChE J., 1994, 40, 1183 CrossRef CAS.
- M. Rigo, P. Canu, L. Angelin and G. Della Valle, Ind. Eng. Chem. Res., 1998, 37, 1189–1195 CrossRef CAS.
- A text book of quantitative inorganic analysis including elementary instrumental analysis by A. I. Vogel, third edition ( 1975), ELBS Longman publications Search PubMed.
- W. C. Wolfe, Anal. Chem., 1962, 34(10), 1328–1330 CrossRef CAS.
- D. Lee, Y. Jeong and K. Soga, Ind. Eng. Chem. Res., 1992, 31, 2642–2647 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
