DOI:
10.1039/C0AY00017E
(Paper)
Anal. Methods, 2010,
2, 513-518
Optimization and validation of spectrofluorimetric method for the determination of clomipramine hydrochloride via ion-pair complexation with alizarin red S
Received
9th January 2010
, Accepted 26th January 2010
First published on
17th February 2010
Abstract
A simple and sensitive spectrofluorimetric method has been developed and validated for the determination of the antidepressant clomipramine hydrochloride in its dosage forms. The method was based on the fluorescent ion-pair complex formation between clomipramine and alizarin red S at pH 1.99. The ion-pair complex showed maximum fluorescence intensity at 561 nm with excitation at 490 nm. Different variables affecting the reaction conditions such as the concentration of alizarin red S, pH, reaction time and the extracting solvents were carefully studied. Under the optimised conditions, the fluorescence intensity was found to be linearly related with clomipramine hydrochloride concentration in the range of 1.0–20 μg ml−1. The detection and quantitation limits were 0.32 and 0.97 μg ml−1, respectively. No interference was observed from the excipients commonly present in dosage forms. The proposed method was successfully applied to the determination of clomipramine in its pharmaceutical tablets with good accuracy and precision; the recovery values were 99.75–100.05%.
1. Introduction
Clomipramine hydrochloride is chemically known as 3-chloro-5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine monohydrochloride with a molecular weight of 351.31. Clomipramine belongs to a group of medications called tricyclic antidepressants.1 It occurs as white to off-white crystalline powder.
Clomipramine is used for the treatment and relief of obsessive and compulsive disorders as well as in depression and other emotional disturbances. It works by increasing the amount of serotonin, a natural substance in the brain that is needed to maintain mental balance. Clomipramine is extensively biotransformed to desmethylclomipramine and other metabolites and their glucuronide conjugates. Desmethylclomipramine is pharmacologically active and participates in both therapeutic and unwanted effects. Clomipramine is considered to be the most potent inhibitor of serotonin uptake among all the tricyclic antidepressants, while its main hepatic metabolite desmethylclomipramine has been shown to be a stronger inhibitor of norepinephrine and a weaker inhibitor of 5-HT uptake than the parent drug. The usual recommended initial dose is 25 mg daily. Despite the beneficial effects of clomipramine hydrochloride the pharmacological doses of the drug had many undesirable side-effects and its overdosage may cause adverse reactions. Therefore, the therapeutical and pharmacological importance of clomipramine has prompted the development of several methods for its determination, both in body fluids and pharmaceuticals.
The assay procedure of the drug in dosage forms is official in British Pharmacopoeia,2 and United States Pharmacopoeia.3 Clomipramine has been determined in its pharmaceutical dosage forms and biological fluids by high performance liquid chromatography (HPLC),4–6 gas chromatography (GC),7 capillary gas chromatography,8 liquid chromatography (LC),9,10 capillary zone electrophoresis (CZE),11,12 flow injection analysis (FIA),13 spectrophotometry,14–19 and spectrofluorimetry.20,21 These methods were associated with some major drawbacks such as inadequate sensitivity, time-consuming, tedious, and dedicated to sophisticated and expensive instruments. Although, spectrofluorimetry is a sensitive and simple technique, however the literature relating to the suitable spectrofluorimetric methods for the determination of the clomipramine is scarce and so far only two spectrofluorimetric methods for determination of clomipramine had been reported which was time consuming.20 For these reasons, the development of a new, sensitive and simple spectrofluorimetric method that overcomes the drawbacks of the existing methodologies was very essential.
The present paper describes a new spectrofluorimetric method for the determination of clomipramine hydrochloride in pharmaceutical formulations. The method is based on the formation of ion-pair complex between clomipramine and alizarin red S at pH 1.99. The ion-pair complex was extracted with chloroform and the fluorescence intensity was measured at 561 nm with excitation at 490 nm. The reaction conditions were optimized and validated as per International Conference on Harmonisation (ICH) guidelines.22
2. Experimental
2.1 Apparatus
All fluorescence spectra were recorded on an F-2500 Hitachi fluorescence spectrophotometer (Tokyo, Japan) equipped with xenon arc lamp. Slit widths were set at 5 nm. All measurements took place in quartz cells with path length 1.0 × 1.0 cm. Excitation and emission wavelengths were set at 460 and 561 nm. Eutech (Cyberscan pH 2100) pH meter was used to measure the pH. All measurements were performed at 25 ± 1 °C.
2.2 Materials and reagents
• Clomipramine hydrochloride pure sample was obtained from Sigma chemical company (St. Louis, MO, USA) and used as received. The following commercially available pharmaceutical dosage forms were subjected to analysis: Clonil-50 tablets (Intas Pharmaceuticals Ltd., Ahmedabad, India) labelled to contain 50 mg each and Clofranil-50 tablets (Sun Pharmaceutical Industries, Mumbai, India) labelled to contain 50 mg each.
• 2.92 × 10−3 Alizarin red S (Fluka Chemie AG, Switzerland, M.W.: 342.26) solution was freshly prepared in distilled water.
• Buffer solutions ranging from pH 1.42–3.49 were prepared by mixing appropriate volumes of 1N sodium acetate and 1N HCl.
All chemicals and solvents used were of analytical grade.
2.3 Procedures
General procedure.
Portions (0.05–1.0 ml) of working standard solution of clomipramine hydrochloride (0.1 mg ml−1) were transferred into a series of 25 ml separating funnels. To each separating funnel, 2.0 ml of 2.92 × 10−3 M alizarin red S solution and 1 ml of buffer solution (pH – 1.99) were added. The contents of the separating funnel were shaken well with 5 ml of chloroform for 2.0 min and then allowed to separate the two layers. The fluorescence intensity of the formed ion-pair complex was measured at 561 nm after excitation at 490 nm. The fluorescence intensity was plotted against the concentration to get the calibration graph. The amount of the drug was evaluated either from the calibration graph or from the corresponding regression equation.
Analysis of commercial tablets.
Ten tablets of each brand were grounded and finely powdered. An amount equivalent to 100 mg of clomipramine hydrochloride was accurately weighed and treated with 30 ml distilled water and left for 10 min for complete extraction of the drug. The aqueous extract was filtered through Whatmann No. 42 filter paper (Whatmann International Limited, Kent, UK) in a 100 ml volumetric flask. The residue was washed well with distilled water and the volume was completed upto the mark. This solution was further diluted to obtain a suitable concentration and analysed by the proposed procedure.
Determination of stoichiometry.
Job's method of continuous variations23 was applied for ascertaining the stoichiometry of the ion-pair complex. This was achieved by combining different volumes i.e. 0.1, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 4.9 ml of clomipramine hydrochloride (2.85 × 10−4 M) with 4.9, 4.5, 4.0, 3.5, 3.0, 2.5, 2.0, 1.5, 1.0, 0.5, and 0.1, of alizarin red S (2.85 × 10−4 M) respectively into a 25 ml separating funnel. The contents of the funnel were shaken vigorously with 5 ml chloroform and then allowed to stand for clear separation of the organic phase. The fluorescence intensity measured at 561 nm was plotted against the mole fraction of drug.
Procedure for reference method20.
One milliliter of working standard solution of clomipramine hydrochloride (0.001–0.01 mg ml−1) was transferred into a 10 ml volumetric flask, followed by 1 ml of ceric ammonium sulfate (0.002 M). The solutions were mixed well and heated in a thermostatically controlled water bath at 100 ± 1 °C for 20 min. The flasks were then cooled and diluted to the mark with distilled water. The fluorescence intensity was measured at 355 nm with excitation at 254 nm. The amount of the drug in a given sample can be estimated either from the calibration graph or the corresponding linear regression equation.
3. Results and discussion
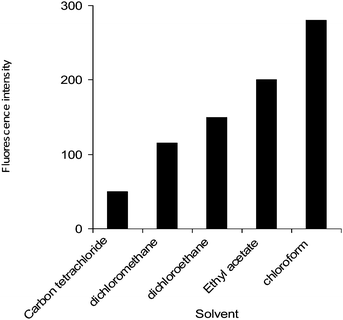
Literature survey reveals that tertiary nitrogen in clomipramine hydrochloride is capable of forming ion-pair complexes with various dyes and reagents such as azocarmine-G, naphthalene blue and naphthalene blue black,15 thymol blue,16 orange I, orange II, croceine orange, lithol Red, fast Red A,17 and picryl chloride.18 The reaction of clomipramine and alizarin red S has not been investigated so far. In the present study clomipramine hydrochloride was found to react with alizarin red S at pH 1.99 resulting in the formation of fluorescent ion-pair complex which is most suitably extracted in chloroform. Fig. 1 shows the excitation and emission spectra of ion-pair complex. The maximum fluorescence intensity was observed at 561 nm when excited at 490 nm. At pH 1.99 the clomipramine gets protonated and hence, cannot be extracted into chloroform. Similarly, the alizarin red S is existing as negatively charged species at pH 1.99 which also cannot be extracted into chloroform. Therefore, the organic layer does not contain either clomipramine or alizarine red S. The fluorescent ion-pair complex formation can be applied to enhance the sensitivity of the method for determination of clomipramine hydrochloride.
3.1 Stoichiometry and reaction mechanism
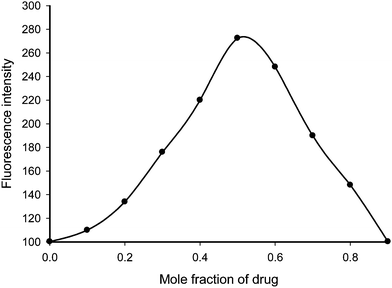
Clomipramine hydrochloride is a derivative of dibenzazepine group possessing nitrogen atom of tertiary amine group in the aliphatic chain which offers a basic characteristic to the drug. The drug is protonated at pH 1.99 and consequently a positive center is created in the drug molecule. Alizarin red S is a sodium salt of 3,4-dihydroxy-9,10-dioxo-2-anthracene sulfonic acid which ionizes in aqueous solution and thus develops a negative charge on it. The Job's plot revealed that the combining molar ratio between clomipramine and alizarin red S is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2). It indicated that one mole of clomipramine, providing one positive centre, reacts with one mole of alizarin red S, providing one negative centre, resulting in the formation of chloroform extractable ion-pair complex. Therefore, based on the literature background and our experimental findings, the possible reaction sequence is shown in Scheme 1. The apparent formation constant and Gibb's free energy (ΔG°) were calculated and found to be 9.544 × 106 and −39.82 kJ mol−1, respectively.
1 (Fig. 2). It indicated that one mole of clomipramine, providing one positive centre, reacts with one mole of alizarin red S, providing one negative centre, resulting in the formation of chloroform extractable ion-pair complex. Therefore, based on the literature background and our experimental findings, the possible reaction sequence is shown in Scheme 1. The apparent formation constant and Gibb's free energy (ΔG°) were calculated and found to be 9.544 × 106 and −39.82 kJ mol−1, respectively.
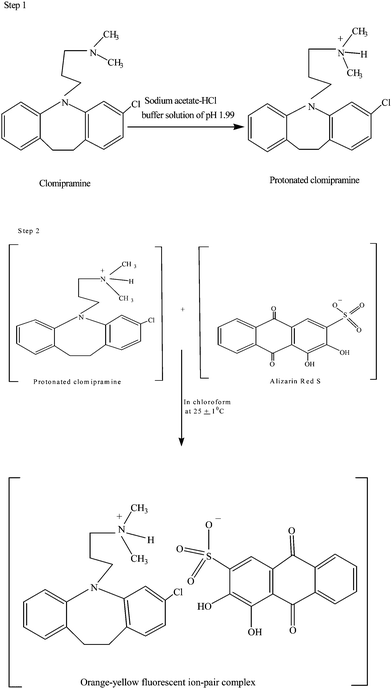 |
| | Scheme 1 Reaction pathway of the proposed method | |
3.2 Study of experimental conditions
Various experimental parameters affecting the formation of ion-pair complex were carefully studied and optimized. Such factors were changed individually while the others were kept constant. The factors include concentration of alizarin red S, pH of aqueous solution, volume of buffer solution, shaking time and solvent for the extraction of ion-pair complex.
Effect of concentration of alizarin red S.
The influence of the concentration of alizarin red S was studied using different volumes of 2.92 × 10−3 M solution of alizarin red S. It was found that the reaction of alizarin red S with clomipramine started upon using 0.5 ml of the dye in the presence of sodium acetate-HCl buffer of pH 1.99; keeping the amount of drug (20 μg) constant. Increasing the volume of alizarin red S, produces a proportional increase in the fluorescence intensity of the reaction product up to 1.9 ml and after that there was no effect on the fluorescence intensity. Therefore, 2.0 ml of 2.92 × 10−3 M of alizarin red S solution was chosen as the optimal volume of the dye.
Effect of pH.
In order to generate a positive centre in clomipramine, the reaction is carried out in acidic medium. Buffer systems (sodium acetate-HCl, potassium biphthalate-HCl) in the pH range 1.42–3.8 were tested. The sodium acetate-HCl buffer system was found to be superior to the potassium biphthalate-HCl buffer since it gave the highest fluorescence intensity. The influence of pH on the reactions was studied by carrying out the reaction in sodium acetate-HCl solution of pH 1.42–3.49. The results indicated that the fluorescence intensity increased as the pH increased and maximum readings were obtained at pH 1.99 after which the fluorescence intensity of the reaction product began to decrease gradually until pH 3.49 (Fig. 3).Therefore, pH of 1.99 was chosen as the optimum pH for the determination process.
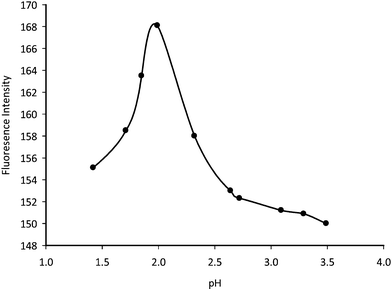 |
| | Fig. 3 Effect of different pH on the fluorescence intensity of the product (clomipramine, 20 μg ml−1). | |
Effect of volume of pH 1.99 buffer solution.
The influence of volume of pH 1.99 buffer solution on the fluorescence intensity was studied in the range of 0.2–1.5 ml. The maximum fluorescence intensity was obtained at 0.9 ml and after that the intensity remained constant. Therefore, 1.0 ml of pH 1.99 buffer solution was adopted as an optimum volume for fluorescence measurement.
Effect of shaking time for extraction.
Different time intervals (0.5–2.5 min) were tested to ascertain the shaking time required for complete extraction of the complex. It was found that the highest fluorescence intensity was reached at 1.5 min and after that the intensity remained constant up to 2.5 min. Therefore, shaking time of 2.0 min was adequate for the extraction of ion-pair complex into chloroform.
3.3 Analytical performance
Calibration and sensitivity.
Under the optimum conditions, calibration curve for the determination of clomipramine hydrochloride was constructed by plotting the fluorescence intensity as a function of the corresponding clomipramine concentration. The regression analysis of the calibration data gave the regression equation as:
where F is the fluorescence intensity, C is the concentration of clomipramine in μg ml−1. The linear relationship was obtained in the concentration range of 1.0–20.0 μg ml−1 with a correlation coefficient of 0.9999. The LOD and LOQ were determined according to ICH guidelines for validation of analytical procedures.22 The LOD and LOQ values were found to be 0.32 μg ml−1 and 0.97 μg ml−1, respectively.
Precision and repeatability.
The within days precision was evaluated through replicate analysis at three concentration levels i.e. 2.0, 10.0 and 20.0 μg ml−1 of clomipramine by performing five experiments on the same day using the same analyte standard solution. The inter-day precision on five successive days was evaluated through replicate analysis of the same concentrations of clomipramine used in intra-day precision. The results are summarized in Table 1. It can be seen from the table that the percent relative standard deviation did not exceed 0.3%, proving the high precision and accuracy of the proposed method.
Table 1 Test of precision of the proposed method
| Proposed method |
Concentration (μg ml−1) |
RSD (%) |
SAEb |
C.Lc |
| Taken |
Founda ± SD |
|
Mean for five independent determinations.
SAE, standard analytical error.
C.L, confidence limit at 95% confidence level and four degrees of freedom (t = 2.776).
|
| Intraday assay |
2.00 |
2.002 ± 0.003 |
0.14 |
0.001 |
0.004 |
| 10.00 |
10.010 ± 0.003 |
0.03 |
0.001 |
0.003 |
| 20.00 |
19.990 ± 0.003 |
0.02 |
0.001 |
0.004 |
| Interday assay |
2.00 |
1.990 ± 0.005 |
0.24 |
0.002 |
0.006 |
| 10.00 |
9.990 ± 0.008 |
0.08 |
0.003 |
0.010 |
| 20.00 |
19.990 ± 0.005 |
0.02 |
0.002 |
0.005 |
Accuracy and recovery.
Recovery studies were performed using standard addition method. For this, known quantities of pure clomipramine hydrochloride were mixed with definite amounts of preanalyzed commercial dosage forms and mixtures were analysed following the proposed method. The results are reported in Table 2. The percent recoveries obtained were quantitative (99.75–100.05%) indicating the good accuracy of the method.
| Concentration (μg ml−1) |
Coefficients of linear regression equation of standard addition |
Recoveryb (%) |
| Clonil-50 (taken) |
Standard added |
Found |
Intercept |
slope |
r
|
|
Coefficient of correlation.
Mean for five independent analyses.
|
| 4.00 |
0,4.0,8.0, 12.0,16.0 |
3.99 |
33.51 |
8.39 |
0.9999 |
99.75 |
| 8.00 |
0,4.0,8.0, 12.0,16.0 |
8.00 |
69.45 |
8.68 |
0.9996 |
100.05 |
Selectivity.
The selectivity of the method was evaluated by investigating the interference liabilities from the common excipients that might be added during pharmaceutical formulation. Tablet excipients such as lactose, starch, glucose, fructose and magnesium stearate did not interfere with the assay. The results of the recovery experiment also indicated that neither the accuracy nor the precision of the proposed method is affected by the coformulated substances.
Robustness.
Robustness was examined by evaluating the influence of small variation in the method variables on its analytical performance. In these experiments, one parameter was changed whereas the others were kept unchanged, and the recovery percentage was calculated each time. It was found that variation in the volume of 2.92 × 10−3 M alizarin red S solution (optimum ± 0.3 ml), reaction temperature, 25 ± 2 °C, volume of pH 1.99 buffer solution (optimum ± 0.2 ml), shaking time (optimum ± 0.5 min.) did not significantly affect the procedures. The proposed spectrofluorimetric method is reliable during normal usage and considered to be robust.
It is evident from the above-mentioned results that the proposed method gave satisfactory results with clomipramine in its bulk. Thus its pharmaceutical dosage forms (Clonil-50, Clofranil-50) were subjected to the analysis of their clomipramine contents by the proposed and the reference methods. The results are summarized in Table 3. The results were compared with those obtained from the reported method.20 No significant differences were found between the calculated and theoretical values of t and F-tests at 95% confidence level proving similar accuracy and precision in the determination of clomipramine by both methods. In addition to this, in both the determinations, the values of lower and upper limits based on the recovery experiments were found to be within ±2%; indicating the compliance of the regulatory guidelines.22
Table 3 Point and interval hypothesis tests: Applicability of the proposed method in pharmaceutical formulations and its comparison with the reference method at 95% confidence level
| Pharmaceutical formulations |
Proposed method |
Reference method |
t- & F valuesb |
θ
L
|
θ
U
|
| Tablets |
Recoverya (%) |
RSD (%) |
Recoverya (%) |
RSD (%) |
|
Mean for five independent analyses.
Theoretical t (ν = 8) and F-values (ν = 4) at 95% confidence level are 2.306 and 6.39, respectively.
A bias, based on recovery experiments, of ±2% is acceptable.
|
| Clonil-50 |
100.14 |
0.061 |
100.04 |
0.05 |
t = 0.270 |
0.989 |
1.010 |
|
F = 1.276 |
| Clofranil-50 |
100.05 |
0.073 |
99.99 |
0.03 |
t = 0.138 |
0.990 |
1.010 |
|
F = 2.422 |
The performance of the proposed method was compared with other existing methods (Table 4). It can be seen from the table that the linear range of the proposed method is quite appreciable and most of the determinations of clomipramine can be done in this range successfully. Moreover, the RSD value of the proposed method is smaller than that of other methods.
Table 4 Comparision of the proposed spectrofluorimetric method with other existing methods for the determination of clomipramine hydrochloride
4. Conclusions
A simple and sensitive spectrofluorimetric method for the determination of clomipramine hydrochloride has been successfully developed and validated. The method involved ion-pair complex formation between clomipramine and alizarin red S dye, and subsequent measuring the fluorescence intensity of the fluorescent reaction product at 561 nm with excitation at 490 nm. The proposed method is specific, accurate, reproducible, and highly sensitive to be applied on the analysis of pharmaceutical formulations. Furthermore, the analysis is relied on a simple apparatus, thus the proposed method is suitable for routine analysis of clomipramine hydrochloride in quality control and clinical laboratories.
Acknowledgements
The authors are grateful to Aligarh Muslim University for providing necessary research facilities. One of the authors Nasheed Afaq is thankful to UGC for award of Research Fellowship to carry out this work.
References
-
R. F. Doerge, Wilson and Gisvold's Textbook of Organic Medical and Pharmaceutical Chemistry, Lippincott, Philadelphia, 8th edn, 1982, pp. 392 Search PubMed.
-
British Pharmacopoeia, H.M. Stationery Office, London: UK, 1998, Vol. I, pp. 1584 Search PubMed.
-
The United States Pharmacopoeia, National Formulary 25, Rockville, MD: USA, 30th edn, 2007, pp. 1795–1796 Search PubMed.
- J. J. Berzas, C. Guiberteau, A. M. Contento and V. Rodriguez, Sensitive and rapid high-performance liquid chromatographic method for simultaneous determination of antidepressants in pharmaceutical formulations, Chromatographia, 2002, 56, 545–551 CAS.
- H. Yoshida, K. Hidaka, J. Ishida, K. Yoshikuni, H. Nohta and M. Yamaguchi, Highly selective and sensitive determination of tricyclic antidepressants in human plasma using high-performance liquid chromatography with post-column (2,2′-bipyridyl) ruthenium (III) chemiluminescence detection, Anal. Chim. Acta, 2000, 413, 137–145 CrossRef CAS.
- L. P. Kostishin, Identification and quantitative determination of clomipramine and melipramine in pharmaceutical preparations by HPLC, Visn. Farm., 2005, 4, 24–27 Search PubMed.
- Z. Cholobowska, E. Chudzikiewicz, P. Koscielniak, W. Pickoszewski and A. Stolarz, Application of gas chromatography with NPD detection to the analysis of tricyclic antidepressants in blood, Chem. Anal., 2003, 48, 255–263.
- J. J. B. Nevado, M. J. V. Llerena, A. M. C. Salcedo and E. A. Nuevo, Determination of fluvoxamine, and clomipramine in pharmaceutical formulations by capillary gas chromatography, J. Chromatogr. Sci., 2000, 38, 200–206 CAS.
- C. Frahnert, M. Rao and K. Grasmader, Analysis of eighteen antidepressants, four atypical antidepressants and active metabolites in serum by liquid chromatography: a simple tool for therapeutic drug monitoring, J. Chromatogr., B, 2003, 794, 35–47 CrossRef CAS.
- M. J. Ruiz-Angel, S. Carda-Broch, E. F. Simo-Alfonso and M. C. Garcia-Alvarez-Coque, Optimised procedures for the reversed-phase liquid chromatographic analysis of formulations containing tricyclic antidepressants, J. Pharm. Biomed. Anal., 2003, 32, 71–84 CrossRef CAS.
- J. R. Flores, J. J. B. Nevado, A. M. C. Salcedo and M. P. C. Diaz, Development of a capillary zone electrophoretic method to determine six antidepressants in their pharmaceutical preparations. Experimental design for evaluating the ruggedness of method, J. Sep. Sci., 2004, 27, 33–40 CrossRef CAS.
- H. S. Kau, C. C. Chen, Y. H. Huang, W. K. Ko, H. L. Wu and S. M. Wu, Method for simultaneous determination of eight cyclic antidepressants by cyclodextrin-modified capillary zone electrophoresis: applications in pharmaceuticals, Anal. Chim. Acta, 2004, 525, 23–30 CrossRef CAS.
- M. Acedo-Valenzuela, T. Galeano-Diaz, N. Mora-Diez and A. Silva-Rodriguez, Response surface methodology for the optimisation of flow-injection analysis with insitu solvent extraction and fluorimetric assay of tricyclic antidepressants, Talanta, 2005, 66, 952–960 CrossRef CAS.
- M. Begum, A. Syeda, M. A. Pasha and A. A. Syed, New spectrophotometric reagents for the determination of certain dibenzazepine drugs, J. Saudi Chem. Soc., 2005, 9, 53–64 Search PubMed.
- J. Begum, K. S. Rao and C. Rambabu, Extractive spectrophotometric determination of clomipramine, Asian J. Chem., 2006, 18, 1437–1442 CAS.
- P. Nagaraja, M. F. Silwadi and A. A. Syed, Extractive spectrophotometric determination of certain dibenzazepine antidepressants in pharmaceutical preparations, Acta Pharm., 2002, 52, 289–297 CAS.
- B. Pitarch, J. Manes and F. Bosch, Extractive-colorimetric determination of mono-, bi-, tri-, and tetracyclic antidepressants using ion pairs, An. R. Acad. Farm., 1986, 52, 279–89 Search PubMed.
- S. A. Hussein, A. M. I. Mohamed and H. Y. Hoda, Spectrophotometric determination of some dibenzazepine drugs with picryl chloride, Talanta, 1989, 36, 1147–9 CrossRef CAS.
- P. Nagaraja, M. F. Silwadi and A. A. Syed, Sensitive spectrophotometric determination of some dibenzazepine drugs with daizotized p-phenylenediamine dihydrochloride, Anal. Lett., 2000, 33, 2913–2926 CAS.
- F. A. Mohamed, H. A. Mohamed, S. A. Hussein and S. A. Ahmed, A validated spectrofluorimetric method for determination of some psychoactive drugs, J. Pharm. Biomed. Anal., 2005, 39, 139–146 CrossRef CAS.
- H. A. Mohamed and I. H. Refaat, Spectrofluorimetric determination of some dibenzazepine drugs, Bull. Pharm. Sci., 1999, 22, 191–196 Search PubMed.
- International Conference on Harmonisation (1995), ICH Harmonised Tripartite Guideline— Text on Validation of Analytical Procedures, Fed. Regist., 1995, 60, 11260 Search PubMed.
-
Acceptable methods In Drugs Directorate Guidelines, Canada Health Protection Branch, Ministry of National Health and Welfare, Draft, Ottawa, Canada, 1992 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 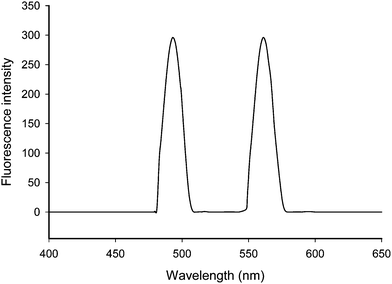
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2). It indicated that one mole of clomipramine, providing one positive centre, reacts with one mole of alizarin red S, providing one negative centre, resulting in the formation of chloroform extractable ion-pair complex. Therefore, based on the literature background and our experimental findings, the possible reaction sequence is shown in Scheme 1. The apparent formation constant and Gibb's free energy (ΔG°) were calculated and found to be 9.544 × 106 and −39.82 kJ mol−1, respectively.
1 (Fig. 2). It indicated that one mole of clomipramine, providing one positive centre, reacts with one mole of alizarin red S, providing one negative centre, resulting in the formation of chloroform extractable ion-pair complex. Therefore, based on the literature background and our experimental findings, the possible reaction sequence is shown in Scheme 1. The apparent formation constant and Gibb's free energy (ΔG°) were calculated and found to be 9.544 × 106 and −39.82 kJ mol−1, respectively.