Sensitive surfactant-mediated spectrofluorimetric determination of sildenafil†
Chien Chun
Wang
d,
Raul A.
Silva
c,
Adriana N.
Masi
bd and
Liliana
Fernandez
*ad
aÁrea de Química Analítica, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Argentina. E-mail: lfernand@unsl.edu.ar; Fax: +542652 430224
bÁrea de Bromatología, Ensayo y Valoración de Medicamentos, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Argentina
cÁrea de Farmacotecnia, Facultad de Química, Bioquímica y Farmacia, Universidad Nacional de San Luis, Argentina
dINQUISAL-CONICET, Chacabuco y Pedernera, 5700, San Luis, Argentina
First published on 10th February 2010
Abstract
Two simple and sensitive surfactant-mediated spectrofluorimetric methods for the determination of sildenafil are proposed in this paper. These methods are based on the interaction of sildenafil with normal micelles of HTAB (hexadecyltrimethylammonium bromide, method A) and the formation of ion-association complexes of sildenafil with SDS (sodium dodecyl sulfate, method B). In both methods, the formed species produce considerable fluorescence enhancement, which allows sildenafil to be quantitatively determined. Linearity was obtained for sildenafil in the concentration range 0.004 to 25 μg mL−1 with a detection limit of 0.0012 μg mL−1 by method A; and a linearity range of 0.005 to 50.0 μg mL−1 with a detection limit of 0.0016 μg mL−1 by method B. The proposed methods have been applied to the analysis of bulk drug, tablets, herbal medicine and beverages. Validation processes were performed by recovery studies and statistical analysis with satisfactory results.
Introduction
Sildenafil, popularly known as Viagra, is a selective inhibitor of cyclic guanosine monophosphate specific phosphodiesterase type 5. Clinically, is an effective drug for treatment of erectile dysfunction (ED) in males. It is also used for pulmonary hypertension, Raynaud's phenomenon, altitude sickness and Duchenne/Becker muscular dystrophy.1–4 As a selective pulmonary vasodilator, sildenafil improves gas exchange, increasing life expectancy and exercise tolerance; this has encouraged its use in premature infants with severe respiratory failure and children with primary and post-surgical pulmonary hypertension, severe lung fibrosis.5,6 As can be expected, application of sildenafil in infants requires an exhaustive control and dosage monitoring. On the other hand, sildenafil shows negative interactions with some medicine used for hypertension and ischemic heart disease treatment7 (e.g., nitroglycerine, doxazosin and terazosin). Unfortunately, ED occurs frequently in patients with these diseases (i.e. up to 60–70%). Since sildenafil was launched on the market as a novel treatment for ED, it has been converted into one of the most widespread drugs of use and abuse. Moreover, sildenafil is found as common adulterant in herbal medicine for treatment of ED and some beverages. The illicit addition of sildenafil in the wide variety of products makes it a potential risk for the society. Therefore, an accurate, sensitive and robust methodology for sildenafil determination is highly required. For this purpose, several methods have been developed,8 such as spectrophotometric,9,10 chromatographic methods,11–15 MEKC,16 voltammetric,17,18 potentiometric19 and HPLC20 methods; but determination of sildenafil based on its fluorescence property has not been reported thus far. HPLC with spectrophotometric detection is mostly applied due to its known advantages related to versatility, but often time-consuming sample pre-treatment steps are required.Surfactants are molecules containing a hydrophobic long-chain with a polar head group which could be ionic or neutral. When these amphiphilic molecules are dissolved in water, they have a tendency to form spontaneous aggregates. Reaching a particular concentration (critical micellar concentration, CMC), surfactant molecules will arrange themselves into organized molecular assemblies, also called micelles. It is widely known the ability of these organized media to provide an appropriate microenvironment able to modify catalytic and luminescence properties of reactants and products. Moreover, surfactants have the advantages of being less harmful to the environment and human health than traditional organic solvents.21 Before a critical concentration is reached, pre-micellar aggregates may still be formed.22–24
In this work, the fluorescence of sildenafil in the presence of hexadecyltrimethylammonium bromide (HTAB) and sodium dodecyl sulfate (SDS) surfactants is studied. Theoretical interaction mechanisms supported by spectral studies are proposed for drug–surfactant systems. Taking into account the great improvement on analytical sensitivity, two new direct methods for quantitative determination of sildenafil in real pharmaceutical samples are developed. Moreover, one of proposed methods has been applied in determining sildenafil in non-registered formulations and common beverages.
Experimental
Instrumental
Shimadzu RF-5301PC spectrofluorimeter (Shimadzu Corporation, Analytical Instrument Division, Kyoto, Japan), equipped with a Xenon discharge lamp and 1 cm quartz cells was used for the fluorescent measurements.Beckman spectrophotometer with 10 mm optical path cells was used to record UV-vis absorption spectra.
A pH meter (Orion Expandable Ion Analyzer, Orion Research, Cambridge, MA, USA) Model EA940 with combined glass electrode was used for monitoring pH adjustment.
Reagents
Sildenafil (as citrate) was kindly provided by Gador S.A. (Buenos Aires, Argentina). Reagents of analytical grade were used: SDS and HTAB were purchased from Tokyo Kasei Industries (Chuo-Ku, Tokyo, Japan). Tris (Mallinckrodt Chemical Works, New York, Los Angeles, St. Louis, USA), NaOH and HCl (Merck, Darmstadt, Germany). High-purity water was obtained from a Millipore (Milford, MA, USA) Milli-Q Plus System.Assay solutions
Sildenafil standard solution containing 2.0 mg mL−1 was prepared dissolving the reagent with ultra pure water. This solution was stable for at least two weeks stored at room temperature.10.0 mM SDS and 10.0 mM HTAB were prepared with an adequate weight of reagents and dissolving in ultra pure water. A 1 × 10−2 mol L−1 HCl solution was prepared by diluting an adequate volume of concentrated acid with ultra pure water.
The pH values in optimization stage were adjusted by the addition of solutions of NaOH(c) or HCl(c) until the target pH value was reached.
Sample solutions
Aqueous extract of mixture herbal (Haploppapus baylahuen, Lycopodium saururus, Baccharis articulata, Thymus vulgaris and Salvia apiana) was obtained by infusion of 5 g (dry weight) of this commercially available mixture with 80 mL of boiling ultra pure water. After 5 min., the mixture was filtered and made to 100 mL with ultra pure water.
An infusion of tea was obtained employing a commercial tea bag (containing 2 g of dry weight Camelia sinensis) and adding 80 mL of boiling ultra pure water. After 5 min., the mixture was filtered. The filtered liquid was made to 100 mL with ultra pure water.
General procedure
Results and discussions
Micelles are highly dynamic aggregates and rates of aggregation processes are nearly at the diffusion-controlled limit.25,26 When surfactant concentration is very low, these monomers behave like independent molecular entities. As surfactant concentration increases, they tend to come closer to each other and form aggregates of different size and shape, this process being thermodynamically favored. Micelles formation can be studied by several methods27 based on the fact that at the vicinity of the CMC occurs a sharp change in experimental parameters: surface tension, absorbance, rate of reaction, conductivity, osmotic pressure, etc. Fluorescence spectroscopy28 is an adequate instrumental technique for CMC determination. Additionally, micellar microenvironment can produce an enhancement on fluorescence emission and improves the sensitivity of analytical methodology.Sildenafil has a basic functional group (NH-piperazine) with a pKa value of 8.7 and a second pKa of 9.6–10.1 due to NH-amide.29,30 Due to its acid-basic characteristics, sildenafil can adopt a positive or negative charge, depending on the pH of the medium.
Sidenafil–HTAB system
When cationic surfactant HTAB is added above a certain limit, the positively charged micellar aggregates begin to form. In alkaline medium, a micellar interface with positive zeta-potential would be the preferred location for the negatively charged molecule of sildenafil to be bound, through electrostatic forces of attraction.31 With further addition of HTAB the aggregates grow until they reach the CMC value, where micelles coexist with surfactant monomers.32 When the monomer concentration is far from the CMC value, the fluorescent intensity does not increase significantly with varying CHTAB. But near to the CMC value, fluorescence signal of sildenafil is enhanced, indicating that drug molecules are bound to the micellar aggregate. The corresponding fluorescence enhancement reaches its maximum when HTAB micelles are formed (CHTAB = CMC). This spectral behaviour is attributed to the change in microenvironment surrounding sildenafil, which is quite different from bulk water. To determine the CMC value (see supporting information†), the rising part and plateau were fitted with linear functions. These two fitted lines cut each other at a point corresponding to the CMC,33 which was ∼0.80 mM, this is in good agreement with the literature reported value from pure aqueous medium.21Sidenafil–SDS system
Since early reports, researchers have already referred to anomalies in the behavior for diluted surfactants; many dye–surfactant systems, especially when the dye has opposite charge to the surfactant, form dye–surfactant aggregates.34,35Below to pH 6, sildenafil is found as cationic form. In the presence of diluted anionic surfactant SDS, where SDS monomers prevail (as dissociated form, DS−), the positively charged molecule of sildenafil is attracted by electrostatic force, forming a sildenafil–DS complex. In Fig. 1, emission spectra of sildenafil–SDS are shown. The emission intensity enhancement accompanied by bathochromic shift, is evidence of sildenafil–DS complex formation. Further addition of SDS produces the formation of some mixed aggregate or micellar structure. This phenomena can be described by these following equations:
| Sildenafil+ + DS− → sildenafil–(DS) | (1) |
| Sildenafil–(DS) + SDS → mixed micelles | (2) |
 | ||
| Fig. 1 Fluorescence spectra of sildenafil–SDS system. Csildenafil = 40 μg mL−1; pH 5; CSDS = 0.0–12.0 mM. λexc = 315 nm; λem = 415 nm. | ||
 | ||
| Fig. 2 Effect of pH on sildenafil–surfactants systems. CSildenafil = 40 μg mL−1. a: CHTAB = 1.0 mM, λexc = 290 nm; λem = 435 nm; b: CSDS = 0.40 mM, λexc = 315 nm; λem = 415 nm. | ||
In Fig. 3, plots of pH influence on sildenafil fluorescence intensity for systems with different surfactant concentrations are presented. Optimal experimental values of pH and surfactants concentration were: for sildenafil–HTAB system, 11.0 at 1.0 mM of HTAB, and for sildenafil–SDS system, 5 at 0.4 mM of SDS.
 | ||
| Fig. 3 Influence of pH at different surfactant concentrations. Fig. 3a) a: pH = 3.5; b: pH = 7.5; c: pH = 11 (λexc = 290 nm; λem = 435nm). Fig. 3b) a: pH = 5.5; b: pH = 3.5; c: pH = 6.5; d: pH = 7.5; e: pH = 8.5; f: pH = 11 (λexc = 315 nm; λem = 415 nm). Csildenafil = 40 μg mL−1. | ||
| Analytical parameters | Spectrofluorimetry | UV-visible Spectrophotometry | |
|---|---|---|---|
| Method A | Method B | ||
| a Excitation slit width = 5 nm; emission slit width = 10 nm. b Excitation slit width = 5 nm; emission slit width = 5 nm. | |||
| λ max (nm) | λ exc = 290 | λ exc = 315 | 225 |
| λ em = 435 | λ em = 415 | ||
| LOL (μg mL−1) | (0.004–14.8)a | (0.005–22.5)a | 5.6–50.0 |
| (0.013–25)b | (0.018–50.0)b | ||
| Slope (μg mL−1) | 67.50a | 44.37a | 0.0441 |
| Intercept | 45.47a | 24.33a | 0.03 |
| Correlation coefficient | 0.9980a | 0.9998a | 0.9973 |
| SD of blank (n = 6) | 0.029a | 0.024a | 0.026 |
| LOQ (μg mL−1) | 0.004a | 0.005a | 5.60 |
| LOD (μg mL−1) | 0.0012a | 0.0016a | 1.76 |
Fig. 4 shows the calibration curve of sildenafil in the presence and absence of surfactants. As can be seen, the linear range (LOL) for sildenafil in aqueous medium has a linearity limit of 20.0 μg mL−1. In presence of HTAB micelles, this limit was extended to 25.0 μg mL−1; employing 0.40 mM of SDS, this limit was extended up to 50.0 μg mL−1. In both methods, an important improvement in analytical sensitivity was achieved.
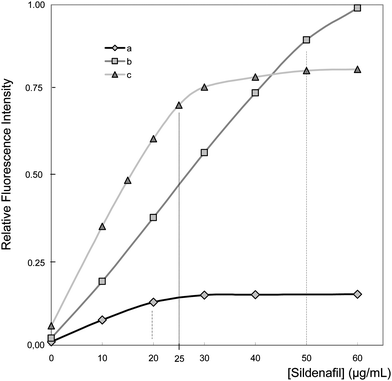 | ||
| Fig. 4 Calibration curve of sildenafil in absence and presence of surfactants. a: aqueous medium; b: 0.40 mM (CSDS ≪ CMC), pH 5, λexc = 315 nm; λem = 415 nm; c: 1.0 mM (CHTAB ≥ CMC), pH 11, λexc = 290 nm; λem = 435 nm. | ||
The deviation of linearity calibration curve of sildenafil in aqueous medium could be explained by the dimerization processes of the drug.32 This aggregation process may conduce to auto-quenching. The presence of surfactants leads to complex formation, diminishing the probability of dimerization of sildenafil molecules.
Table 2 shows some reported methods for sildenafil determination. The proposed methods have the best detection limits, only exceeded by ESI mass-spectrometry with prior HPLC separation. Additional advantages of the developed methodologies are associated with the instrumental and operational simplicity, adequately sensitivity and having the widest linearity range.
| Method | Detection system | LOL (μg mL−1) | LOD (μg mL−1) | Reference |
|---|---|---|---|---|
| Extractive spectrophotometric methods | UV-visible spectrophotometry | Method A 1.25–25 | 0.16 | 9 |
| Method B 1.5–60 | 0.18 | |||
| HPLC | UV-visible spectrophotometry | 0.01–1 | Not available | 12 |
| HPLC-MS | Electrospray positive ionization (ESI) mass-spectrometry | 0.000125–0.04 | 0.00005 | 16 |
| Micellar electrokinetic chromatography | UV-visible spectrophotometry | 0.080–0.9 | 0.017 | 17 |
| Adsorptive stripping square-wave voltammetry | Voltammetry | 0.029–0.32 | Not available | 18 |
| Polymer membrane sensors | Potentiometry | 6.6–600 | 3.3 | 19 |
| This work – Surfactant-mediated spectrofluorimetry | Spectrofluorimetry | Method A 0.004–25 | 0.0012 | — |
| Method B 0.005–50 | 0.0016 |
| Formulation | Label claim (mg/tablet) | Added (mg) | Found (mg) ± RSD (%)a | Recovery (%)a,b | ||
|---|---|---|---|---|---|---|
| A | B | A | B | |||
| a Average of 6 replicates. b 100 × [(found − base)/added)]. c Base value. A and B = developed methods A and B respectively. | ||||||
| Vorst® | 25 | — | 24.98c ± 1.34 | 24.66c ± 0.80 | — | — |
| 5 | 29.83 ± 1.50 | 29.73 ± 1.16 | 97.0 | 101.4 | ||
| 10 | 34.95 ± 1.66 | 34.67 ± 1.02 | 98.7 | 100.1 | ||
| 50 | — | 48.98c ± 1.52 | 48.68c ± 1.16 | — | — | |
| 5 | 54.01 ± 0.97 | 53.75 ± 0.54 | 100.6 | 101.4 | ||
| 10 | 58.95 ± 1.36 | 58.78 ± 1.12 | 98.7 | 101.0 | ||
| Magnus® | 25 | — | 25.28c ± 1.80 | 25.02c ± 0.90 | — | — |
| 5 | 30.24 ± 0.73 | 30.11 ± 2.03 | 99.2 | 101.8 | ||
| 10 | 35.37 ± 0.66 | 34.97 ± 1.64 | 100.9 | 99.5 | ||
| 50 | — | 50.99c ± 0.52 | 50.79c ± 0.88 | — | — | |
| 5 | 55.95 ± 0.80 | 55.87 ± 1.08 | 99.2 | 98.6 | ||
| 10 | 60.95 ± 1.00 | 60.89 ± 1.12 | 99.62 | 101.0 | ||
Moreover, the developed methods were applied to sildenafil determination in local herbal medicine for ED treatment and common beverages. Method B presented severe limitations due to spectral matrix interferences; probably, the equilibrium of complex formation between sildenafil and SDS is perturbed by the high salinity levels of samples. Table 4 shows the obtained results of sildenafil quantification by method A. In this case, method A proved to be adequate for sildenafil quantification in non-registered formulations.
| Samples a | Added (μg) b | Found (μg) ± RSD (%)c | Recovery (%) |
|---|---|---|---|
| a 1: Lycopodium saururus. Sample volume used = 1 mL. 2: Commercial herbal mixture of Haploppapus baylahuen, Lycopodium saururus, Baccharis articulata, Thymus vulgaris, Salvia apiana. Sample volume used = 0.1 mL. 3: Commercial powder orange juice. Sample volume used = 0.1 mL. 4: Camelia sinensis. Sample volume used = 0.1 mL. b Final volume = 10 mL. c Average of 3 replicates. | |||
| 1 | 5.00 | 5.04 ± 2.50 | 100.86 |
| 10.00 | 10.06 ± 1.05 | 100.60 | |
| 2 | 5.00 | 4.94 ± 3.00 | 98.84 |
| 10.00 | 9.99 ± 1.09 | 99.99 | |
| 3 | 5.00 | 5.06 ± 2.90 | 101.20 |
| 10.00 | 10.00 ± 0.77 | 100.00 | |
| 4 | 5.00 | 5.07 ± 2.20 | 101.40 |
| 10.00 | 9.96 ± 0.75 | 99.67 |
Conclusions
Fluorescent properties of sildenafil have been studied in the presence of HTAB and SDS surfactants. Since sildenafil could adopt cationic or anionic form, the working pH was a fundamental parameter for its interaction with surfactants. Use of surfactants provides a simple means to enhance the fluorescence of sildenafil (5.5-fold and 7.3-fold increases achieved by HTAB and SDS, respectively) as well as avoiding the self-quenching process of the drug. Therefore, improved LOD and extents on linearity of working curve to determine sildenafil were achieved employing these surfactants. Proposed methods use simple reagents and low-cost instruments. They have proper sensitivity to permit sildenafil determination even down to 0.0012 μg mL−1. These methods are simple, sensitive, accurate, and with good precision allowing the analysis of sildenafil in pharmaceutical quality control. Additionally, method A proved to be effective to sildenafil quantification as adulterant in herbal medicine and beverages.Acknowledgements
The authors wish to thank INQUISAL-CONICET (Instituto de Química de San Luis - Consejo Nacional de Investigaciones Científicas y Tecnológicas), FONCYT (Fondo Nacional de Ciencia y Tecnología) and National University of San Luis (Project 22/Q828) for the financial support.References
- M. Boolell, M. J. Allen, S. A. Ballard, S. Geti-Attee, G. J. Muirhead, A. M. Naylor, I. H. Osterloh and C. Jingell, Int. J. Impot. Res., 1996, 8, 47–52 CAS.
- F. Montorsi, T. E. D. McDermott, R. Morgan, A. Olsson, A. Schultz, H. J. Kirkeby and I. H. Osterloh, Urology, 1999, 53, 1011–1018 CrossRef CAS.
- H. P. Rang, M. M. Dale, J. M. Ritter and P. K. Moore, in Pharmacology, Churchill Livingstone, Edinburgh, London, New York 5th ed., 2003 Search PubMed.
- J. D. Corbin and S. H. Francis, J. Androl., 2003, 24, S38–S41 CAS.
- D. Abrams, I. Schulze-Neick and A. G. Magee, Heart, 2000, 84, E4 CrossRef CAS.
- L. S. Shekerdemian, H. B. Ravn and D. J. Penn, Am. J. Resp. Crit. Care. Med., 2002, 165, 1098–1102.
- R. A. Kloner, Am. J. Cardiol., 2005, 96, 42–46 CrossRef.
- S. Singh, B. Prasad, A. A. Savaliya, R. P. Shah, V. M. Gohil and A. Kaur, TrAC, 2009, 28, 13–28 Search PubMed.
- N. D. Dinesh, P. Nagaraja, N. M. Made Gowda and K. S. Rangappa, Talanta, 2002, 57, 757–764 CrossRef CAS.
- G. Altiokka, Z. Atkosar, E. Sener and M. Tunçel, J. Pharm. Biomed. Anal., 2001, 25, 339–342 CrossRef CAS.
- C. Pistos, I. Papoutsis, A. Dona, M. Stefanidou, S. Athanaselis, C. Maravelias and C. Spiliopoulou, Forensic Sci. Int., 2008, 178, 192–198 CrossRef CAS.
- Ming-Thau Sheua, An-Bang Wua, Geng-Cheng Yehb, Angel Hsiaa and Hsiu-O Ho, J. Chromatogr., B, 2003, 791, 255–262 CrossRef CAS.
- E. Mikami, T. Ohno and H. Matsumoto, Forensic Sci. Int., 2002, 130, 140–146 CrossRef CAS.
- C. Man, N. Nor, R. Lajis and G. Harn, J. Chromatogr., A, 2009, 1216, 8426–8430 CrossRef CAS.
- Y. Wang, J. Wang, Y. Cui, J. P. Fawcett and J. Gu, J. Chromatogr., B, 2005, 828, 118–121 CrossRef CAS.
- J. J. Berzas Nevado, J. Rodríguez Flores, G. Castañeda Peñalvo and N. Rodríguez Fariñas, J. Chromatogr., A, 2002, 953, 279–286 CrossRef CAS.
- J. Rodrıguez, J. J. Berzas, G. Castañeda and N. Rodríguez, Talanta, 2004, 62, 427–432 CrossRef CAS.
- K. Tyszczuk and M. Korolczuk, Voltammetric method for the determination of sildenafil citrate (Viagra) in pure form and in pharmaceutical formulations, Bioelectrochemistry, 2009 DOI:10.1016/j.bioelechem.2009.08.005.
- A. M. Othmana, N. M. H. Rizk and M. S. El-Shahawi, Anal. Chim. Acta, 2004, 515, 303–309 CrossRef CAS.
- S. Trefi, V. Gilard, S. Balayssac, M. Malet-Martino and R. Martino, Magn. Reson. Chem., 2009, 47, S163–S173 CAS.
- E. Pramauro and E. Pelizzetti, Wilson & Wilson's, in Comprehensive analytical Chemistry: Surfactants in Analytical Chemistry –Applications of Organizad Amphiphilic Media, ed. S. G. Weber, Elsevier, The Netherlands, 1996, vol. 31, ch. 3, pp 131–202 Search PubMed.
- C. E. Drennan, R. J. Hughes, V. C. Reinsborough and O. O. Soriyan, Can. J. Chem., 1998, 76, 152–157 CrossRef CAS.
- P. Wängnerud and B. Jönsson, Langmuir, 1994, 10, 3542–3549 CrossRef.
- S. Niu, K. R. Gopidas and N. J. Turro, Langmuir, 1992, 8, 1271–1277 CrossRef CAS.
- P. J. Sams, E. Wyn-Jones and J. Rassing, Chem. Phys. Lett., 1972, 13, 233–236 CrossRef CAS.
- E. A. G. Aniansson, S. N. Wall, M. Almgren, H. Hoffmann, I. Kielmann, W. Ulbricht, R. Zana, J. Lang and C. Tondre, J. Phys. Chem., 1976, 80, 905–922 CrossRef CAS.
- P. W. Atkins, in Physical Chemistry; W.H. Freeman and Co., New York, 1998 Search PubMed.
- D. C. Saha, K. Ray and T. N. Misra, Spectrochim. Acta, Part A, 2000, 56, 797–801 CrossRef CAS.
- E. G. C. Clark and A. C. Moffat, in Clark's Isolation and Identification of Drugs, 3th ed., The Pharmaceutical Press, London, 2004, pp 1559–1560 Search PubMed.
- M. M. Al Omari, M. B. Zughul, J. E. D. Davies and A. A. Badwan, J. Pharm. Biomed. Anal., 2006, 41, 857–865 CrossRef CAS.
- M. Haldar and Chowdhury, Chem. Phys. Lett., 1999, 312, 432–439 CrossRef CAS.
- M. Bielska, A. Sobczyńska and K. Prochaska, Dyes Pigm., 2009, 80, 201–205 CrossRef CAS.
- A. Dominguez, A. Fernández, N. Gonzalez, E. Iglesias and L. Montenegro, J. Chem. Educ., 1997, 74, 1227–1231 CrossRef CAS.
- P. Mukerjee and K. J. Mysels, J. Am. Chem. Soc., 1955, 77, 2937–2943 CrossRef CAS.
- H. Sato, M. Kawasaki and K. Kasatani, 1981, 17, pp. 243–248.
Footnote |
| † Electronic supplementary information (ESI) available: Figure 1. Fluorescence emission spectra of sildenafil–HTAB. Figure 2. Influence of HTAB concentration on sildenafil fluorescence intensity. Figure 3. UV-spectra of sildenafil–SDS system. See DOI: 10.1039/b9ay00208a |
| This journal is © The Royal Society of Chemistry 2010 |
