Flow injection analysis of paracetamol using a biomimetic sensor as a sensitive and selective amperometric detector
Mariana C. Q.
Oliveira
a,
Marcos R. V.
Lanza
b,
Auro A.
Tanaka
c and
Maria D. P. T.
Sotomayor
*a
aAnalytical Chemistry Department, Institute of Chemistry, São Paulo State University (UNESP), 14800-900, Araraquara, SP, Brazil. E-mail: mpilar@iq.unesp.br; Tel: +55-16-33016620
bDepartment of Molecular Chemistry and Physics, University of São Paulo, 13560-970, São Carlos, SP, Brazil
cCenter of Physical Sciences and Technology, Federal University of Maranhão, 65085-580, São Luís, MA, Brazil
First published on 16th March 2010
Abstract
This work describes the coupling of a biomimetic sensor to a flow injection system for the sensitive determination of paracetamol. The sensor was prepared as previously described in the literature (M. D. P. T. Sotomayor, A. Sigoli, M. R. V. Lanza, A. A. Tanaka and L. T. Kubota, J. Braz. Chem. Soc., 2008, 19, 734) by modifying a glassy carbon electrode surface with a Nafion® membrane doped with iron tetrapyridinoporphyrazine (FeTPyPz), a biomimetic catalyst of the P450 enzyme. The performance of the sensor for paracetamol detection was investigated and optimized in a flow injection system (FIA) using a wall jet electrochemical cell. Under optimized conditions a wide linear response range (1.0 × 10−5 to 5.0 × 10−2 mol L−1) was obtained, with a sensitivity of 2579 (±129) μA L μmol−1. The detection and quantification limits of the sensor for paracetamol in the FIA system were 1.0 and 3.5 μmol L−1, respectively. The analytical frequency was 51 samples h−1, and over a period of five days (320 determinations) the biosensor maintained practically the same response. The system was successfully applied to paracetamol quantification in seven pharmaceutical formulations and in water samples from six rivers in São Paulo State, Brazil.
Introduction
Pharmaceutical substances and personal care products have recently been recognized as an important class of organic pollutants due to their physical–chemical properties, which allow their persistence and bioaccumulation in the environment, provoking negative effects in aquatic or terrestrial ecosystems at concentrations as low as a few nanograms per liter.1–3Many studies have reported the presence of a large number of pharmaceuticals, such as anti-inflammatories, analgesics, β-blockers, lipid regulators, antibiotics, anti-epileptics and estrogens, at average concentrations in the μg L−1 range, in sewage treatment plant effluents, surface and groundwater and even in drinking water,4–9 indicating their poor degradability in sewage treatment plants.5,8,9
Concern about pharmaceuticals has often focused on antibiotics, which may promote resistance in natural bacterial populations, and on hormones, which may induce estrogenic responses as well as alterations in the reproduction or development of aquatic organisms.1,2,10,11 Wastewaters are the main disposal route of pharmaceuticals into the environment. Nevertheless, other different sources of pharmaceutical release can be proposed to explain the appearance of these xenobiotics in waters and soils, such as inadequate treatment of manufacturing waste, direct disposal of unconsumed drugs in households and the use of manure for topsoil dressing.1,4,5,10 These compounds are considered as a new category of persistent pollutants.3
There is therefore interest in the development of simple, sensitive, selective and reliable methods for the determination of these chemicals, not only in the aquatic environment, but also during production of pharmaceuticals and in dissolution studies.12
Paracetamol (acetaminophen, N-acetyl-p-aminophenol, 4-acetamidophenol or tylenol) is included in this new class of environments pollutants. It is widely used for young children, old people, and in pregnancy as an antipyretic and analgesic drug, to control mild to moderate pain or reduce fever. Its action is similar to that of aspirin, so it is an appropriate alternative for patients who are sensitive to acetylsalicylic acid.13
Thus, it is very important to establish fast, simple and accurate methodologies for the detection of paracetamol in quality control analyses (in pharmaceutical formulations), for medical control (in biological fluids such as urine, blood or plasma) and more recently for application in environment control in samples from sewage treatment plant effluents, surface and groundwater and even in drinking water.14 It is known that overdose ingestions of acetaminophen can lead to accumulation of toxic metabolites, which may cause severe and sometimes fatal hepatotoxicity and nephrotoxicity.15–17
Many analytical methodologies, using both batch and flow modes, have been proposed for the determination of paracetamol, including titrimetry,18 spectrophotometry (spectrofluorimetry, near infrared reflectance spectrometry, Raman spectrometry and Fourier transform infrared spectrophotometry),19–26 chromatography,27,28 chemiluminescence29–31 and enzymatic analysis.32,33 Electrochemical methods34–36 are an attractive alternative, since they are fast, inexpensive, and can be adapted for portable, disposable and miniaturized applications.
Paracetamol can be readily oxidized at carbon paste or glassy carbon electrodes,37 however these amperometric procedures are non-selective, since the potential involved in this process ranges from 0.6 to 0.8 V, and various substances are electroactive in this potential interval. However, use of modified electrodes, such as biosensors32,37 and more recently biomimetic sensors,38 can enable operation at potentials much lower than those normally used, thus decreasing the interference.37
Flow injection analysis (FIA) has been routinely used since its inception to provide automated control of sample handling.39 FIA involves the injection of a reproducible sample volume into a continuously flowing carrier solution, followed by quantification at a detector device. In addition to automated control, FIA offers other advantages including convective mass transport, matrix exchange and increased precision. The combination of FIA with electrochemical and selective sensors is an attractive tool because of the flexibility of the former and the diagnostic power of the latter.40 Flow-through electrochemical sensors allow the continuous real-time monitoring of analytes, and are among the most important types of analytical tools that have received extensive attention, in pharmaceutical analysis as well as in other environmental and biochemical applications.13,37,40,41
This paper reports on the development of the first (as far as we are aware) biomimetic sensor coupled to a FIA system for analysis of paracetamol in pharmaceutical formulations and aquatic samples.
Experimental
Chemicals and solutions
All chemicals used in the construction and application of the sensor were analytical grade reagents. All chemicals used in the chromatographic experiments were high performance liquid chromatography (HPLC) grade reagents.The biomimetic complex, iron(III) tetrapyridinoporphyrazine (FeTPyPz), was prepared and purified as previously described.42
Acetic acid and N,N–dimethylformamide (DMF) were acquired from Synth (São Paulo, Brazil). Paracetamol (acetaminophen) was obtained from Sigma (St. Louis, USA). 5% (m/v) Nafion® solution was from Aldrich (Milwaukee, USA). Sodium acetate was acquired from Merck (Darmstadt, Germany), and all HPLC grade solvents were from Tedia (Rio de Janeiro, Brazil).
Paracetamol and acetate buffer solutions were prepared with water purified in a Millipore Milli-Q system, and the pH was determined using an Orion® pH meter (Model 3 Star pH).
Biomimetic sensor construction
The biomimetic sensor was constructed as previously described.38 First, a solution containing 5 mg mL−1 of the FeTPyPz in DMF was prepared. Then, the surface of a glassy carbon (GC) electrode (Metrohm®, Switzerland), with a geometrical area of 0.126 cm2, was cleaned according to the procedure described in the literature.43 After cleaning the electrode, 100 μL of FeTPyPz solution was mixed with 50 μL of 5% (m/v) Nafion® solution, and an aliquot of 50 μL of this mixture placed on the surface of the electrode. Finally, the solvent was evaporated at room temperature during 6 h, forming a thin green film.Flow manifold and electrochemical measurements
The biomimetic sensor was inserted into a flow-through wall-jet amperometric cell and used as the working electrode (Fig. 1). An Ag|AgCl(KClsat) electrode was the reference, and a platinum wire was the auxiliary electrode. The electrodes were connected to a potentiostat (Palm-sense®, Palm Instruments BV, The Netherlands) interfaced to a microcomputer for potential control and data acquisition.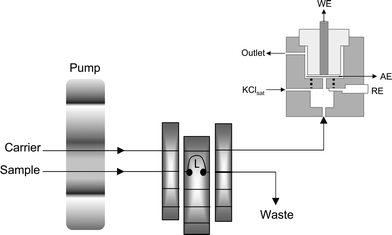 | ||
| Fig. 1 Schematic diagram of the flow injection system for amperometric determination of paracetamol. L: sample loop (75 μL); WE: working electrode (biomimetic sensor); AE: auxiliary electrode (platinum); RE: home-made Ag|AgCl(KClsat reference electrode). | ||
A peristaltic pump (Ismatec®) was used to provide the flow of the 0.1 mol L−1 (pH 3.6) acetate buffer carrier solution. The standards and samples containing paracetamol were injected into the carrier using a sliding central bar sampling valve.44
HPLC analysis
In order to validate the results obtained using the proposed sensor, they were compared to those obtained using a chromatographic method based on HPLC.45 Chromatographic analyses were performed using a Shimadzu® Model 20A liquid chromatograph coupled to an UV/Vis detector (SPD-20A), autosampler (SIL-20A) and a degasser DGU-20A5, controlled by a personal computer. A C18 column (250 × 4.6 mm, Shim-Pack CLC–ODS) was used, inside an oven (Shimadzu® CTO–10AS) maintained at 30 °C. The mobile phase was a mixture of methanol and water in a ratio of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 (v/v). The flow rate was 1.0 mL min−1, the sample injection volume 20 μL, and the detector absorption wavelength 254 nm.
87 (v/v). The flow rate was 1.0 mL min−1, the sample injection volume 20 μL, and the detector absorption wavelength 254 nm.
Pharmaceutical formulation analysis
Quantification of paracetamol in seven commercial samples was carried out according to the external standards method. Samples of four commercial solutions were analyzed after simple dilution with deionized water, without additional treatment. These were: Paracetamol (Ache®, Lot L0804193, validity 08/10), labeled value of 200 g L−1 (1.32 mol L−1 paracetamol); Tylenol® for children (Janssen-Cilag, Lot LFL169, 04/10), fruit flavor with labeled value of 160 mg of paracetamol for each 5 mL of syrup; Tylenol® in drops (Janssen-Cilag, Lot FL121, 04/11), labeled value of 200 g L−1; Tylenol® for babies, concentrated oral suspension (Janssen-Cilag, LAL008, 11/09), fruit flavor with labeled value of 100 g L−1 of paracetamol. Three additional samples of tablets were also analyzed: Tylenol® DC (Janssen-Cilag, Lot LCL008, 01/11), labeled value per tablet of 500 mg of paracetamol and 65 mg of caffeine; Tylenol® AP (Janssen-Cilag, Lot LFL105, 02/10), labeled value per tablet of 650 mg of paracetamol; Paracetamol (EMS®, Lot L15655, 05/10), labeled value of 750 mg of paracetamol per tablet.Aquatic sample analysis
Six water samples from rivers in São Paulo State, Brazil, were enriched with paracetamol and then analyzed using the proposed sensor in order to evaluate the matrix effect on recovery values.Results and discussion
Biomimetic sensor for paracetamol
The optimization and performance, in batch mode, of the biomimetic sensor used in this work has been reported previously.38Table 1 provides the characteristics of this sensor, where the FeTPyPz complex acts as a biomimetic catalyst of the P450 enzyme.46| Parameter | Response |
|---|---|
| a RSD: relative standard deviation. | |
| Linear range/mol L−1 | 4.0 × 10−6 to 4.2 × 10−4 |
| Sensitivity/μA L mol−1 | 5798 ± 73 |
| Correlation coefficient | 0.9994 (n = 10) |
| Detection limit/μmol L−1 | 1.2 |
| Quantification limit/μmol L−1 | 4.0 |
| Measurement repeatability (RSDa, n = 7 and [Paracetamol] = 2.4 × 10−4 mol L−1) | 3.4 |
| Reproducibility in the construction of the sensors (RSDa, n = 4) | 5.0 |
| Applied potential [mV vs. Ag|AgCl(KClsat)] | 450 |
| Response time/s | 0.5 |
| Sensor lifetime/days | 180 |
| Operational stability/measurements | 50 (kept 95% of the initial signal) |
| Paracetamol diffusion coefficient/cm2 s−1 | 1.066 × 10−6 |
| Apparent Michaelis-Menten constant (KappMM)/mol L−1 | 2.15 × 10−2 mol L−1 |
The sensitivity, operational stability and response time shown by the sensor in batch mode indicated that the device should be able to be satisfactorily coupled to a flow system. Thus, in the next step the sensor was installed in a FIA system shown in Fig. 1. An advantage of flow conditions is that the electrochemical products are more efficiently removed from the electrode surface, avoiding poisoning of the sensor and increasing its lifetime.47 Further experiments, using the FIA system, were performed in order to optimize parameters including flow rate, injected sample volume (Vi) and applied potential.
FIA system optimization
Optimization of the applied potential was necessary because a home-made Ag|AgCl(KClsat) reference electrode was used in the wall jet cell. Fig. 2 shows the results obtained under flow conditions. The maximum response of the sensor was achieved at an applied potential of 500 mV, thus this potential was selected during subsequent experiments. Although, in the batch system,1 higher sensitivity was obtained at 450 mV vs. a commercial Ag|AgCl(KClsat) electrode (Table 1), under flow conditions a small increment of +50 mV was observed.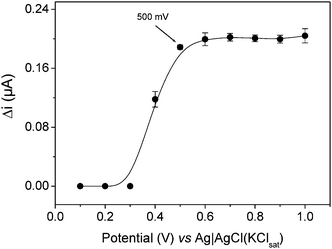 | ||
| Fig. 2 Influence of the applied potential on the response to 1.0 × 10−4 mol L−1 paracetamol, with Vi of 75 μL, flow rate of 1.25 mL min−1 and using a 0.1 mol L−1 acetate buffer at pH 3.6 as carrier. The error bars correspond to the standard deviation for seven replicates. | ||
The flow rate dependence of the signal response of the biomimetic sensor can be seen in Fig. 3. The signal response for paracetamol increases up to a flow rate of 1.25 mL min−1 because mass transfer, accompanied by electronic transfer, increases with increasing flow rate. The subsequent decrease in the signal response with increased flow rate is due to limitations of the chemical reaction, since as the sample passes through the electrode faster, the fraction of the substrate oxidized by its chemical reaction with the FeTPyPz is smaller. This behavior can be explained considering the short response time (0.5 s), and the response mechanism of the sensor to be characteristic of a typical catalytic electro-oxidation (chemical/electrochemical).38,48 Therefore an optimized flow rate of 1.25 mL min−1 was used.
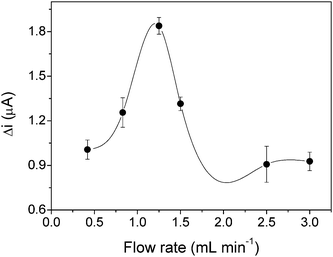 | ||
| Fig. 3 Effect of the flow rate on the response to 1.0 × 10−4 mol L−1 paracetamol, with Vi of 75 μL, and using a 0.1 mol L−1 acetate buffer at pH 3.6 as carrier. The error bars correspond to the standard deviation for seven replicates. | ||
The effect of the injected sample volume (Vi) on the sensor response was studied. The signal response (Fig. 4) increased with increasing Vi, perhaps related to a longer sample contact time with the catalytic zone of the biomimetic sensor. However, at higher sample volume a lower analytical frequency and lower measurement repeatability were obtained; as evaluated by the standard deviation for seven replicates. A Vi of 75 μL was therefore selected, on the basis of better repeatability.
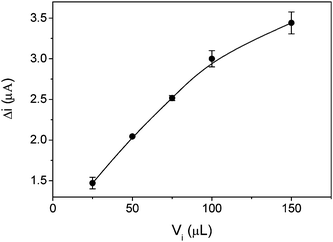 | ||
| Fig. 4 Effect of injected sample volume (Vi) on the response to 4.0 × 10−4 mol L−1 paracetamol. The experiments were carried out with 0.1 mol L−1 acetate buffer at pH 3.6 as carrier, with a flow rate of 1.25 mL min−1 and applying a potential of 500 mV vs. Ag|AgCl. The error bars correspond to the standard deviation for seven replicates. | ||
Analytical characteristics of the proposed FIA system
Fig. 5 presents the FIA signals as a function of paracetamol concentration under optimized conditions, and it can be observed that there is no memory effect for the sensor response. The analytical curve obtained from the data in Fig. 5 showed a wide linear response range, from 1.0 × 10−5 to 5.0 × 10−2 mol L−1, described by| Δi = 2702 (±77) [Paracetamol] + 0.02 (±0.01) | (Eq. 1) |
![FIA signals obtained for a biomimetic sensor under flow conditions for different paracetamol analyses. Experimental conditions: Vi = 75 μL, 0.1 mol L−1 acetate buffer at pH 3.6 (1.25 mL min−1) and applied potential of 500 mV vs. Ag|AgCl. [Paracetamol]: a = 1.0 × 10−5 mol L−1; b = 5.0 × 10−5 mol L−1; c = 1.0 × 10−4 mol L−1; d = 5.0 × 10−4 mol L−1; e = 1.0 × 10−3 mol L−1; f = 5.0 × 10−3 mol L−1; g = 1.0 × 10−2 mol L−1; h = 2.5 × 10−2 mol L−1; i = 5.0 × 10−2 mol L−1.](/image/article/2010/AY/b9ay00283a/b9ay00283a-f5.gif) | ||
| Fig. 5 FIA signals obtained for a biomimetic sensor under flow conditions for different paracetamol analyses. Experimental conditions: Vi = 75 μL, 0.1 mol L−1 acetate buffer at pH 3.6 (1.25 mL min−1) and applied potential of 500 mV vs. Ag|AgCl. [Paracetamol]: a = 1.0 × 10−5 mol L−1; b = 5.0 × 10−5 mol L−1; c = 1.0 × 10−4 mol L−1; d = 5.0 × 10−4 mol L−1; e = 1.0 × 10−3 mol L−1; f = 5.0 × 10−3 mol L−1; g = 1.0 × 10−2 mol L−1; h = 2.5 × 10−2 mol L−1; i = 5.0 × 10−2 mol L−1. | ||
Detection and quantification limits, determined from the standard deviation of ten independent measurements of the blank in accordance with IUPAC recommendations,52 were 1.04 × 10−6 mol L−1 and 3.46 × 10−6 mol L−1, respectively. The detection limit value is lower than that obtained for another biosensor for paracetamol, based on the HRP enzyme.32
The repeatability of the biomimetic sensor was investigated for injections of 4.0 × 10−4 mol L−1 paracetamol solution, and the relative standard deviation between injections was 1.2% (n = 7). Under these conditions, the biosensor can be operated with an analytical frequency of 51 injections h−1. The FIA system reproducibility was evaluated by comparison of the sensitivities obtained using three sensors prepared on different days. The RSD value obtained was 5.0%, the same as that obtained for the sensor in batch mode.
Finally, Fig. 6 shows the operational stability of the sensor under flow conditions. This parameter was investigated using eight consecutive injections of 1.0 × 10−3 mol L−1 paracetamol standard solution, every 30 min for 4 h each day, over a 5 day period, totaling 64 injections per day or 320 injections over the 5 days of the evaluation. During this time the sensor presented the same response.
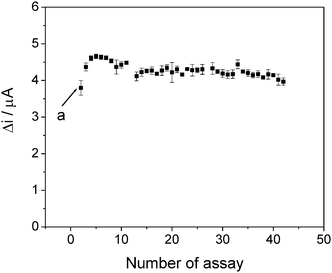 | ||
| Fig. 6 Relative response obtained for 1.0 × 10−3 mol L−1 paracetamol at an applied potential of 500 mV vs. Ag|AgCl, as a function of the number of assays. (a) Conditioning of the system. Each point corresponds to an average of eight injections. | ||
The flow was maintained during periods when no injection was carried out, in order to evaluate the resistance of the film on the electrode surface. After 5 days the film was still firmly adhered to the electrode surface, with no change in its appearance. This is important, since an ability for at least eight hours continuous monitoring is often required of automated methodologies.
The analytical parameters for paracetamol quantification using the FIA system are shown in Table 2. All characteristics demonstrate the viability of the application of this biomimetic sensor for determination of paracetamol in pharmaceutical formulations, and in environmental and industrial samples.
| Parameter | Response |
|---|---|
| Applied potential vs. Ag|AgCl(KClsat) | 0.5 V |
| Flow rate/mL min−1 | 1.25 |
| Vi/μL | 75 |
| Linear range/mol L−1 | 1.0 × 10−5–5.0 × 10−2 |
| Sensitivity/μA L μmol−1 | 2579 ± 129 |
| Quantification limit/μmol L−1 | 3.5 |
| Detection limit/μmol L−1 | 1.0 |
| Measurement repeatability (RSD, n = 7 and [Paracetamol] = 4.0 × 10−4 mol L−1) | 1.2% |
| Reproducibility in the construction of the sensors (RSD, n = 3) | 5.0% |
| Operational stability | 320 measurements or 5 days (kept 93% of the initial signal) |
| Analytical frequency | 51 samples h−1 |
Application
The application of the proposed sensor was tested in a similar way to the batch mode,38 using two different sample types. The first type, the commonest, were the seven commercial formulations. The second type were the river water samples, which were enriched with paracetamol at two levels of concentration in order to demonstrate the applicability of the proposed FIA system to environmental samples.Table 3 shows the results obtained for the determination of paracetamol in the commercial formulations. The concentrations were determined by the FIA system using the external calibration method, and compared with those obtained with the official chromatographic method. The results were not significantly different, at a confidence level of 95%, suggesting that the proposed methodology is an efficient and rapid alternative for paracetamol analyses.
| Sample | Label value | Experimental value | Error (%) | |
|---|---|---|---|---|
| Comparative method (HPLC) | Proposed sensor | |||
| Tylenol® (LFL121, 04/11) | 200 mg mL−1 | 190 | 196 | +3.2 |
| Paracetamol (L0804193, 08/10) | 200 mg mL−1 | 197 | 203 | +3.0 |
| Tylenol® (LFL169, 04/10) | 160 mg/5 mL | 162 | 163 | +0.6 |
| Tylenol® (LAL008, 11/09) | 100 mg mL−1 | 92 | 96 | +4.3 |
| Tylenol DC (LCL008, 01/11) | 500 mg/tablet | 500 | 488 | −2.4 |
| Tylenol AP (LFL105, 02/10) | 650 mg/tablet | 656 | 680 | +3.6 |
| Paracetamol (L15655, 05/10) | 750 mg/tablet | 765 | 800 | +4.6 |
Fig. 7 shows the response profile obtained for analyses of the six river water samples enriched with paracetamol at two concentration levels. It can be seen in Table 4 that very good recoveries were obtained, with values close to 100% and near those obtained with the HPLC method.
![FIA responses obtained for the sensor calibration and for measurements of the six river waters enriched with paracetamol at two levels of concentration. A–F: 5.0 × 10−2 mol L−1 paracetamol; A′–F′: 1.0 × 10−4 mol L−1 paracetamol. A and A′: Tietê river at the Usina da Barra club; B and B′: Tietê river on the outskirts of Igaraçu city; C and C′: Tietê river by the bridge in Barra Bonita city; D and D′: Jacaré Guaçú; E and E′: Jacaré Pepira; F and F′: Jaú. [Paracetamol]: the same as a–i in Fig. 5.](/image/article/2010/AY/b9ay00283a/b9ay00283a-f7.gif) | ||
| Fig. 7 FIA responses obtained for the sensor calibration and for measurements of the six river waters enriched with paracetamol at two levels of concentration. A–F: 5.0 × 10−2 mol L−1 paracetamol; A′–F′: 1.0 × 10−4 mol L−1 paracetamol. A and A′: Tietê river at the Usina da Barra club; B and B′: Tietê river on the outskirts of Igaraçu city; C and C′: Tietê river by the bridge in Barra Bonita city; D and D′: Jacaré Guaçú; E and E′: Jacaré Pepira; F and F′: Jaú. [Paracetamol]: the same as a–i in Fig. 5. | ||
| River | % Recovery | |
|---|---|---|
| FIAa | HPLC | |
| a Average of recovery values for the two concentration levels. | ||
| Jacaré Pepira | 100.0 ± 2.0 | 96 |
| Jacaré Guaçú | 96.4 ± 3.6 | 94 |
| Jaú | 99.8 ± 1.3 | 96 |
| Tietê river on the outskirts of Igaraçu city | 97.8 ± 1.9 | 94 |
| Tietê river by the bridge in Barra Bonita city | 96.9 ± 0.5 | 94 |
| Tietê river at the Usina da Barra club | 95.8 ± 5.6 | 92 |
Conclusions
This work describes for the first time the determination of paracetamol using a biomimetic sensor as a sensitive and selective detector within a flow injection system. The FIA arrangement presents a wide response range and high sensitivity, and was satisfactorily applied to the analysis of commercial pharmaceutical formulations. It was also demonstrated that the drug can be quantified in aqueous samples, such as river water.Acknowledgements
The authors gratefully acknowledge financial support from FAPESP. MCQO is indebted to FAPESP for a fellowship. The authors acknowledge Profa. Dra. Mary Rosa Rodrigues de Marchi and Mr. Thiago Bernardo Cavassani from GRESCO (IQCAr–UNESP) for instruction in use of the HPLC technique.References
- B. Halling-Sorensen, S. N. Nielsen, P. F. Lanzky, F. Ingerslev, H. C. H. Lutzhoft and S. E. Jorgensen, Chemosphere, 1998, 36, 357–393 CrossRef CAS.
- J. P. Bound and N. Voulvoulis, Chemosphere, 2004, 56, 1143–1155 CrossRef CAS.
- C. G. Daughton, Environ. Impact Assess. Rev., 2004, 24, 711–732 CrossRef.
- T. Heberer, Toxicol. Lett., 2002, 131, 5–17 CrossRef CAS.
- M. Stumpf, T. A. Ternes, R. D. Wilken, S. V. Rodrigues and W. Baumann, Sci. Total Environ., 1999, 225, 135–141 CrossRef.
- R. Céspedes, S. Lacorte, D. Raldú, A. Ginebreda, D. Barceló and B. Piña, Chemosphere, 2005, 61, 1710–1719 CrossRef CAS.
- D. Bendz, N. A. Paxéus, T. R. Ginn and F. J. Loge, J. Hazard. Mater., 2005, 122, 195–204 CrossRef CAS.
- N. Lindqvist, T. Tuhkanen and L. Kronberg, Water Res., 2005, 39, 2219–2228 CrossRef CAS.
- R. Andreozzi, R. Marotta and N. Paxéus, Chemosphere, 2003, 50, 1319–1330 CrossRef CAS.
- M. D. Hernando, M. Mezcua, A. R. Fernández-Alba and D. Barceló, Talanta, 2006, 69, 334–342 CrossRef CAS.
- K. Fent, A. A. Weston and D. Caminada, Aquat. Toxicol., 2006, 76, 122–159 CrossRef CAS.
- M. L. Ramos, J. F. Tyson and D. J. Curran, Anal. Chim. Acta, 1998, 364, 107 CrossRef CAS.
- F. S. Felix, C. M. A. Brett and L. Angnes, J. Pharm. Biomed. Anal., 2007, 43, 1622 CrossRef CAS.
- D. Loffler, J. Rombke, M. Meller and T. A. Ternes, Environ. Sci. Technol., 2005, 39, 5209 CrossRef.
- F. L. Martin and A. E. McLean, Drug Chem. Toxicol., 1998, 21, 477 CrossRef CAS.
- C. A. Mugford and J. B. Tarloff, Toxicol. Lett., 1997, 93, 15 CrossRef CAS.
- H. T. Nagasawa, D. W. Shoeman, J. F. Cohen and W. B. Rathbun, J. Biochem. Toxicol., 1996, 11, 289 CrossRef CAS.
- M. K. Srivastava, S. Ahmed, D. Singh and I. C. Shukala, Analyst, 1985, 110, 735 RSC.
- Z. Boushsain, S. Garrigues, A. Morales-Rubio and M. de la Guardia, Anal. Chim. Acta, 1996, 330, 59 CrossRef CAS.
- G. Amann, G. Gubitz, R. W. Frei and W. Santi, Anal. Chim. Acta, 1980, 116, 119 CrossRef CAS.
- K. Molt and M. Egelkraut, GIT Fachz. Lab., 1988, 32, 1311 Search PubMed.
- T. H. King, C. K. Mann and T. J. Vickers, J. Pharm. Sci., 1985, 74, 443 CAS.
- Z. Boushsain, S. Garrigues and M. de la Guardia, Analyst, 1996, 121, 635 RSC.
- M. A. Evans and R. D. Harbinson, J. Pharm. Sci., 1977, 66, 1628 CAS.
- A. Ivaska and T. H. Ryan, Collect. Chem. Commun, 1981, 46, 2865 Search PubMed.
- M. L. Ramos, J. F. Tyson and D. J. Curran, Anal. Chim. Acta, 1998, 364, 107 CrossRef CAS.
- M. J. Stewart, P. I. Adriaenssens, D. R. Jarvie and L. F. Prescott, Ann. Clin. Biochem, 1979, 16, 89 CAS.
- A. N. Papas, M. Y. Alpert, S. M. Marchese and J. W. Fitzgerald, Anal. Chem., 1985, 57, 1408 CrossRef CAS.
- I. I. Koukli, A. C. Calokerinos and T. P. Hadjiioannou, Analyst, 1989, 114, 711 RSC.
- A. G. Alapont, L. L. Zamora and J. M. Calatayud, J. Pharm. Biomed. Anal., 1999, 21, 311 CrossRef CAS.
- K. Vandyke, M. Sacks and N. Qazi, J. Biolumin. Chemilumin., 1998, 13, 339 CrossRef CAS.
- I. C. Vieira, K. O. Lupetti and O. Fatibello-Filho, Quim. Nova, 2003, 26, 39 CAS.
- O. Fatibello-Filho, K. O. Lupetti and I. C. Vieira, Talanta, 2001, 55, 685 CrossRef.
- J. Wang, T. Golden and P. Tuzhi, Anal. Chem., 1987, 59, 740 CrossRef CAS.
- P. Masawat, S. Liawruangrath, Y. Vaneesorn and B. Liawruangrath, Talanta, 2002, 58, 1221 CrossRef CAS.
- W. Peng, T. Li, H. Li and E. Wang, Anal. Chim. Acta, 1994, 298, 415 CrossRef CAS.
- G. A. Messina, I. E. De Vito and J. Raba, Anal. Chim. Acta, 2006, 559, 152 CrossRef CAS.
- M. D. P. T. Sotomayor, A. Sigoli, M. R. V. Lanza, A. A. Tanaka and L. T. Kubota, J. Braz. Chem. Soc., 2008, 19, 734 CAS.
- J. Ruzicka and E. H. Hansen, Anal. Chim. Acta, 1975, 78, 145 CrossRef CAS.
- P. Cervini and E. T. G. Cavalheiro, J. Braz. Chem. Soc., 2008, 19, 836 CAS.
- A. D. Vidal, P. O. Barrales and A. M. Díaz, Microchim. Acta, 2003, 141, 157 CrossRef.
- A. A. Tanaka, C. Fierro, D. A. Scherson and E. Yeager, Mater. Chem. Phys., 1989, 22, 431 CrossRef CAS.
- P. Calvo-Marzal, S. S. Rosatto, P. A. Granjeiro, H. Aoyama and L. T. Kubota, Anal. Chim. Acta, 2001, 441, 207 CrossRef CAS.
- H. Bergamin, F. J. X. Medeiros, B. F. Reis and E. A. G. Zaggatto, Anal. Chim. Acta, 1978, 101, 9 CrossRef.
- G. Lunn; HPLC-Methods for Pharmaceutical Analysis, John Wiley & Sons, Inc.: New York, 2000, vol. 1 Search PubMed.
- M. Sono, M. P. Roach, E. D. Coulter and J. H. Dawson, Chem. Rev., 1996, 96, 2841 CrossRef CAS.
- S. S. Rosatto, M. P. T. Sotomayor, L. T. Kubota and Y. Gushikem, Electrochim. Acta, 2002, 47, 4451 CrossRef CAS.
- A. J. Bard and L. R. Faulkner; Electrochemical Methods: Fundamentals and Applications, 2nd ed., John Wiley & Sons, Inc.: New York, 2001 Search PubMed.
- M. J. A. Cañada, M. I. P. Reguera, A. R. Medina, M. L. F. Córdova and A. M. Díaz, J. Pharm. Biomed. Anal., 2000, 22, 59 CrossRef CAS.
- J. A. M. Pulgarin and L. F. G. Bermejo, Anal. Chim. Acta, 1996, 333, 59 CrossRef.
- W. T. Suarez, H. J. Vieira and O. Fatibello-Filho, Eclética Quim, 2005, 30, 21 Search PubMed.
- A. Currie, Anal. Chim. Acta, 1999, 391, 105 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
