Simultaneous quantification of azadirachtin and 3-tigloylazadirachtol in Brazilian seeds and oil of Azadirachta indica: application to quality control and marketing
Moacir Rossi
Forim
,
Maria Fátima das Graças Fernandes
da Silva
*,
Quezia Bezerra
Cass
,
João Batista
Fernandes
and
Paulo Cezar
Vieira
Departamento de Química, Universidade Federal de São Carlos, CP 676, 13565-905, São Carlos, SP, Brazil
First published on 27th May 2010
Abstract
A rapid, sensitive and selective HPLC-UV method was developed for quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in Neem kernels and oil, with a good linearity over a range of 5.0–60.0 and 2.5–50.0 μg mL−1 respectively, with r2 > 0.999 for all curves. The limits of detection (LOD) for both limonoids were 25 ng mL−1 for kernels and 0.10 μg mL−1 for oil, and quantification (LOQ) for 1 and 2 were 1.5 μg mL−1 for both matrices. The quantification method was applied to Neem seeds and oil from different areas of Brazil. The low quality of Brazilian oil (concentrations of 1 = 228.5 to 630 mg kg−1 and 2 = 117.1 to 582.9 mg kg−1) could be attributed to mechanical extraction, since the content of azadirachtin and 3-tigloylazadirachtol in seeds was (1 = 2048.8 to 5117.1 mg kg−1, 2 = 224.7 to 1116.5 mg kg−1) similar to values obtained from Indian seeds (1 = 556.9 to 3030.8 mg kg−1, 2 = 43.1 to 590.6 mg kg−1).
1. Introduction
The Neem tree, Azadirachta indica A. Juss, is native of the Indian sub-continent, but also grows well in other tropical and subtropical areas around the world.1,2 It was introduced successfully in Northern, Northeastern and Western regions of Brazil.3 Detection of azadirachtin from all parts of Neem trees have been described in the literature,4 but it has been frequently reported to be present at highest concentrations in mature seeds.5 Unfortunately, Rembold (1989)6 introduced the names azadirachtin A, B, C and D, describing these as isomers. Azadirachtin A is identical to azadirachtin, therefore the original name stands. Since the structure of azadirachtin B was determined by Klenk et al. (1986)7 and named 3-tigloylazadirachtol, that name stands. Azadirachtin C was never fully described, thus the name is unused. Azadirachtin D is correctly 1-tigloyl-3-acetyl-11-hydroxymeliacarpin. These three compounds belong to different compound groups and are not isomers.1 Later, the authors isolated the limonoids from the meliacarpin groups, naming them as azadirachtin E, F, G, etc, however, the correct names are 1-detigloylazadirachtin, 1-hydroxy-3-tigloyl-11,19-deoxo-11-hydroxymeliacarpin, 3-tigloyl-13,14-desepoxy-17-hydroxyazadirachtol, respectively (Kraus, 1995, 2002).8,9Neem tree plantations have been established in Brazil, specifically to supply oil from fruits for the production of natural insecticides and cosmetic products. The main problems are due to the issues of standardization and quality control. The oil should contain a specified concentration of active ingredient as an assurance that the product will perform as intended. In addition, the concentration of azadirachtin in Neem oil depends on the quality of seeds used. Thus, it would be better to determine the active components first in the seeds. In this case, the precision of analysis depends on the extraction methods. There are several methods to obtain Neem oil from the seeds, such as mechanical pressing, supercritical fluid extraction, and solvent extraction. Mechanical extraction is the most widely used method to extract the Neem oil from seed.10 However, the oil produced with this method usually has a low price, since it contains water and a lower amount of azadirachtin. Extraction using supercritical fluid produces oil of a very high purity; however, this analytical methodology is very expensive to be routinely used. Extraction using solvent has several advantages. It gives higher yield than mechanical extraction, and has a relatively low operating cost compared with supercritical fluid extraction.10
These factors have provided a stimulus to develop a rapid and sensitive method for quantitative determination of azadirachtin (1) and 3-tigloylazadirachtol (2) (Fig. 1) in Brazilian Neem kernels and oil, for quality control and better marketing of seeds and oil. Since many analytical high-performance liquid chromatography (HPLC) methods fail to separate 1 and 2, reported concentrations of both limonoids may actually represent the sum of these two active components.11 Thus, our study focused on the simultaneous quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in a single run and the analysis of the analytes by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) to confirm their purity. In order to assess an efficient extraction in a shorter time, a new method of extraction using n-hexane and methanol, mechanical agitation and centrifugation was developed and both limonoids were obtained in large amounts. This extraction method is in development on a pilot-scale and will be available soon for industry with low investment cost.
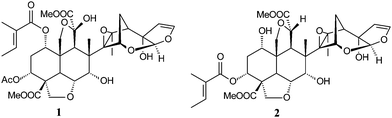 | ||
| Fig. 1 Structure of azadirachtin (1) and 3-tigloylazadirachtol (2). | ||
2. Experimental
2.1. Chemicals and materials
Methanol, acetonitrile and tetrahydrofuran (Mallinckrodt, St. Louis, MO, USA) used for the mobile phase was HPLC grade; prior to use they were filtered through a polytetrafluoroethylene (PTFE) membrane Millipore filter (0.45 μm) and degassed by sonication for 15 min. All other solvents used in extraction and isolation were of analytical grade (Mallinckrodt Baker, SA, Xalostoc, Mexico). Deuterated chloroform (CDCl3) was from Aldrich Chemical Company, Inc. (Milwaukee, Wis., USA). Water was purified with Millipore Milli-Q apparatus (Millipore, São Paulo, SP, Brazil) and degassed by sonication for 15 min prior to use. Column chromatography was performed with either Kieselgel 60 (230–400 mesh, Merck KGaA, 64271, Darmstadt, Germany) or Sephadex LH-20 (25–100 μm, Pharmacies Fine Chemical Co. Ltd., Uppsala, Sweden). The solid phase extraction (SPE) cartridge used was cyanopropyl SPE cartridge (Bakerbond Spe Cyano, J. T. Backer).2.2. Instrumentation
The HPLC system consisted of Shimadzu LC-10ATVP pump (Kyoto, Japan), a FCV-10ALVP solenoid valve, a DGU-14A degasser, a SIL-10AF auto sample injector, a SPD-10ALVP multi-wavelength absorbance detector, a SCL-10AVP interface. Data acquisition was performed using CLASS LC10 software. A commercial analytical octadecylsilane (C18) column (Phenomenex-Luna II; 150 × 4.6 mm i.d., 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm) was employed for the screening analysis of Neem kernels methanolic extract. A second HPLC system consisted of Shimadzu LC-6AD pump (Kyoto, Japan), a SPD-10ALVP multi-wavelength absorbance detector, Rheodyne 7725i injection valve with a 200 μL loop. Data acquisition was performed using CLASS-VP software. Semi preparative Phenomenex Luna Phenyl Hexyl column (250 × 6 mm column, 10 μm; Phenomenex, Torrance, CA, USA) was used for azadirachtin (1) and 3-tigloylazadirachtol (2) separation. SPE clean-up was performed using a vacuum manifold processor (Varian, Harbor City, CA, USA) using a vacuum pump TE-058 (Tecnal, Piracicaba, SP, Brazil). One and two-dimensional nuclear magnetic resonance (1D and 2D NMR) spectra were recorded on a Bruker Avance DRX-400 spectrometer (Karlsruhe, Germany) (1H, 400 MHz; 13C, 100 MHz). An ultrasonic bath (Brason 1510R, Danbury, USA) was use to de-gas solvents. Low resolution electrospray mass spectra were carried out on a Micromass Quattro LC-triple quadrupole instrument (Manchester, UK), which was operated in the negative ion mode, scanning from m/z 100–700. The mass conditions were optimized by direct injection. The source voltage was 3.90 kV; capillary voltage, 26–28 V; extractor, 4 V; RF lens, 0.23 V; capillary temperature, 130 °C; desolvation temperature, 300 °C. Data acquisition and processing were carried using the MassLynx 3.0 software supplied with the instrument.2.3. Extraction and isolation of azadirachtin (1) and 3-tigloylazadirachtol (2) standards
Fresh, ripe fruits of A. indica were collected from the experimental campus of the Agronomic Institute of Paraná, Xambrê-PR, Brazil, in April, 2005. The plant was identified by Dr Sueli de Souza Matinez. A voucher specimen is deposited at the Herbarium of the State University of Maringá PR, Brazil, number 11709-HUM. Fully matured fruits were manually depulped and the mesocarp (seed coat) adhering to the hard epicarp was removed to obtain the seed kernels. The air-dried powdered seed kernels (800 g) were extracted using hexane by percolation processing at room temperature repeated four times, and for 1 h each time, using mechanical agitation and centrifugation. The supernatant oil was transferred into a separate vial, this fraction did not contain azadirachtin (1) and 3-tigloylazadirachtol (2), and thus it was discarded. The precipitate was extracted using methanol by percolation processing at room temperature repeated five times, and for 12 h each time, using mechanical agitation and centrifugation. Crude methanolic extract (52 g) was obtained after filtration and evaporation of the organic solvent under vacuum at 40 °C. A portion of the resultant methanolic extract (25 g) was chromatographed over silica gel (230–400 mesh, CHCl3–acetonitrile–MeOH gradient) yielding a fraction containing both 1 and 2. This fraction was chromatographed by gel permeation (Sephadex LH 20, MeOH) to give further fractions. The 1H NMR (200 MHz) of the all fractions showed characteristics of azadirachtin (1) and 3-tigloylazadirachtol (2) only in fraction 6, and then, it was subjected to semi preparative HPLC [Phenomenex Luna Phenyl Hexyl, 250 × 6 mm column, 10 μm; acetonitrile–MeOH–THF–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61), flow rate 1.8 mL min−1, UV = 217 and 254 nm] to yield azadirachtin (1; 47 mg) in fraction 6 and 3-tigloylazadirachtol (2; 23 mg) in fraction 7.
61), flow rate 1.8 mL min−1, UV = 217 and 254 nm] to yield azadirachtin (1; 47 mg) in fraction 6 and 3-tigloylazadirachtol (2; 23 mg) in fraction 7.
2.4. Sample preparation
2.4.1.1. Chromatography method development. The final chromatographic system was chosen after a series of screening analyses. Seed kernel concentrate extract was reconstituted in 400 μL of hexane and subjected to clean-up procedures. The clean-up of each extract was performed by applying this solution in a 1 mL 100 mg−1 cyanopropyl SPE cartridge (Bakerbond Spe Cyano, J. T. Backer) previously conditioned with 5 mL of hexane and it was not dried. The residue of concentrate extract was dissolved in an additional 600 μL of hexane and also passed through the same cyanopropyl SPE cartridge and it was then washed with 4 mL of hexane. The solvent was removed from the cartridge using a vacuum manifold pump and afterwards the tube was dried. This fraction did not contain azadirachtin (1) and 3-tigloylazadirachtol (2), and thus it was discarded. The residue of concentrate extract after hexane washing was twice reconstituted in 500 μL of MeOH, and both fractions were passed through the same cyanopropyl SPE cartridge. The solvent was removed from the cartridge by vacuum. Eluent was evaporated on the Speed-Vac (Savant SpeedVac Plus SC 10 A, São Paulo, Brazil) at 43 °C and re-suspended in 1.0 mL of MeOH for HPLC analysis. The method selectivity was determined by comparison of the chromatograms of extracts with chromatograms of standards of 1 and 2, and the identification was achieved by comparing the retention time of peaks. The optimal conditions consisted of an isocratic solvent system containing acetonitrile–MeOH–THF–H2O (34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61; THF: tetrahydrofuran), employing a reverse-phase analytical C18 column (Phenomenex-Luna II; 150 × 4.6 mm i.d., 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm), at flow rate of 0.4 mL min−1, UV = 217 nm and injector fitted with a 20 μL loop.
61; THF: tetrahydrofuran), employing a reverse-phase analytical C18 column (Phenomenex-Luna II; 150 × 4.6 mm i.d., 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm), at flow rate of 0.4 mL min−1, UV = 217 nm and injector fitted with a 20 μL loop.
2.4.1.2. HPLC-MS/MS. The individual peaks of azadirachtin (1) and 3-tigloylazadirachtol (2) were identified by HPLC-MS/MS. All analyses were carried out using an Alliance 2795 HPLC (Waters, Manchester, UK) coupled with a Quattro Premier T-Wave mass spectrometer. A reverse-phase analytical C18 column (Phenomenex-Luna II; 150 × 4.6 mm, 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm) was used. Mobile phase consisted of an isocratic mixture of acetonitrile–MeOH–THF–H2O (34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61) at flow rate of 0.4 mL min−1 and injector fitted with a 20 μL loop. To deliver the samples into the mass spectrometer, a splitter was used at a rate of 2
61) at flow rate of 0.4 mL min−1 and injector fitted with a 20 μL loop. To deliver the samples into the mass spectrometer, a splitter was used at a rate of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (waste:MS). Samples were ionized using an electrospray (ES) ion source operating in negative mode. The temperatures of block source and probe were set at 135 °C and 300 °C, respectively. Optimization of the flow rates of drying gases as well as the three main ionization parameters (capillary, cone, and extractor voltages) were performed using direct infusion into the ES source. The flows of nebulizer and desolvation gases (nitrogen) were 51 and 350 L h−1, respectively. The capillary was then set at 2.94 kV for all experiments.
1 (waste:MS). Samples were ionized using an electrospray (ES) ion source operating in negative mode. The temperatures of block source and probe were set at 135 °C and 300 °C, respectively. Optimization of the flow rates of drying gases as well as the three main ionization parameters (capillary, cone, and extractor voltages) were performed using direct infusion into the ES source. The flows of nebulizer and desolvation gases (nitrogen) were 51 and 350 L h−1, respectively. The capillary was then set at 2.94 kV for all experiments.
2.4.2.1. Chromatography method development. Hydraulic press Neem seed oil was homogenized and 15 mg was dissolved in 200 μL hexane and subjected to the same clean-up procedures as those for seed kernels above. The eluent was evaporated on Speed-Vac (Savant SpeedVac Plus SC 10 A, São Paulo, Brazil) at 43 °C and re-suspended in 1.0 mL of MeOH for HPLC analysis. The method selectivity was determined in the same way as for the seed kernels. The optimal conditions consisted of an isocratic solvent system containing MeOH–acetonitrile–THF–H2O (36.75
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35
7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9
4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51), employing a reverse-phase analytical C18 column (Phenomenex-Luna II; 150 × 4.6 mm i.d., 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm), at flow rate of 0.8 mL min−1, UV = 217 nm and injector fitted with a 20 μL loop.
51), employing a reverse-phase analytical C18 column (Phenomenex-Luna II; 150 × 4.6 mm i.d., 5 μm) equipped with a precolumn (Phenomenex; 4 × 3 mm, 5 μm), at flow rate of 0.8 mL min−1, UV = 217 nm and injector fitted with a 20 μL loop.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61) for analysis of seed kernels, and the other, MeOH–acetonitrile–THF–H2O (36.75
61) for analysis of seed kernels, and the other, MeOH–acetonitrile–THF–H2O (36.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35
7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9
4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51) for oil, and peak area responses were obtained. A calibration curve for each limonoid was constructed by plotting the peak area against the concentration (average of three runs).
51) for oil, and peak area responses were obtained. A calibration curve for each limonoid was constructed by plotting the peak area against the concentration (average of three runs).
2.5. Preparation of samples from different areas of Brazil
Neem seed kernels were also collected in March 2006, from different cities of eight Brazilian states, Xambrê and Paranavaí (Paraná), Brejinho do Nazaré (Tocantins), Timbaúba, Petrolina and Recife (Pernambuco), Itinga (Minas Gerais), Catanduva, Ariranha and Urupês (São Paulo), Fortaleza (Ceará), Três Lagoas (Mato Grosso) and Juazeiro (Bahia). Powdered seed kernels were subjected to solvent extraction as described in section 2.4.1. Each seed kernel concentrate extract was reconstituted in hexane and subjected to clean-up procedures. The clean-up of each extract was performed as described in section 2.4.1.1. Eluent of each sample was evaporated by vacuum (Savant SpeedVac Plus CS 10A, São Paulo, Brazil) at 43 °C, and re-suspended in 500 μL of MeOH for HPLC analysis.Neem oil was obtained from Dalquim Ind. Química Ltda (imported oil), Itajaí, SC; Allgreem Biotecnologia (imported oil), São Paulo, SP; Agronomic Institute of Paraná, Maringá (plant from Barra da Bahia/BA); Bioneem Tec. Consult. Ind. e Com. Ltda, Itinga, MG; Base Fértil Comercial Agrícola (plant from Cuiabá/MT), São Paulo, SP; Baraúna Co. Ind. Ltda, Catanduva, SP; Usina Cruangi, Petrolina, PE. Oils were subjected to clean-up procedures as described in section 2.4.2.1. The eluent in each sample was evaporated under vacuum (Savant SpeedVac Plus CS 10A, São Paulo, Brazil) at 43 °C, and re-suspended in 500 μL of MeOH for HPLC analysis.
3. Results and discussion
3.1. Method development and solid phase extraction
The standards used in this study were purified as described in section 2.3, and identified on the basis of spectral data, particularly 1H and 13C NMR, HSQC, HMBC, ESI-MS/MS, and by comparison of the 13C NMR spectrum with those from literature.12–14 In an attempt to establish a suitable method for detection and quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in Neem kernels by HPLC, investigations therefore initially focused on finding the best conditions for extraction and separation of both limonoids from the Neem kernels. These conditions included extraction with methanol in centrifuge tubes by vortex agitation (section 2.4.1). To obtain initial information about retention time of the analytes [1 and 2] and the compounds in the crude methanol extracts, a screening analysis was performed. Thus, 1.0 mg crude extract in 1 mL of MeOH was subjected to HPLC analyses as shown in section 2.4.1.1, using a gradient elution with acetonitrile in water (5–100% in 60 min). The eluent was monitored at 217 nm detection wavelength at ambient temperature. Based on the chromatogram, clean-up procedures (section 2.4.1.1) were carried out on all concentrated extracts. The final chromatographic system for analyses of the methanolic extract obtained after SPE clean-up was chosen after some screening analysis. The optimal conditions consisted of an isocratic solvent system containing acetonitrile–MeOH–THF–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61), employing a reverse-phase analytical C18 column (section 2.4.1.1). Azadirachtin (1) (retention factor k = 4.6) was eluted before 3-tigloylazadirachtol (2) (k = 5.1) (selectivity factor α = 1.1; resolution factor RS = 1.9) (Fig. 2). The individual peaks were identified by their retention time and spiking with the corresponding standards. In order to confirm the peaks, a fraction of methanolic extract obtained after SPE clean-up was also analyzed by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS, section 2.4.1.2). There were no interfering peaks co-eluting with the compounds of interest, and it was confirmed that the first eluted peak was azadirachtin (1) and the second one was 3-tigloylazadirachtol (2) by their second-generation ion product spectra. Azadirachtin (1) showed deprotonated molecules ([M − H]−) at m/z 719 and 3-tigloylazadirachtol (2) ([M − H]−) at m/z 661.
61), employing a reverse-phase analytical C18 column (section 2.4.1.1). Azadirachtin (1) (retention factor k = 4.6) was eluted before 3-tigloylazadirachtol (2) (k = 5.1) (selectivity factor α = 1.1; resolution factor RS = 1.9) (Fig. 2). The individual peaks were identified by their retention time and spiking with the corresponding standards. In order to confirm the peaks, a fraction of methanolic extract obtained after SPE clean-up was also analyzed by high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS, section 2.4.1.2). There were no interfering peaks co-eluting with the compounds of interest, and it was confirmed that the first eluted peak was azadirachtin (1) and the second one was 3-tigloylazadirachtol (2) by their second-generation ion product spectra. Azadirachtin (1) showed deprotonated molecules ([M − H]−) at m/z 719 and 3-tigloylazadirachtol (2) ([M − H]−) at m/z 661.
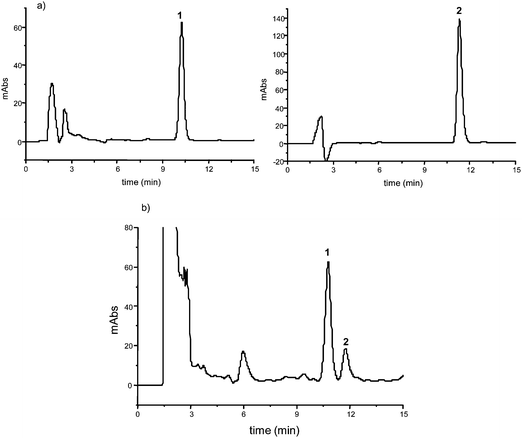 | ||
| Fig. 2 Chromatograms of the analysis of azadirachtin (1) and 3-tigloylazadirachtol (2) in methanolic extract of kernels from Azadirachta indica: a) standards (20 and 40 μg mL−1, respectively; injected volume 20 μL; optimal conditions section 2.4.3); b) SPE extract (20 mg mL−1; injected volume 20 μL; optimal conditions section 2.4.1.1). | ||
The Neem seed oil was also subjected to clean-up procedures (section 2.4.2.1). The final chromatographic system for analysis of the methanolic extract obtained after SPE clean-up was also chosen after some screening analyses. Neem oil contains different endogenous compounds15 that were not present in the Neem kernels extract, and they coeluted with 1 and 2. Having discovered that the separation of this sample could not be achieved using the same mobile phase used for Neem kernels extract, new conditions were examined. The optimal conditions consisted of an isocratic solvent system containing MeOH–acetonitrile–THF–H2O (36.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35
7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9
4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51), employing a reverse-phase analytical C18 column (section 2.4.2.1). The individual peaks were identified by HPLC-MS/MS, and Azadirachtin (1) (retention factor k = 5.1) once again eluted before 3-tigloylazadirachtol (2) (k = 6.7) (selectivity factor α = 1.3; resolution factor RS = 6.0) (Fig. 3).
51), employing a reverse-phase analytical C18 column (section 2.4.2.1). The individual peaks were identified by HPLC-MS/MS, and Azadirachtin (1) (retention factor k = 5.1) once again eluted before 3-tigloylazadirachtol (2) (k = 6.7) (selectivity factor α = 1.3; resolution factor RS = 6.0) (Fig. 3).
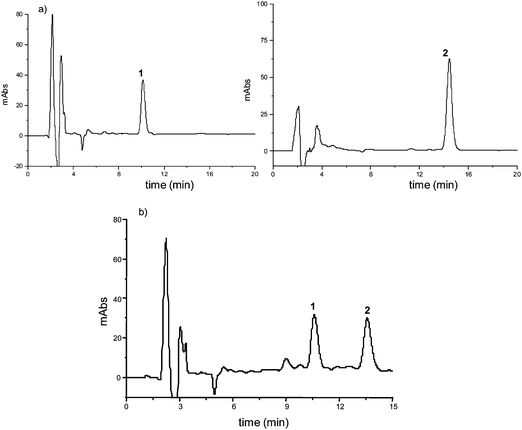 | ||
| Fig. 3 Chromatograms of the analysis of azadirachtin (1) and 3-tigloylazadirachtol (2) in seed oil from Azadirachta indica: a) standards (10 and 20 μg mL−1, respectively; injected volume 20 μL; optimal conditions section 2.4.3); b) SPE extract (15 mg mL−1; injected volume 20 μL; optimal conditions section 2.4.2.1). | ||
3.2. Method validation
Regression analysis of the least-square line for data acquired from the external standard calibration curves showed good linearity over the 5.0–60.0 μg mL−1 concentration range of azadirachtin (1), and over 2.5–50.0 μg mL−1 for 3-tigloylazadirachtol (2) using the isocratic solvent system containing acetonitrile–MeOH–THF–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61) (Fig. 4), and at MeOH–acetonitrile–THF–H2O (36.75
61) (Fig. 4), and at MeOH–acetonitrile–THF–H2O (36.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35
7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9
4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51) (Fig. 5), with a correlation coefficient above 0.999 for all curves.
51) (Fig. 5), with a correlation coefficient above 0.999 for all curves.
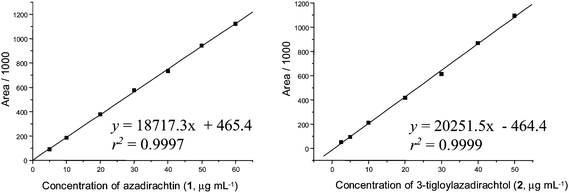 | ||
Fig. 4 Mean calibration curves for azadirachtins 1 (mean ± 1.4 RSD, n = 3) and 3-tigloylazadirachtol (2) (mean ± 1.2 RSD, n = 3); elution: acetonitrile–MeOH–THF–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61). 61). | ||
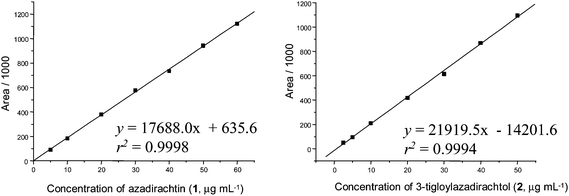 | ||
Fig. 5 Mean calibration curves for azadirachtins 1 (mean ± 0.76 RSD, n = 3) and 3-tigloylazadirachtol (2) (mean ± 1.3 RSD, n = 3); elution: MeOH–acetonitrile–THF–H2O (36.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35 7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51). 51). | ||
The intra- and inter-day precisions were assessed using the data of three quality controls analyzed over a 3-day period. The results are given in Table 1 and are expressed as Relative Standard Deviations (RSD) with three different concentrations. The accuracy evaluated from back calculation was expressed as the percent deviation between amount found and amount added of standard at the three concentrations examined. RSD values (Table 1) for azadirachtin (1) ranged from 0.77 to 3.72% and accuracy from 97.2 to 102.1%, for 3-tigloylazadirachtol (2) varied from 0.35 to 2.90% and accuracy from 96.1 to 104.7%, for both tested matrices indicating that the developed HPLC method is reproducible and accurate.
| [μg mL−1] | 1st day | 2nd day | 3rd day | Intra-day (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Kernels | RSD | Accuracy | RSD | Accuracy | RSD | Accuracy | RSD | Accuracy |
| Limonoid 1 | ||||||||
| 6.0 | 1.19 | 101.6 | 2.62 | 98.6 | 1.85 | 102.1 | 1.89 | 100.8 |
| 25.0 | 1.01 | 97.3 | 1.50 | 99.7 | 1.81 | 99.8 | 1.44 | 98.9 |
| 54.0 | 1.23 | 101.7 | 1.97 | 99.9 | 0.99 | 98.9 | 1.40 | 100.2 |
| Limonoid 2 | ||||||||
| 3.0 | 1.07 | 99.7 | 0.94 | 100.3 | 1.06 | 101.7 | 1.02 | 100.6 |
| 25.0 | 1.66 | 99.6 | 0.36 | 101.1 | 0.35 | 100.8 | 0.79 | 100.5 |
| 45.0 | 1.03 | 100.3 | 0.42 | 99.0 | 0.47 | 99.5 | 0.64 | 99.6 |
| Oil | ||||||||
| Limonoid 1 | ||||||||
| 6.0 | 1.29 | 97.2 | 0.77 | 99.5 | 1.47 | 98.0 | 1.17 | 98.2 |
| 25.0 | 1.02 | 98.0 | 1.38 | 100.6 | 1.81 | 100.9 | 1.40 | 99.8 |
| 54.0 | 0.92 | 100.5 | 1.66 | 101.6 | 3.72 | 100.5 | 2.10 | 100.9 |
| Limonoid 2 | ||||||||
| 3.0 | 1.95 | 98.4 | 0.63 | 101.0 | 1.55 | 100.5 | 1.38 | 100.0 |
| 25.0 | 1.13 | 99.7 | 2.65 | 96.1 | 2.01 | 98.9 | 1.93 | 98.2 |
| 45.0 | 1.86 | 104.7 | 0.70 | 101.3 | 2.90 | 101.7 | 1.82 | 102.6 |
The LOQ for azadirachtin (1) and 3-tigloylazadirachtol (2) were 1.5 μg mL−1 for both matrices, presenting RSD of 18.1% and 15.7%, respectively, while the LOD for both limonoids were determined at 25 ng mL−1 for kernels and 0.10 μg mL−1 for oil, reflecting the high sensitivity of the proposed method.
External standard calibration curves were obtained and validated by the standard addition method. This method of calibration is particularly important to evaluate the matrix interference, the recovery in the clean-up procedure, and recommended for conditions in which the matrix without the analytes cannot be used for preparing the calibration solutions.16 The parallelism between the regression lines from both curves showed the selectivity of the method. The standard addition curves revealed a good linearity too, with a similar correlation coefficient. The calibration curves are shown in Fig. 6. These curves were assessed by using only azadiractin (1) since both limonoids are chemically and chromatographically very similar, and due to the small amount of 3-tigloylazadirachtol (2) as standard.
![Mean calibration azadirachtin 1 addition curves for kernels [mean ± 2.8 RSD, n = 3; elution: acetonitrile–MeOH–THF–H2O (34 : 4 : 1 : 61)] and seed oil [mean ± 1.6 RSD, n = 3; elution: MeOH–acetonitrile–THF–H2O (36.75 : 7.35 : 4.9 : 51)] from Azadirachta indica.](/image/article/2010/AY/c0ay00008f/c0ay00008f-f6.gif) | ||
Fig. 6 Mean calibration azadirachtin 1 addition curves for kernels [mean ± 2.8 RSD, n = 3; elution: acetonitrile–MeOH–THF–H2O (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61)] and seed oil [mean ± 1.6 RSD, n = 3; elution: MeOH–acetonitrile–THF–H2O (36.75 61)] and seed oil [mean ± 1.6 RSD, n = 3; elution: MeOH–acetonitrile–THF–H2O (36.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.35 7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51)] from Azadirachta indica. 51)] from Azadirachta indica. | ||
The mean extraction efficiency for azadirachtin (1) from kernels and oil varied from 93.4% to 101.9% and 91.9% to 102.2%, respectively, and showed RSD ranging from 0.11 to 4.64%, and 0.56 to 2.77% (n = 3), respectively (Table 2). So, the proposed method can be considered suitable for quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in plant material and oils.
No significant degradation of azadirachtin (1) was detected in samples investigated over 24 h (sampling at 2 hourly intervals) at an ambient temperature, and over seven days at −20 °C, compared to initial values (RSD 0.64% and accuracy of 100.9%; RSD 0.46% and accuracy 101.5%, respectively).
3.3. Application to kernels and oils samples from different areas of Brazil
The HPLC method has been applied to quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in Azadirachta indica seeds and oil from different areas of Brazil (Table 3). These limonoids in the samples were expressed as mg kg−1 of seed kernels or oil. Both limonoids were found in all samples, and as expected, azadirachtin (1) was observed in higher concentration than 3-tigloylazadirachtol (2) in all seed kernels analyzed. The concentration of azadiracthin (1) in kernels analyzed ranged from 2048.8 to 5117.1 mg kg−1, the lowest being 1516.4 mg kg−1 in seeds from Ariranha (SP). 3-Tigloylazadirachtol (2) was present in smaller proportion, but in contrast was very different, ranging from 224.7 to 1116.5 mg kg−1. Temperature and humidity appear not influence the limonoid content in the kernels, since in the northeast [highest being 4718.3 mg kg−1 for 1 in Petrolina, PE, and 1116.5 mg kg−1 for 2 in Juazeiro, BA] and in the south [highest being 5117.1 mg kg−1 for 1 in Xambrê, PR, and 1090.3 mg kg−1 for 2 in Paranavaí, PR] of Brazil, concentrations of both limonoids were similar. However, it is premature to draw any conclusions about the role of environmental conditions on the variation of limonoids 1 and 2 content, until plants with the same developmental stage have been evaluated.| Area | Number of annual fruits production | Limonoid 1 (Mean ± S.D.; mg kg−1) | Limonoid 2 (Mean ± S.D.; mg kg−1) |
|---|---|---|---|
| a Results are expressed as average of three experiments and three individual analyses. b Azadirachta indica developed from germination of seeds. c A. indica grafted on Melia azedarach. | |||
| Fortaleza/CE | Several fruits production | 2048.8 ± 31.4 | 429.5 ± 15.0 |
| Recife/PE | Several fruits production | 2347.0 ± 17.9 | 224.7 ± 1.9 |
| Timbaúba/PE | 1st fruits production | 2770.6 ± 23.7 | 437.0 ± 3.6 |
| Timbaúba/PE | Several fruits production | 3578.3 ± 83.8 | 396.4 ± 7.1 |
| Petrolina/PE | Several fruits production | 4718.3 ± 34.9 | 557.5 ± 6.4 |
| Petrolina/PE | 1st fruits production | 3870.7 ± 38.2 | 657.6 ± 1.4 |
| Brejinho do Nazaré/TO | 1st fruits production | 3475.8 ± 73.1 | 818.5 ± 7.7 |
| Brejinho do Nazaré/TO | 2nd fruits production | 3180.6 ± 55.7 | 673.2 ± 9.5 |
| Brejinho do Nazaré/TO | 3rd fruits production | 4230.7 ± 25.6 | 685.9 ± 2.0 |
| Juazeiro/BA | Several fruits production | 2912.2 ± 51.2 | 1116.5 ± 13.3 |
| Itinga/MG | Several fruits production | 3454.1 ± 15.8 | 445.3 ± 0.3 |
| Itinga/MG | 1st fruits production; planted land far river | 3377.0 ± 32.4 | 791.4 ± 13.7 |
| Itinga/MG | 1st fruits production; planted land near river | 3999.9 ± 10.4 | 681.4 ± 6.8 |
| Três Lagoas/MT | 1st fruits production | 4760.2 ± 104.7 | 678.6 ± 10.8 |
| Catanduva/SP | Several fruits production | 4320.9 ± 70.8 | 728.5 ± 6.6 |
| Ariranha/SP | Several fruits production | 1516.4 ± 52.1 | 441.3 ± 4.6 |
| Urupês/SP | Several fruits production | 3167.1 ± 47.7 | 505.0 ± 19.4 |
| Xambrê/PR (plant no grafted)b | 1st fruits production | 5117.1 ± 48.8 | 835.6 ± 13.4 |
| Xambrê/PR (grafted plant)c | 1st fruits production | 4949.6 ± 70.0 | 271.1 ± 4.3 |
| Paranavaí/PR | Several fruits production | 4459.0 ± 67.8 | 1090.3 ± 13.3 |
The Chinaberry tree, Melia azedarach, is native to Asia and was widely grown in Brazil as an ornamental plant. These trees have a better potential to survive low temperatures than the Neem trees. Azadirachta indica has been grafted to stems of M. azedarach and the tree has adapted well to cool climates. These grafts growing in southern regions of Brazil are part of a breeding and selection program of the Agronomic Institute of Paraná (Brazil) aimed at the development of vigorous Neem trees to be established in cooler regions. Azadirachtin (1) and 3-tigloylazadirachtol (2) has been frequently reported to be present at highest concentration in the mature seeds.5,17 However, the concentrations of 1 and 2 may vary due to fluctuating nutrients in the natural sources, or susceptibility of the compounds to environmental influences as such heat, light, etc., or when the tree is grafted onto a second one, such as M. azedarach as a rootstock. The advantage of grafting A. indica onto M. azedarach is that it is suitable for use in cool climates, however, it is unclear how grafting might affect the content of azadirachtin (1) and 3-tigloylazadirachtol (2) in fruits as compared with those harvested from a non-grafted Neem tree. Thus, analysis of these fruits are very important for their quality control, since the content of 1 and 2 is linked to the insecticidal activity of the oil. Inspection of data clearly shows that azadirachtin (1) (4949.6 mg kg−1) was nearly in equal proportion in kernels from grafted plant and that developed from germination of non-grafted seeds, and at the same developmental stage (1 = 5117.1 mg kg−1). 3-Tigloylazadirachtol (2) was present in smaller proportion in grafted plant (2 = 271.1 mg kg−1 grafted; 2 = 835.6 mg kg−1 non-grafted). The lowest concentrations of 3-tigloylazadirachtol (2) were found in seeds from Recife (PE; 2 = 224.7 mg kg−1) and in grafted kernels. In the case of the latter, this could be attributed to the influence of grafting on the biosyntheses of this limonoid in this organ, however, in the former it is not clear in comparison with other seeds from Pernambuco state (2 = 396.4 to 657.6 mg kg−1).
The analysis of Neem oil (Table 4) is more interesting since it was not found to have markedly different concentrations between azadirachtin (1) (ranging from 228.5 to 1577.4 mg kg−1) and 3-tigloylazadirachtol (2) (117.1 to 1171.0 mg kg−1), suggesting that mechanical extraction fails to extract mainly the limonoid 1, which is present in high concentration in seeds. Both limonoids 1 and 2 were found in high concentration in samples of oil imported from India (1 = 1577.4 mg kg−1, 2 = 1171.0 mg kg−1). These results indicated that the low quality of Brazilian oil (1 = 228.5 to 630.3 mg kg−1, 2 = 117.1 to 582.9 mg kg−1) can be attributed to mechanical extraction, since the content of azadirachtin (1) and 3-tigloylazadirachtol (2) in seeds from Indian plants (1 = 556.9 to 3030.8 mg kg−1, 2 = 43.1 to 590.6 mg kg−1)13 is similar to that of Brazilian seeds (1 = 2048.8 to 5117.1 mg kg−1, 2 = 224.7 to 1116.5 mg kg−1).
| Area | Limonoid 1 (Mean ± S.D.; mg kg−1) | Limonoid 2 (Mean ± S.D.; mg kg−1) |
|---|---|---|
| a Results are expressed as average of three experiments and three individual analyses. b Neem seed oil was obtained by mechanical pressing; Imported oil is a fortified azadiachtin formulation. | ||
| Petrolina/PE | 500.8 ± 4.2 | 416.8 ± 5.9 |
| Barra da Bahia/BA | 475.5 ± 2.5 | 368.3 ± 2.6 |
| Itinga/MG | 228.5 ± 2.4 | 582.9 ± 11.7 |
| Itinga/MG | 630.3 ± 13.5 | 566.1 ± 8.3 |
| Cuiabá/MT | 349.6 ± 4.3 | 117.1 ± 0.6 |
| Catanduva/SP | 461.9 ± 15.4 | 313.4 ± 4.9 |
| Catanduva/SP | 445.0 ± 5.1 | 165.5 ± 2.1 |
| India (imported) | 1125.3 ± 25.9 | 827.0 ± 11.3 |
| India | 1058.8 ± 19.1 | 875.2 ± 4.5 |
| India | 859.0 ± 12.3 | 647.2 ± 6.3 |
| India | 1577.4 ± 33.9 | 1171.0 ± 25.4 |
| India | 958.2 ± 23.4 | 847.9 ± 12.7 |
| India | 1082.0 ± 7.3 | 827.3 ± 15.6 |
4. Conclusion
The present HPLC method is simple, repeatable, sensitive, and accurate. It has been successfully applied to the quantification of azadirachtin (1) and 3-tigloylazadirachtol (2) in Brazilian Neem seeds and oil. Neem trees begin their reproductive stage at around three to five years of age but the maximum production of fruits does not occur until they are ten years old. The graft of A. indica on M. azedarach analyzed was eight years old, and it proved to have sufficient azadirachtin (1) and 3-tigloylazadirachtol (2), confirming that this graft forms an excellent basis for the breeding of vigorous Neem trees to be established in cooler regions. Finally, these results suggest that the program of the Agronomic Institute of Paraná (Brazil) will provide useful Neem trees to be used in integrated pest management systems in southern regions of Brazil.In addition, faster screening of seeds has potential commercial utility in the selection of kernels to produce a Neem oil abundant in azadirachtin (1) and 3-tigloylazadirachtol (2). This study showed the high quality of the Brazilian Neem seeds and that seed cake, a waste product from the Neem oil industry, could serve as an inexpensive and readily available source of limonoids 1 and 2, which should be extracted with methanol (or ethanol) and returned to oil. Industry requires oil abundant in azadirachtin every time it is manufactured; in this case this HPLC method may assure standardized oil and good revenues for Brazilian industries.
Acknowledgements
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and to the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), for their financial support.References
- E. D. Morgan, Azadirachtin, a scientific gold mine, Bioorg. Med. Chem., 2009, 17, 4096–4105 CrossRef CAS.
- H. Schmutterer, Properties and potential of natural pesticides from the neem tree, Azadirachta indica, Annu. Rev. Entomol., 1990, 35, 271–297 CrossRef CAS.
- S. S. Martinez, O nim - Azadirachta indica - natureza, usos múltiplos, produção, 1st ed., IAPAR - Instituto Agronômico do Paraná, Londrina, Brazil, 2002, pp. 42 Search PubMed.
- K. M. S. Sundaram, Azadirachtin biopesticide: A review of studies conducted on its analytical chemistry, environmental behavior and biological effects, J. Environ. Sci. Health, Part B, 1996, 31, 913–948 CrossRef.
- A. Akhila and K. Rani, Chemistry of the Neem tree (Azadirachta indica A. Juss.), Prog. Chem. Org. Nat. Prod., 1999, 78, 47–149 Search PubMed.
- H. Rembold, Isomeric azadirachtins and their model of action. In Focus on phytochemical pesticides. Volume 1, The Neem Tree, ed. J. Jacobson, CRR Press Inc., 1989, p. 47 Search PubMed.
- A. Klenk, M. Bökel and W. Kraus, 3-Tigloylazadirachtol (tigloyl = 2-methylcrotonoyl), an insect growth regulating constituent of Azadirachta indica, J. Chem. Soc., Chem. Commun., 1986, 523–524 RSC.
- W. Kraus, In The Neem tree Azadirachta indica A. Juss. and other Meliaceous plants; Schmutterer, H., Ed.; VCH Verlagsgesellschaft: Weinheim, 1995, pp 35–88 Search PubMed.
- W. Kraus, In The Neem tree Azadirachta indica A. Juss. and other Meliaceous plants; Schmutterer, H., Ed., 2nd ed.; Neem Foundation: Mumbai, 2002, pp 39–110 Search PubMed.
- M. Y. Liauw, F. A. Natan, P. Widiyanti, D. Ikasari, N. Indraswati and F. E. Soetaredjo, Extraction of Neem oil (Azadirachta indica A. Juss) using n-hexane and ethanol: studies of oil quality, kinetic and thermodynamic, ARPN J. Eng. Appl. Sci., 2008, 3, 49–54 Search PubMed.
- M. B. Isman, Neem and other botanical insecticides: barriers to commercialization, Phytoparasitica, 1997, 25, 339–344 Search PubMed.
- R. Thejavathi, S. R. Yakkundi and B. Ravindranath, Determination of azadirachtin by reversed-phase high-performance liquid chromatography using anisole as internal standard, J. Chromatogr., A, 1995, 705, 374–379 CrossRef CAS.
- O. P. Sidhu, V. Kumar and H. M. Behl, Variability in Neem (Azadirachta indica) with respect to azadirachtin content, J. Agric. Food Chem., 2003, 51, 910–915 CrossRef CAS.
- W. Kraus, R. Maile, B. Vogler and B. Wundrak, 1-Tigloyl-3-acetylazadirachtol, a new limonoid from the marrango tree, Azadirachta excelsa Jack (Meliaceae), J. Indian Chem. Soc., 1997, 74, 870–873 CAS.
- H. Mei, Y. Hsieh, C. Nardo, X. Xu, S. Wang, K. Ng and W. A. Korfmacher, Investigation of matrix effects in bioanalytical high-performance liquid chromatography/tandem mass spectrometric assays: application to drug discovery, Rapid Commun. Mass Spectrom., 2003, 17, 97–103 CrossRef CAS.
- L. R. Snyder, J. J. Kirkland and J. L. Glajch, Practical HPLC Method Development, second ed., Wiley, New York, 1997 Search PubMed.
- M. R. Forim, V. E. Cornélio, M. F. G. F. da Silva, E. Rodrigues-Filho, J. B. Fernandes, P. C. Vieira, S. S. Matinez, M. P. Napolitano and R. A. Yost, Chemical characterization of Azadirachta indica grafted on Melia azedarach and analyses of azadirachtin by HPLC-MS-MS (SRM) and meliatoxins by MALDI-MS, Phytochem. Anal., 2010 DOI:10.1002/pca.1208.
| This journal is © The Royal Society of Chemistry 2010 |
