Quantification of four tobacco-specific nitrosamines in cigarette filter tips using liquid chromatography-tandem mass spectrometry
P. M.
Clayton
*,
A.
Cunningham
and
J. D. H.
van Heemst
British American Tobacco, Group R&D, Regents Park Road, Southampton, SO15 8TL, UK. E-mail: Peter_Clayton@BAT.com
First published on 4th June 2010
Abstract
Tobacco-specific N-nitrosamines (TSNA) have been suggested by some scientists to play an important role in tobacco smoke carcinogenesis. We have developed and validated an LC-MS/MS method for the determination of TSNA, notably N-nitrosoanabasine (NAB), N-nitrosoanatabine (NAT), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN), extracted from smoked cigarette filter tips. Reporting limits of 0.44, 0.89, 0.91 and 0.91ng mL−1 for NAB, NAT, NNK and NNN respectively were achieved. The newly developed method may find application in the filter analysis methodology for estimating the mouth-level exposure to NAB, NAT, NNK and NNN for cigarette smokers. TSNA levels were determined in mainstream smoke collected on industry standard Cambridge Filter Pads following smoking on a smoking machine. TSNA yields were compared with TSNA levels extracted from cigarette filter tips. However, the observation of the progressive post-smoking accumulation of TSNA during ambient storage of smoked cigarette filter tips potentially compromises use of this technique as an estimate of mouth-level exposure. Storage of smoked cigarette filter tips at sub-ambient temperatures reduced substantially the post-smoking synthesis of TSNA.
Introduction
Tobacco-specific N-nitrosamines (TSNA) are a group of chemicals which include N-nitrosoanabasine (NAB), N-nitrosoanatabine (NAT), (4-methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN). They are considered by some scientists to be important carcinogens in tobacco smoke;1 their chemical structures are shown in Table 1. TSNA are formed from tobacco alkaloids during the curing process or pyrosynthetically in a burning cigarette.1,2 More specifically, NNK is formed from nicotine; NNN is derived from nornicotine, which in turn can also be formed from nicotine. NAB and NAT are derived from the minor tobacco alkaloids anabasine and anatabine respectively.3–5 The TSNA are of interest because of their link with carcinogenesis; various studies have implicated TSNA as sources of carcinogenesis in animal models.6 The International Agency for Research on Cancer has classified NNK and NNN as Group 1 carcinogens – ‘carcinogenic to humans’.7 NAB is classified as a weak esophageal carcinogen in rats, whereas NAT is a Group 3 carcinogen – ‘not classifiable for human carcinogenicity’, showing no carcinogenicity in animal studies featuring rats.8| Compound | Chemical structure |
|---|---|
| N-nitrosoanabasine (NAB) |
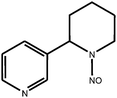
|
| Monoisotopic mass: 191 u | |
| N-nitrosanatabine (NAT) |
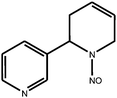
|
| Monoisotopic mass: 189 u | |
| (4-methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NNK) |
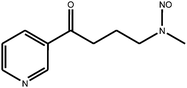
|
| Monoisotopic mass: 207 u | |
| N-nitrosonornicotine (NNN) |
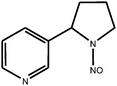
|
| Monoisotopic mass: 177 u |
In recent years the use of liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) has proven to offer a sensitive and robust technique for the determination of TSNA in cigarette smoke. The use of this technique in the analysis of mainstream smoke produced using smoking machines together with a Cambridge Filter Pad (CFP) was described by Wu and co-workers9 and developed further by Wagner et al.10 and Wu et al.11 In the publication by Wagner et al.10 the intra-assay method precisions were between 2.7–3.4%, 2.9–3.3%, 2.5–3.4% and 2.9–4.7% for NAB, NAT, NNK, and NNN respectively; while the inter-assay method precisions were between 5.1–7.3%, 5.9–6.9%, 5.8% and 6.7–8.5% for NAB, NAT, NNK, and NNN respectively. This showed the LC-MS/MS method was capable of good repeatability and reproducibility.
In a recent study12 on twenty-five UK cigarette brands the amounts of TSNA captured on CFP under ISO smoking conditions (puffing parameters of 35 mL volume, 2 s duration and one puff taken every 60s)13 ranged between 1.08 to 44.2 ng/cigarette for NAB, 7.8 to 148 ng/cigarette for NAT, 5.2 to 499.9 ng/cigarette for NNK and 6.5 to 257.6 ng/cigarette for NNN. The ranges in these values illustrates the large variation that can arise in mainstream smoke yields of TSNA from different cigarette brands. The amounts of TSNA captured on CFP during ISO smoking conditions may not be indicative of the amounts of TSNA available to human smokers because smoking machine yields are not predictive of the exposure humans obtain when smoking.14 The ISO smoking regime is less intense than other laboratory smoking regimes used to test cigarettes, for instance the Health Canada Intense smoking regime.15 The filter analysis methodology was developed in order to estimate the amounts of nicotine and ‘tar’16 that smokers are exposed to at mouth-level while smoking in their everyday environment. The filter analysis methodology is fully described in a recent publication17 and is briefly summarised here.
In the filter analysis methodology, a smoking machine is used to smoke cigarettes using a range of defined smoking regimes (puff volume, puff duration and puff interval) which encompasses a range of human puffing characteristics. Smoke particulate matter collected on CFP and cigarette filter tips are analysed for nicotine and ‘tar’ allowing the relationship to be established between chemicals retained in the filter tip and that which has been collected on the CFP. Subsequent analysis of filter tips originating from human smokers allows for estimations to be made for the mouth-level exposure which smokers received when they consumed cigarettes. The method is predicated on the filtration efficiency remaining relatively constant at human flow rates; the relationship between filtration efficiency and flow rate was examined by Shepperd and co-workers.18
Prior to the adoption of LC-MS methodology, TSNA were quantified using GC-TEA (gas chromatography-thermal energy analyzer). In this technique the N-nitroso compound emerging from the GC column is decomposed by pyrolysis into a nitrosyl radical (NO˙). Ozone formed by an electric discharge is introduced where it reacts with the nitrosyl radical yielding an electronically excited NO2* species. The excited NO2* rapidly decay to its ground state with the emission of light in the near infrared region of the spectrum. The emission of this chemiluminescence radiation facilitates the detection and quantitation of N-nitroso compounds.19,20 The advantage of using GC-TEA to quantify TSNA is that the technique is specific for TSNA but suffers from a lack of sensitivity especially for NAB and NNK in low TSNA tobacco products. The GC-TEA method is further disadvantaged by its inability to differentiate between co-eluting nitroso components and it requires extensive sample preparation.21 An innovation recently published is the use of gas chromatography-ion-trap tandem mass spectrometry which was used to measure four TSNA adsorbed onto model surfaces from secondhand tobacco smoke.21 The methodology gave excellent performance (method precisions of 3.0% and 2.8% [intra-assay] and 8.8 and 7.2% [inter-assay] for NNK and NNN respectively.
The liquid chromatography/electrospray ionisation tandem mass spectrometric method detailed here differs from the procedure described by Wu9 in that the extraction procedure is simplified and deuterated internal standards (rather than 13C analogues) were used to compensate for matrix effects. This paper also reports on whether post-smoking formation of TSNA in filter tips occurs (as has been observed occurring with CFP9 and also in moist snuff following storage)22 and what steps may be taken to minimise such effects. This publication extends the filter analysis methodology to facilitate the estimation of mouth-level exposure to TSNA for human smokers. A direct estimation of TSNA mouth-level exposure would be advantageous because TSNA levels in mainstream smoke do not necessarily correlate with ‘tar’ delivery across a range of different cigarette products.23 Estimation of TSNA exposure is accomplished by the extraction of TSNA from smoked filter tips followed by LC-MS/MS analysis. A recent publication has reported human TSNA estimates based on tip nicotine levels TSNA.24
An alternative approach to the use of the filter analysis methodology for estimating mouth-level exposure to TSNA has been recently published by Polzin and co-workers.25 In this technique, tobacco-blend derived solanesol – a high boiling point alcohol which is deposited in filters during smoking – was quantified. TSNA were extracted from CFP to establish the relationship between filter tip solanesol and TSNA deposited on CFP. Filters obtained from human smokers were analysed for solanesol which allowed the estimation of mouth-level TSNA exposure.
The filter analysis methodology is a technique complementary to the measurement of TSNA biomarkers in urine.26 The analysis of cigarette filter tips allows a non-invasive estimation of the yield that smokers obtain from their cigarettes. Recently cigarette filter-based methodologies were the subject of a comprehensive review article.27
Experimental section
Reagents and standards
TSNA analytical standards were purchased from Kinesis (St. Neots, UK) as pre-mixed stock solution in methanol: methylene chloride (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) containing NAB (9.8 μg mL−1), NAT (19.8 μg mL−1), NNK (20.1 μg mL−1), NNN (20.1 μg mL−1).
20) containing NAB (9.8 μg mL−1), NAT (19.8 μg mL−1), NNK (20.1 μg mL−1), NNN (20.1 μg mL−1).
Deuterated TSNA internal standards (Table 2) were also purchased from Kinesis as pre-mixed stock solution in methanol: methylene chloride (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) containing d4-NAB (39.27 μg mL−1), d4-NAT (78.47 μg mL−1), d4-NNK (79.26 μg mL−1), d4-NNN (78.45 μg mL−1).
20) containing d4-NAB (39.27 μg mL−1), d4-NAT (78.47 μg mL−1), d4-NNK (79.26 μg mL−1), d4-NNN (78.45 μg mL−1).
| Compound | Chemical structure |
|---|---|
| d4-NAB |
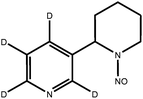
|
| Monoisotopic mass: 195 u | |
| d4-NAT |
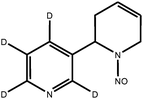
|
| Monoisotopic mass: 193 u | |
| d4-NNK |
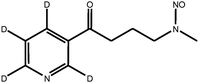
|
| Monoisotopic mass: 211 u | |
| d4-NNN |
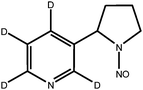
|
| Monoisotopic mass: 181 u |
Acetonitrile: HPLC grade (Rathburn Chemicals, Wakerburn, UK).
Ammonium acetate: ReagentPlus™, 99.99+% (Sigma-Aldrich Inc., MO, USA).
Methanol: HPLC grade (Rathburn Chemicals, Wakerburn, UK).
Equipment
Burghart RMB20 rotary smoking machine (Burghart GmbH, Hamburg). Glass fibre Cambridge Filter Pads (Whatman, Maidstone, UK) 92 and 44 mm diameter.Applied Biosystems (Warrington, UK) API 5000 [Gauge repeatability and reproducibility, recovery experiment] or API 4000 Q Trap LC-MS/MS instrument [filter tip ageing experiment] with ESI probe and Analyst Software version 1.4.
1200 Series LC system (Agilent Technologies, Wokingham, UK) consisting of in-line degasser, binary pump, autosampler, column thermostat unit.
LC column: Luna 3 μ C18 100 A; 100 × 2 mm with SecurityGuard guard column: C18 4 mm × 2 mm (Phenomenex, UK).
Extraction solution
0.625 mL of deuterated stock standard was made up to 5 L with methanol. The concentration of deuterated internal standards were: 4.91 ng mL−1 d4-NAB, 9.81 ng mL−1 d4-NAT, 9.91 ng mL−1 d4-NNK and 9.81 ng mL−1 d4-NNN.Calibration smoking
Five cigarettes of a commercial 7 mg ISO ‘tar’ yield16 cigarette from Germany were smoked onto a CFP using the smoking regimes described previously.17 A 44 mm CFP was used for all smoking regimes apart the most intense regime which utilised a larger diameter 92 mm CFP in order to prevent the pad being overloaded by the amount of smoke. Following the completion of the smoking cycle, the cigarette butts were removed from the smoking machine and cut to 10 mm sections from the ‘mouth’-end (referred to as part-filter tips), and extracted using the extraction solution. The CFP from each smoking cycle were extracted in 20 mL of extraction solution (40 mL used to extract 92 mm CFP).Generation of part-filter tips for ageing experiment
In order to quantify any post-smoking formation of TSNA within the samples, smoked part-filter tips were generated using two smoking regimes:high intensity smoking: puff volume = 60 mL, puff duration = 1.5 s, puff interval = 30 s
low intensity smoking: puff volume = 40 mL, puff duration = 2.0 s, puff interval = 60 s
The cigarettes used were a commercial 7 mg ISO ‘tar’ yield cigarette from Germany. Following smoking, part-filter tips were placed in aluminium screw-top cans and stored for up to four weeks under a variety of temperatures: i) 22 °C, ii) + 4 °C, iii) −17 °C. Four replicates (each of 5 tips) were analysed after 7, 14, 21 and 28 days for each of the storage environments.
Extraction of part-filter tips for ageing experiment
Five part-filter tips were placed in a 150 mL round bottom flask. Extraction solution (20 mL) was added and flasks were shaken on an orbital shaker (160 rpm) for 30 min and then an aliquot of the extract was analysed.Extraction of CFP
The CFP were extracted in 20 mL extraction solution. Each CFP was placed in a 50 mL centrifuge tube shaken (200 rpm) for 30 min and then an aliquot of the extract was analysed.Extraction of single part-filter tips in recovery experiment
To determine the extraction recoveries of TSNA from part-filter tips a standard addition recovery experiment was undertaken. Smoked part-filter tips, containing TSNA delivered from smoke, were spiked with a solution containing TSNA and extracted. These were compared to smoked part-filter tips which remained unspiked. A commercial 7 mg ISO ‘tar’ yield cigarette brand from Germany was smoked according to the ISO smoking regime. Part-filters were spiked with 100 μL of a methanol solution containing 250 ng mL−1 NAB, 500 ng mL−1 NAT, NNK, NNN (higher level spiking) or 100 μL of a methanol solution containing 125 ng mL−1 NAB, 250 ng mL−1 NAT, NNK, NNN (lower level spiking). Five part-filter tips were spiked at the higher level and five were spiked at the lower level.After spiking, the part-filter tips were allowed to equilibrate for 10 min. The part-filter tips were then individually extracted in 4 mL of extract solution (200 rpm) for 30 min, the resultant solution was then analysed. A further five part-filters tips were extracted without spiking to determine the amounts of TSNA deposited from smoke.
LC-MS/MS conditions
Separation was accomplished by reverse-phase gradient HPLC using a Luna C18 column with guard column maintained at 40 °C. The elution gradient used is described in Table 3, the injection volume was 5 μL.| Time/min (post-injection) | 5 mM aqueous ammonium acetate (%) | 5 mM ammonium acetate in 95% acetonitrile/5% water (%) |
|---|---|---|
| 0 | 95 | 5 |
| 4 | 30 | 70 |
| 4.1 | 95 | 5 |
| 10 | 95 | 5 |
Detection of TSNA was by tandem mass spectrometry using positive mode electrospray ionization; the conditions employed are shown in Tables 4 and 5.
| Parameter | Setting |
|---|---|
| Source temperature | 450 °C |
| Ionisation source polarity | positive |
| Electrospray voltage | +5500 V |
| Curtain gas | 25 psi |
| Ion source gas 1 | 50 psi |
| Ion source gas 2 | 50 psi |
| Collision gas | 6 psi |
| Declustering potential | 60 V |
| Entrance potential | 20 V |
| Compound | Precursor (m/z) | Product (m/z) | CE/eV | CXP/V | Neutral loss (u) |
|---|---|---|---|---|---|
| a CE – collision energy, CXP – collision cell exit potential. | |||||
| NAB | 192 | 162 | 18 | 12 | 30 |
| d4-NAB | 196 | 166 | 18 | 12 | 30 |
| NAT | 190 | 79 | 45 | 12 | 111 |
| d4-NAT | 194 | 83 | 45 | 12 | 111 |
| NNK | 208 | 122 | 19 | 9 | 86 |
| d4-NNK | 212 | 126 | 18 | 15 | 86 |
| NNN | 178 | 148 | 16 | 9 | 30 |
| d4-NNN | 182 | 152 | 16 | 10 | 30 |
Preparation of standards
The stock standard solution was diluted to produce a mixture containing 49.0, 99.0, 100.5 and 100.5 ng mL−1 NAB, NAT, NNK and NNN respectively. This solution was further diluted to give a range of concentrations of 24.5, 4.9, 2.45 and 0.495 ng mL−1 for NAB.900 μL of each of the five above mixtures was added to 100 μL of a mixture containing 49.1, 98.1, 99.1 and 98.1 ng ml−1 d4-NAB, d4-NAT, d4-NNK, d4-NNN respectively, prepared by dilution of the deuterated TSNA Internal Standard mix. The resultant set of five internally standardised calibration standards were used in analyses. The prepared standards were stored in the dark at +4 °C for up to 1 month.
Data analysis
Peak area determinations were processed using Analyst version 1.4 software. Where possible this was accomplished automatically, however, all peaks were manually inspected and re-integrated where necessary. Data was exported into MINITAB version 15.1 (Minitab Ltd, Coventry, UK) for further statistical evaluation.Results and discussion
Production mass spectra and chromatograms
Mass spectra with the proposed structures of the fragments are shown in Fig. 1–4. There are some notable differences to the electron impact (EI) spectra of the NNK and NNN21 due to the fact that a different ionisation technique was used in this study. For example, the EI mass spectrum of NNK shows a signal at m/z 207 (M+˙, formed by loss of a single electron from NNK), whereas the ESI spectrum of NNK shows a signal at m/z 208 ([M + H]+˙, formed by protonation of NNK).![Mass spectrum of [NAB + H]+ (collision energy:18 eV).](/image/article/2010/AY/b9ay00306a/b9ay00306a-f1.gif) | ||
| Fig. 1 Mass spectrum of [NAB + H]+ (collision energy:18 eV). | ||
![Mass spectrum of [NAT + H]+ (collision energy:45 eV).](/image/article/2010/AY/b9ay00306a/b9ay00306a-f2.gif) | ||
| Fig. 2 Mass spectrum of [NAT + H]+ (collision energy:45 eV). | ||
![Mass spectrum of [NNK + H]+ (collision energy:19 eV).](/image/article/2010/AY/b9ay00306a/b9ay00306a-f3.gif) | ||
| Fig. 3 Mass spectrum of [NNK + H]+ (collision energy:19 eV). | ||
![Mass spectrum of [NNN + H]+ (collision energy:16 eV).](/image/article/2010/AY/b9ay00306a/b9ay00306a-f4.gif) | ||
| Fig. 4 Mass spectrum of [NNN + H]+ (collision energy:16 eV). | ||
In the spectrum of NAB (Fig. 1) the peak at m/z 192 represents the pseudo-molecular ion ([M + H]+˙). The fragment at m/z 162 is a result of α-cleavage, producing the indicated ion at m/z 162 and the NO˙ radical.26 It is believed the peak at m/z 133 is the result of α-cleavage and the resulting ring-opening of the piperidyl group in m/z 162, followed by charge-site initiation cleavage, which moves the electron pair onto the nitrogen atom, breaking the remaining C–N bond in the former piperidyl group.
In the spectrum of NAT (Fig. 2) the peak at m/z 190 (pseudo-molecular ion ([M + H]+˙)) is absent because of the high collision energy (CE = 45 eV) used to fragment this molecule. Important fragments are: m/z 159 (thought to arise as a result of double α-cleavage, loss of NO˙ followed by a subsequent loss of H˙ resulting in a stable ion); m/z 132 (double charge-site initiation cleavage in m/z 159 and subsequent loss of hydrogen cyanide); m/z 106 (charge-site initiation cleavage in m/z 132 resulting in loss of ethyne) and m/z 79 (pyridine radical ion, observed previously).26
In the spectrum of NNK (Fig. 3) the peak at m/z 208 represents the pseudo-molecular ion ([M + H]+˙). Important fragments are: m/z 178 (double α-cleavage, loss of NO˙); m/z 148 (thought to be charge-site initiation cleavage of m/z 208); m/z 122 (pseudo-α-cleavage in m/z 178, followed by loss of H˙, charge-site initiated cleavage, methyl rearrangement onto the carbonyl group and the resulting electron-pair relocation and double-bond formation; subsequent loss of ethene and hydrogen cyanide);28m/z 106 (thought to be pseudo-α-cleavage in m/z 178, followed by radical-site initiation α-cleavage at the carbonyl group) and m/z 57.
In the spectrum of NNN (Fig. 4) the peak at m/z 178 represents the pseudo-molecular ion ([M + H]+˙). Important fragments are: m/z 148 (double α-cleavage, loss of NO˙, observed previously);26m/z 120 (thought to be α-cleavage and the resulting ring-opening of the pyrrolidyl group in m/z 148, followed by pseudo-α-cleavage resulting in the loss of ethene) and m/z 105 (hydrogen rearrangement and loss of ˙CH3 in m/z 120).26
Method sensitivity and standard calibration lines
The reporting limits of the method were 0.44, 0.89, 0.91, 0.91 ng mL−1 for NAB, NAT, NNK and NNN respectively (which is defined as the nominal concentration of the lowest calibration standard used in the standard calibration regression). Given that five part-filter tips were extracted in 20 mL, this was equivalent to 1.76, 3.56, 3.64, 3.64 ng/tip for NAB, NAT, NNK and NNN respectively.The method was found to be linear over the calibration ranges of 0.44–44, 0.89–89, 0.91–91 and 0.91–91 ng mL−1 for NAB, NAT, NNK and NNN respectively. A weighting of concentration−1 was applied to all regression lines (not forced through the origin). All standard calibration points were ±15% of the nominal value. There was no requirement to extrapolate measurements above or below the calibration range. All analytes of calibration standards were found to be stable in methanol under shorter term shortage (24 h, 22 °C). The mass chromatograms of the four TSNA following extraction are presented in Fig. 5. The presence of peak shouldering, most clearly observed in the mass chromatogram of NAT, has been noted before and has been explained as arising from the existence of E and Z nitrosamine isomers.9,29
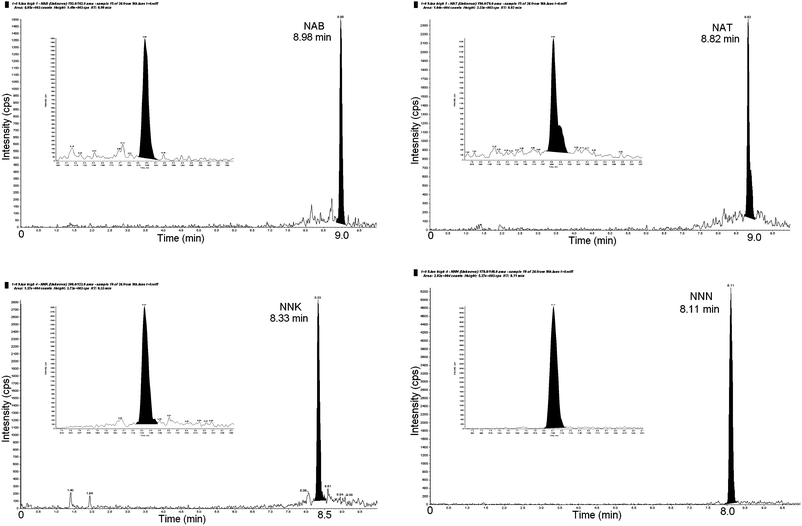 | ||
| Fig. 5 Chromatograms of four TSNA extracted from part-filter tips. | ||
Recovery of TSNA extracted from part-filter tips
The results of the recovery experiments are shown in Table 6.| Sample | NAB | NAT | NNK | NNN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | conc./ng mL−1 | CV (%) | rec (%) | conc./ng mL−1 | CV (%) | rec (%) | conc./ng mL−1 | CV (%) | rec (%) | conc./ng mL−1 | CV (%) | rec (%) |
| a conc. = concentration, CV = coefficient of variation, based on five replicates = 100% × standard deviation/average amount. b rec. = recovery = 100% × (conc. − conc.tips/unspiked)/conc.added), for conc.added values, see the Experimental section. c Recoveries were in the range 90–110% for both high and low spiked part-filter tips; percentage recoveries in this range are considered acceptable for an LC-MS/MS analytical method. | ||||||||||||
| Tips unspiked | 0.35 | 5.2 | na | 3.23 | 5.8 | na | 3.77 | 9.4 | na | 1.94 | 6.6 | na |
| Tips low spiked | 3.55 | 2.8 | 102 | 9.69 | 5.2 | 103 | 10.3 | 4.9 | 105 | 8.64 | 4.6 | 107 |
| Tips high spiked | 6.74 | 3.8 | 102 | 15.6 | 4.8 | 99 | 15.5 | 3.2 | 94 | 14.2 | 2.7 | 98 |
Method performance
To evaluate the method, a Gauge Repeatability and Reproducibility (Gauge R&R) study was undertaken as follows.Part-filter tips were generated using two different smoking regimes, lower and higher intensity regimes (described above in section on Generation of part-filter tips for ageing experiment). Smoking was completed on two separate days. Analyses of all samples were completed on two LC-MS/MS (API5000) instruments with independent operators. Five replicates were analysed per day/instrument/regime. The results of the Gauge R&R study are summarized in Table 7, these data are based on the use of a single smoking machine.
| % contribution | ||||
|---|---|---|---|---|
| NAB | NAT | NNK | NNN | |
| a These results show that the repeatability and reproducibility of the analysis of the four TSNA extracted from the part-filter filter tips was acceptable. | ||||
| Total Gauge R&R | 16.6 | 22.8 | 23.3 | 15.3 |
| Repeatability | 15.5 | 11.5 | 23.3 | 14.1 |
| Reproducibility | 6.1 | 19.7 | 0 | 6.0 |
| (variation attributable to LC-MS/MS instruments) | 6.1 | 16.2 | 0 | 6.0 |
Part-filter tip ageing experiments
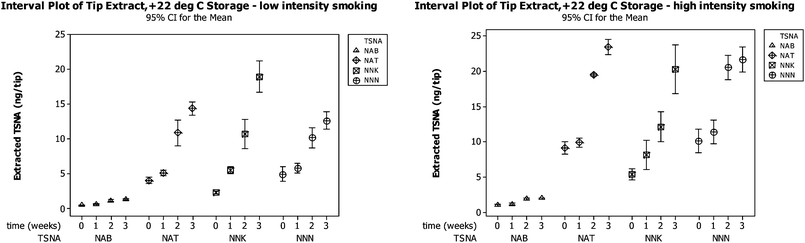
In the filter analysis methodology,17 part-filter tips are collected from smokers and forwarded for analysis. This will inevitably result in delays where filter tips are stored under ambient conditions. To assess whether post-smoking formation of TSNA occurred in tips following periods of storage, part-filter tips were stored for periods up to four weeks.Fig. 6 shows the effect of storage on part-filter tips under ambient conditions of 22 °C for up to three weeks. Each time-point consisted of 4 replicate measurements (each of 5 smoked tips). It is clear there were statistically significant increases in the levels of the four TSNA over three weeks. In both high and low intensity smoking experiments the NNK tip levels exhibited the greatest proportional increase over 0–21 days (see Experimental section for details of high and low intensity smoking).
The results of this experiment prompted an examination of environmental conditions where the post-smoking synthesis of TSNA might be minimized. The changes in TSNA levels extracted from part-filter tips following storage at +22 °C, +4 °C and −17 °C for up to 4 weeks are depicted in Fig. 7. Four replicates were extracted (only 2 replicates available at week 4, low intensity smoking, no data for week 4, high intensity smoking).
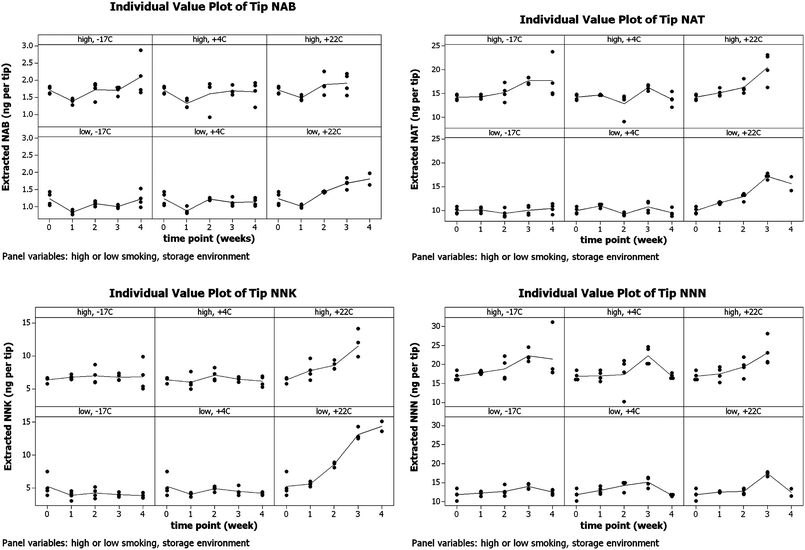 | ||
| Fig. 7 Effect of storage temperature on TSNA levels extracted from part-filter tips (2 smoking intensities) followed by storage at −17 °C, +4 °C and +22 °C for up to 4 weeks. | ||
The experiment confirmed that TSNA accumulate in smoked filter tips over a 4 week period at room temperature. The effect is probably a result of the considerable excess of tobacco alkaloids and nitrate/nitrite ions present in the smoked filter tip. The post-smoking synthesis of TSNA would appear to be much reduced when smoked filter tips are stored at sub-ambient temperature. Interestingly, similar observations have been reported on the effects of storage on TSNA levels in moist snuff; TSNA levels increased significantly after 4 weeks of storage at ambient room temperature, however following storage at +4 °C these components did not increase.22
Conclusion
An analytical method for the simultaneous determination of NAB, NAT, NNK and NNK extracted from part-filter tips has been developed and validated. The method is sensitive and has favourable repeatability and reproducibility characteristics (as determined by a Gauge R&R study). The method is also suitable for the analysis of TSNA extracted from CFP as used in many smoking machines. The method may find application in filter analysis studies which can be used to estimate the mouth-level exposure of TSNA received by smokers under natural conditions. However, the post smoking formation of TSNA in filter tips, in particular NNK, may restrict the practical use of this method unless sub-ambient storage conditions are used pre-analysis.References
- J. D. Adams, S. J. Lee, N. Vinchkoski, A. Castonguay and D. Hoffmann, Cancer Lett., 1983, 17, 339–346 CrossRef CAS.
- S. C. Moldoveanu, N. P. Kulshreshtha and J. M. Wilkins, Study of the pyrosynthesis of NNN and NNK in mainstream cigarette smoke, 2001, 55th Tobacco Science Research Conference, Greensboro, North Carolina Search PubMed.
- S. S. Hecht, C. H. B. Chen, R. Young and D. Hoffmann, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 1981, 11, 57–66 Search PubMed.
- K. D. Brunnemann and D. Hoffmann, Toxicology, 1991, 21, 235–240 CAS.
- A. Wiernik, A. Christakopoulos, L. Johansson and I. Wahlberg, Recent advances in tobacco science, 1995, 21, 39–80 Search PubMed.
- S. S. Hecht and D. Hoffmann, Cancer Surveys, 1989, 8, 273–294 Search PubMed.
- International Agency for Research on Cancer, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans – Tobacco habits other than smoking; betel-quid and areca-nut chewing; and some related nitrosamines, 1985, volume 37, ISBN 92 832 1537 0, IARC Press, Lyon Search PubMed.
- S. S. Hecht, Biochemistry, Chem. Res. Toxicol., 1998, 11, 559–603 Search PubMed.
- W. Wu, D. L. Ashley and C. H. Watson, Anal. Chem., 2003, 75, 4827–4832 CrossRef CAS.
- K. A. Wagner, N. H. Finkel, J. E. Fossett and I. G. Gillman, Anal. Chem., 2005, 77, 1001–1006 CrossRef CAS.
- J. Wu, P. Joza, M. Sharifi, W. S. Rickert and J. H. Lauterbach, Anal. Chem., 2008, 80, 1341–1345 CrossRef CAS.
- E. O. Gregg, C. Hill, M. Hollywood, M. Kearney, K. McAdam, D. J. McLaughlin, S. Purkis and M. Williams, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 2004, 21, 117–138 Search PubMed.
-
ISO 3308
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000
Routine analytical cigarette-smoking machine—Definitions and standard conditions, 2000, International Organisation for Standardization, Geneva Search PubMed.
2000
Routine analytical cigarette-smoking machine—Definitions and standard conditions, 2000, International Organisation for Standardization, Geneva Search PubMed. - R. R. Baker, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 2002, 20, 23–41 Search PubMed.
- M. E. Counts, M. J. Morton, S. W. Laffoon, R. H. Cox and P. J. Lipowicz, Regulatory Toxicology and Pharmacology, 2005, 41, 185–227 CrossRef CAS.
-
ISO 4387
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000
Cigarettes—Determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine, 2000, International Organisation for Standardization, Geneva Search PubMed.
2000
Cigarettes—Determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine, 2000, International Organisation for Standardization, Geneva Search PubMed. - F. K. St. Charles, M. Ashley, C. J. Shepperd, P. Clayton and G. Errington, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 2009, 23, 232–243 Search PubMed.
- C. J. Shepperd, F. K. St. Charles, M. Lien and M. Dixon, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 2006, 22, 176–184 Search PubMed.
- D. H. Fine, F. Rufeh, D. Lieb and D. P. Rounbehler, Anal. Chem., 1975, 47, 1188–1191 CrossRef CAS.
- K. D. Brunnemann, B. Prokopczyk, M. V. Djordjevic and D. Hoffmann, Critical Reviews in Toxicology, 1996, 26, 121–137 Search PubMed.
- M. Sleiman, R. L. Maddalena, L. A. Gundel and H. Destaillats, J. Chomatogr. A, 2009, 1216, 7899–7905 Search PubMed.
- M. V. Djordjevic, J. Fan, L. P. Bush, K. D. Brunnemann and D. Hoffmann, J. Agric. Food Chem., 1993, 41, 1790–1794 CrossRef CAS.
- Y. S. Dind, L. Zhang, R. B. Jain, N. Jain, R. Y. Wang, D. L. Ashley and C. H. Watson, Cancer Epidemiology, Biomarkers & Prevention, 2008, 17, 3366–3371 Search PubMed.
- C. J. Shepperd, A. C. Eldridge, D. C. Mariner, M. McEwan, G. Errington and M. Dixon, Regulatory Toxicology and Pharmacology, 2009, 55, 97–109 CrossRef CAS.
- G. M. Polzin, W. Wu, X. Yan, J. M. McCraw, S. Abdul-Salaam, A. D. Tavakoli, L. Zhang, D. L. Ashley and C. H. Watson, Nicotine & Tobacco Research, 2009, 11, 868–874 Search PubMed.
- D. Kavvadias, G. Scherer, M. Urban, F. Cheung, G. Errington, C. J. Shepperd and M. McEwan, J. Chromatogr. B, 2009, 877, 1185–1192 CrossRef CAS.
- J. L. Pauly, R. J. O'Connor, G. M. Paszkiewicz, K. M. Cummings, M. V. Djordjevic and P. G. Shields, Cancer Epidemiol. Biomarkers Prev., 2009, 18, 3321–3333 Search PubMed.
- F. W. McLafferty, and F. Turecek, Interpretation of Mass Spectra. University Science Books, 4th edition, 1993, ISBN 0-935702-25-3 Search PubMed.
- S. S. Hecht, T. E. Spratt and N. Trushin, Carcinogenesis, 1997, 18, 1851–1854 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |