CZE separation of nitrogenous drugs in cationic form
Francesca
Buiarelli
,
Marina
Franco
*,
Renata
Jasionowska
and
Giulia
Pelagalli
Dipartimento di Chimica, Università “Sapienza”, 00185, Rome, Italy. E-mail: marina.franco@uniroma1.it
First published on 22nd April 2010
Abstract
A rapid and efficient capillary zone electrophoresis method was developed for Quality Control analysis of pharmaceutical preparations containing antihistamines, decongestants and anticholinergic remedies: chlorpheniramine, diphenhydramine, ephedrine, isopropamide, pheniramine. These analytes were separated in cationic form optimizing the experimental conditions in 60 mM tetraborate buffer pH = 9.2 as a BGE (Background Electrolyte) on a Beckman P/ACE System MDQ instrument. The effective capillary length was 48 cm, I.D. = 75 μm, the applied voltage 15 kV, and the temperature 25 °C. Detection was performed by a DAD (Diode Array Detector) at 210 nm. Separation time was less than 8 min. After experimental conditions optimization, the proposed method was validated. Precision of migration time (tm) ranging from 0.19% to 0.29% and corrected peak area (Ac) from 2.54% to 3.68%. The linearity of detector response was tested in the range 5–40 μg ml−1 obtaining the 0.9962 ≤ r2 ≤ 0.9982. LOD and LOQ, accuracy (recovery) and ruggedness were evaluated for each analyte demonstrating the good reliability of the method. Analysis of the pharmaceutical real sample was performed.
1. Introduction
In the last years, the application of CE separations to the Quality Control of pharmaceuticals became very important. Actually the official HPLC methods, used in industrial Quality Control, are very time-consuming: every component of the pharmaceutical mixture is extracted and determined separately; consequently the complete analysis is performed by several steps.For these reasons it is important to develop new methods employing techniques to analyze all components in a single step.
The CE offers many advantages: versatility, high efficiency and short analysis time.
A capillary zone electrophoresis (CZE) method1 has been proposed by Chinese researchers for pseudoephedrine, paracetamol, and dextromethorphan separation in phosphate buffer at pH = 7. The method gave the satisfactory analytical results and was applied for pseudoephedrine determination in tablets and in human urine. Other Authors2 describe an accurate and rapid method for separation by CZE in tetraborate buffer (pH = 8.5) of four drugs: codeine, diphenydramine, ephedrine and noscapine present in a syrup formulation. The method was validated and proposed as routine analysis in Quality Control of pharmaceutical preparations.
In forensic field a CZE-MS method was developed and applied to determine the main drugs of abuse (ephedrine and others) in hair samples.3
Separation of three analytes (dextrometorphan, phenylephrine and diphenhydramine) was studied and optimized by CZE and MEKC ways comparing selectivity of different media.4 The best results were obtained in the MEKC technique.
A comparison study of CZE and MEKC separation is described.5 Seven cold remedy active compounds (including chlorpheniramine maleate) were satisfactorily baseline-resolved by MEKC mode. After validation, the analysis of a pharmaceutical preparations containing paracetamol and chlorpheniramine maleate was performed.6
Another paper reported the CZE separation in non-aqueous media: a good resolution for drugs analysis was obtained by CE in non-aqueous media.7 A complex mixture of basic and acidic analytes, 12 compounds, was resolved in about 10 min demonstrating the suitability of the method.
Authors8 have studied possible analysis in acetonitrile–methanol media of four basic compounds (dextromethorphan, diphenhydramine, chlorpheniramine and pseudoephedrine) in five cold medicines.
Determination of two active compounds of interest (chlorpheniramine maleate and ephedrine hydrochloride) has been developed by HPLC technique9 and successfully applied for analysis of nasal drops. The same components have been determined in tablets by other authors.10 They optimized and validated the MEKC mode obtaining good results.
There are no publications about simultaneous determination of five analytes of interest.
The purpose of this study was to develop, optimize and validate a CZE method for the separation of most commonly-used antihistamines, decongestants and long-acting anticholinergic drugs such as: chlorpheniramine maleate (CPM), diphenhydramine hydrochloride (DPH), ephedrine hydrochloride (E), isopropamide iodide (II), pheniramine maleate (PM).
2. Experimental
2.1. Apparatus and operational conditions
The analyses were carried out on a P/ACE system from Beckman Instrument Fullerton, CA (USA) with a UV-DAD detector.The fused silica capillary (58 cm total length, effective length 48 cm, I.D. = 75 μm) was supplied by SGE (Melbourne, Australia). The new capillary, before using, was rinsed with distilled water (5 min), sodium hydroxide 0.1 M (5 min) and distilled water (5 min). Then conditioning was performed washing with appropriate buffer (5 min) and performing a run without injection (15 min). Daily, before starting measurements, the capillary was washed with distilled water (2 min), sodium hydroxide (1 min), distilled water (2 min) and conditioned with appropriate buffer (5 min). Between runs, buffer rinse was performed for 2 min. Detection wavelength was set at 210 nm. The sample injections were performed in a hydrodynamic mode (3 s under 0.5 psi).
For ruggedness evaluation electrophoretic separations were carried out on the Spectraphoresis 1000 instrument from Thermo-Quest Corporation, CA (USA).
2.2. Chemicals and materials
All reagents were of analytical grade purity.Standards of isopropamide iodide, ephedrine hydrochloride, diphenhydramine hydrochloride, pheniramine maleate, chlorpheniramine maleate, sodium tetraborate, sodium hydrogen carbonate, Trizma-base were purchased from SIGMA-ALDRICH (Steinheim, Germany).
The real sample was FIENAMINA tablets commercially available.
For solution filtering the syringe filters 0.45 μm (Millex HV, Millipore, MA, USA) were used.
2.3. Buffers, standard and real sample preparation
The Carbonate buffer solution was prepared titrating sodium bicarbonate solution with sodium hydroxide to pH = 9.2.Tris-borate buffer was obtained titrating with boric acid a solution of Trizma-base to pH = 9.0. Borate buffer was prepared dissolving the sodium tetraborate salt in distilled water.
Standard stock solutions of studied analytes (at 1 mg ml−1 concentration) were prepared in distilled water and kept at T = 4 °C. The calibration solutions were obtained by dilution of the stock solution with a 10 mM solution of borate buffer to give a desired analyte concentration (5 to 40 μg ml−1).
For the real sample preparation, twenty tablets were weighed and grounded in a mortar. The quantity of powder equivalent to one tablet was weighed and dissolved in a volumetric flask of 100 ml with distilled water. After sonication for 10 min in an ultrasonic bath, the supernatant solution was centrifuged for 5 min and filtered through the 0.45 μm syringe filter. Finally the solution was diluted with a 10 mM tetraborate buffer to give a concentration in the calibration range.
3. Results and discussion
3.1. Method development
In order to propose a suitable method for routine analysis, it was necessary to evaluate the experimental conditions for the best resolution of studied analytes. In this step of work, parameters such as pH, BGE concentration and applied voltage were explored.The molecules of interest are all basis (except isopropamide). Their pKa values are collected in Table 1.
To optimize the BGE concentration, separations of the standard mixture solution in borate buffer at different molarity were performed (30–70 mM). In Fig. 1 migration time versus BGE concentration is reported. Peak resolution, analysis time and peak broadening were evaluated and 60 mM borate buffer at pH = 9.2 was selected as a compromise value.
 | ||
| Fig. 1 Plot of migration time vs. different buffer concentration Experimental conditions: borate buffer at 30, 50, 60 and 70 mM pH = 9.2; 15 kV; T = 25 °C; λ = 210 nm (Beckman Instrument). | ||
For the best CZE separation of studied analytes, the optimal experimental conditions are:
- Uncoated fused silica capillary I.D.= 75 μm; total length = 58 cm; effective length = 48 cm;
- BGE solution 60 mM tetraborate buffer pH = 9.2;
- Applied voltage 15 kV;
- Injection mode: 0.5 psi for 3 s;
- Detection wavelength 210 nm;
- Temperature 25 °C;
In these operating conditions the analysis time is 7.5 min (see Fig. 2)
 | ||
| Fig. 2 Electropherogram of standard mixture. Peak identification: 1) II; 2) E; 3) PM; 4) CPM; 5) DPH. Experimental conditions: borate buffer 60 mM pH = 9.2; 15 kV (i = 82 μA); T = 25 °C; λ = 210 nm (Beckman Instrument). | ||
3.2. Method validation
In the optimized experimental conditions the proposed method was validated examining the following parameters: repeatability of migration time (intra-day and inter-day) and of corrected peak area, linearity range of detector response, LOD, LOQ, accuracy (recoveries) and ruggedness.The data are collected in Table 2. The RSD% values were calculated for five consecutive injections of the standard solution at 10 μg ml−1.
| ANALYTES | Operator 1 RSD (%) | Operator 2 RSD (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | |||||
| tm | Ac | tm | Ac | tm | Ac | tm | Ac | |
| II | 0.19 | 3.22 | 0.48 | 4.66 | 0.50 | 2.80 | 0.44 | 4.62 |
| E | 0.24 | 2.54 | 0.81 | 3.78 | 0.47 | 2.76 | 0.51 | 1.37 |
| PM | 0.26 | 2.68 | 0.86 | 4.05 | 0.43 | 3.30 | 0.48 | 2.22 |
| CPM | 0.26 | 3.68 | 0.88 | 5.53 | 0.47 | 0.95 | 0.46 | 2.57 |
| DPH | 0.29 | 3.42 | 0.92 | 4.90 | 0.45 | 3.07 | 0.58 | 5.35 |
Five standard solutions containing five analytes of interest were injected in triplicate. The resulting equations and relative linear correlation coefficient (r2) values are collected in Table 3.
| ANALYTES | EQUATIONS | r2 |
|---|---|---|
| II | y = 55.779x − 9.474 | 0.9969 |
| E | y = 47.394x − 15.862 | 0.9981 |
| PM | y = 61.323x − 28.441 | 0.9982 |
| CPM | y = 61.001x − 56.948 | 0.9982 |
| DPH | y = 95.510x − 104.550 | 0.9982 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ranged between 0.7 μg ml−1 to 1.0 μg ml−1.
1 ranged between 0.7 μg ml−1 to 1.0 μg ml−1.
LOQ, the lowest concentration of analytes that can be quantified with good precision, defined as signal to noise ratio S/N of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ranged between 2.1 μg ml−1 to 3.0 μg ml−1.
1 ranged between 2.1 μg ml−1 to 3.0 μg ml−1.
Active compounds: ephedrine hydrochloride 15 mg, chlorpheniramine maleate 10 mg
Excipients: hydrogenate oil, magnesium stearate, hydroxypropylmethylcellulose, parabens.
In optimized experimental conditions the real sample was injected and the resulting electropherogram (Fig. 3) does not reveal interfering peaks.
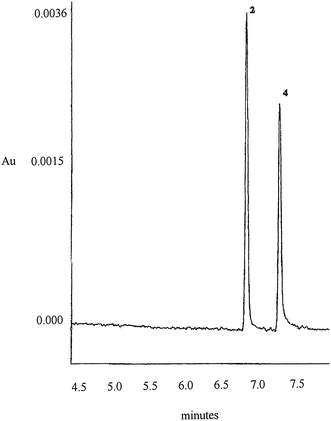 | ||
| Fig. 3 Electropherogram of real sample Peak identification and experimental conditions as in Fig. 2. | ||
| FIENAMINA | QUANTITY FOUND (mg/tab) | QUANTITY DECLARED (mg/tab) | RECOVERY (%) |
|---|---|---|---|
| Ephedrine hydrochloride | 13.86 ± 0.06 | 15.00 | 86.6 |
| Chlorpheniramine maleate | 7.93 ± 0.06 | 10.00 | 96.6 |
- measurements on different apparatus;
- performance by different operators;
- change of environmental conditions.
Measurements on another instrument, the Spectraphoresis 1000 apparatus, in the optimal experimental conditions were performed, injecting the standard mixture in hydrodynamic mode for 1 s. In this instrument the cartridge form is different and the capillary total length is 44 cm, the effective length 36 cm (shorter than optimal length of capillary utilized on the Beckman instrument). Consequently, on this instrument, it was necessary to apply a lower voltage (10 kV).
In Fig. 4 the resulting electropherogram is reported. The analysis time is shorter (about 6 min). The precision data of migration time and corrected peak area of analytes are reported in Table 5.
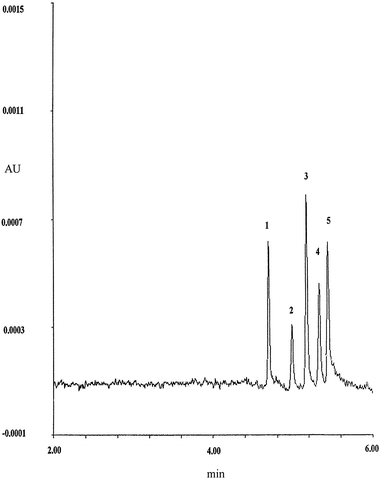 | ||
| Fig. 4 Electropherogram of the standard mixture. Peak identification as in Fig. 2. Experimental conditions: borate buffer 60 mM pH = 9.2; 10 kV (i = 42 μA); T = 25 °C; λ = 210 nm (Spectraphoresis 1000 instrument). | ||
The RSD% values were calculated for five consecutive injections of the standard solution at 20 μg ml−1 (intermediate concentration).
The results obtained by different operators (intra-laboratory) were satisfactory. Precision (run-to-run) of the migration time ranged from 0.19% to 0.50% and for the corrected peak area from 0.95% to 3.68%.
The environmental conditions (external temperature) did not influence the analytical results because the temperature control system allows capillary cooling. The calibration data obtained several months later gave the linear response in the same range with 0.9978 ≤ r2 ≤ 0.9997 and intraday precision of tm and Ac were respectively 0.43%÷0.50% and 0.27%÷1.69%
4. Conclusion
A fast and accurate CZE method has been developed for the simultaneous separation of five of the most common antihistamines, decongestants and anticholinergic remedies.The best resolution was obtained in about 8 min applying a simple buffer 60 mM tetraborate, pH = 9.2 at 25 °C and constant voltage of 15 kV in an uncoated fused silica capillary of L = 58 cm and I.D.= 75 μm. The method was validated and applied to real sample analysis in these experimental conditions. The main advantages of the CE method with respect to the HPLC are short analysis time and low reagents and solvent consumption. This is important not only for economic reasons, but also for the environmental impact aspect. The developed CZE method is cheap, easy, did not require sample pre-treatment, and offers a valid alternative for Quality Control analysis in the pharmaceutical industry.
References
- L. Zhang, Q. Hu, G. Chen and Y. Fang, Anal. Chim. Acta, 2000, 424(2), 257–262 CrossRef CAS.
- M. R. Gomez, L. Sombra, R. A. Olsina, L. D. Martinez and M. F. Silva, Farmaco, 2005, 60, 85–90 CrossRef CAS.
- R. Gottardo, F. Bortolotti, G. De Paoli, J. Pascali, I. Miksik and F. Tagliaro, J. Chromatogr., A, 2007, 1159(1–2), 185–9 CrossRef CAS.
- M. R. Gomez, R. A. Olsina, L. D. Martinez and M. F. Silva, J. Pharm. Biomed. Anal., 2002, 30(3), 791–799 CrossRef CAS.
- L. Suntornsuk, Electrophoresis, 2001, 22(1), 139–143 CrossRef CAS.
- L. Suntornsuk, O. Pipitharome and P. Wilairat, J. Pharm. Biomed. Anal., 2003, 33(3), 441–449 CAS.
- H. Sirèn, T. Hiissa and Y. Min, Analyst, 2000, 125(9), 1561–1568 RSC.
- Dong Yuming, Chen Xiaofeng and et al. , Journal of Pharm. And Biomed. Analysis, 2005, 39(1–2), 285–9 Search PubMed.
- He Jiatian, Zhao Xinghong, Chen Yao and L. Fang, Yaowu Fenxi Zazhi, 2008, 28(1), 143–145 Search PubMed.
- F. Buiarelli, F. Coccioli, R. Jasionowska and A. Terracciano, Electrophoresis, 2008, 29, 3519–3523 CrossRef CAS.
- SciFinder Scholar™ Copyright© 2007 American Chemical Society (CAS Chemical Abstract Service).
- S. P. Porras, M.-L. Riekkola and E. Kenndler, Chromatographia, 2001, 53(5/6), 290–294 CAS.
- Feng Luan, Weiping Ma, Zang and et al, Pharm. Res., 2005, 22(9), 1454–1460 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
