Correlation between acidic potassium permanganate chemiluminescence and in vitro cell culture assay: Physiologically meaningful antioxidant activity
Xavier A.
Conlan
*a,
Nicole
Stupka
*a,
Geoffrey P.
McDermott
b,
Neil W.
Barnett
b and
Paul S.
Francis
ab
aInstitute for Technology research and Innovation, Deakin University, Geelong, Victoria 3217, Australia. E-mail: xavier@deakin.edu.au; Tel: +61 3 52271416
bSchool of Life and Environmental Sciences, Deakin University, Geelong, Victoria 3217, Australia
First published on 21st December 2009
Abstract
There is great interest in the activity of antioxidant molecules, including polyphenols, from food and plant sources. Acidic potassium permanganate chemiluminescence signal intensity was shown to predict the ability of polyphenols to positively act on cellular redox state and attenuate oxidative stress in cultured skeletal muscle cells.
Introduction
The health benefits of antioxidant-rich ‘Mediterranean’ type diets are well recognised.1 Epidemiological studies have repeatedly shown that diets based on whole grains, polyunsaturated plant oils (e.g. olive oil) and a moderate intake of red wine are associated with a lower incidence of cardiovascular disease, cancer, diabetes, osteoporosis and brain disorders.2,3 These are thought to result from the presence of specific micronutrients, including antioxidant and anti-inflammatory compounds (such as resveratrol4 and oleocanthal5) present in red wine and olive oil, respectively. However, relating a ‘Mediterranean diet’ and the intake of plant antioxidant phytochemicals to good health outcomes after adjusting for confounding lifestyle factors is challenging.6 Epidemiological studies do not reveal the cellular mechanisms underlying the health benefits of plant antioxidants and do not account for the diversity in the composition and concentration of nutrients found in various plant foods or the same plant food with different geographical origins.7These foods (e.g. grains, olives and wine) contain a diverse range of chemical components, including polyphenolics, a large and complex family of molecules believed to have health promoting benefits, because of their antioxidant properties.8 They can also enhance cellular endogenous antioxidant defences by the activation of genes encoding for endogenous antioxidant enzymes and molecules.9
The research challenge lies in identifying, prioritising, and possibly isolating these antioxidant molecules to better understand their role in human health and nutrition. There is a lot of interest in prioritising plant phenols based on their antioxidant activities. Current approaches involve arduous sample fractionation into smaller ‘crude’ extracts, followed by cell culture assays, with the bioactive identified only after a positive antioxidant response in the cell, or examination of the antioxidant activities of fractions or specific compounds using various chemical ‘test tube’ assays.10
It is a substantial challenge to identify bioactives with antioxidant potential from complex mixtures. Several screening systems based on chromatographic separation coupled with chemical antioxidant tests (predominantly involving 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radicals) have been developed.11,12 These systems enable the antioxidant potential of a molecule of interest to be quantified, but the relationship between activities established by these chemical tests and the action of the compounds in biological systems has been the subject of much debate.10,13 However, in vitro cell culture assays for antioxidant potential have been shown to be significant in relation to in vivo studies.14
The chemiluminescence reaction with acidic potassium permanganate has recently been utilised as a rapid chemical test of the total antioxidant status of wines, fruit juices and teas.15,16 The emission, which emanates from an excited manganese(II) species formed in the reaction,17 is elicited by many organic molecules, but a particularly intense response is obtained for readily oxidisable phenols and related compounds.18 This approach is particularly promising for screening components of complex samples after chromatographic separation, because the fast reaction kinetics avoid the band broadening and associated loss of resolution produced by conventional chemical assays.
However, the full potential of this chemiluminescence reagent to screen for bioactives with antioxidant activity cannot be realised until the relationship between the response for individual compounds and their activity in biological systems is explored. We have therefore compared the relative chemiluminescence signal intensity for a series of bioactive compounds with their antioxidant activity in human primary skeletal muscle cell cultures, to demonstrate the feasibility of this approach.
Experimental section
Acidic potassium permanganate chemiluminescence
The chemiluminescence reagent was prepared by adding 1 × 10−3 M potassium permanganate and 1% (w/v) sodium polyphosphate to deionised water and adjusting the pH to 2.5 using sulfuric acid. The reactions were performed using a flow injection analysis manifold with chemiluminescence detector, as previously described.15Primary skeletal muscle cell culture
All experimental procedures were formally approved by the Deakin University and Barwon Health Human Research Ethics Committees. Primary skeletal muscles cells (myoblasts) were isolated by enzymatic digestion of vastus lateralis muscle biopsies from healthy elderly donors undergoing elective hip replacement surgery. Myoblasts were expanded in growth media on extra-cellular matrix (ECM)-coated culture flasks (#E1270, Sigma-Aldrich; ECM diluted 1:75 in skeletal muscle growth medium (SkGM)) in 5% O2 and 5% CO2 at 37 °C until passage 3 and experiments were carried out at passage 4. Myoblasts were cultured in 5% oxygen representing physiological oxygen levels. Low oxygen culture conditions provide a reducing redox environment compared to standard 20% oxygen culture conditions.Detection of reactive oxygen species in muscle cells
Fresh stock solutions (50 mM) of resveratrol, rosmarinic acid, gallic acid, o-coumaric acid, cinnamic acid and flavone (Sigma Aldrich) were prepared in deionised water. Myoblasts were seeded into 96-black well, clear bottom plates in 5% CO2, and either 5% or 20% O2, at 37 °C and proliferated until 80% confluent in growth media. To stimulate the formation of multinucleated muscle fibres (myotubes), cells were cultured in differentiation media for 5 days. For the chronic phenol treatment experiments, phenol stocks were diluted in differentiation media to the concentration indicated in Fig. 1 and myotubes were treated for 2 days. For the acute phenol treatment experiments, myotubes were cultured in differentiation media for a further 2 days. At 7 days differentiation, myotubes were loaded with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) (#C6827, Molecular Probes) in phosphate buffered saline for 30 min. Then for the acute phenol treatment experiments, phenol stocks were diluted in Dulbecco's Modified Eagle Medium (DMEM) (minus phenol red) and then control and phenol-treated myotubes were exposed to 50 µM H2O2 or vehicle to assess the acute effects on phenols on reactive oxygen sepcies (ROS) levels in the basal state or in response to oxidative stress. For the chronic (2 days) phenol treatment experiments, to determine whether the phenols may accumulate within myotubes and minimize the potentially confounding effects of phenols neutralizing H2O2 in the cell culture media rather than intracellularly, all myotubes were treated only with DMEM (minus phenol red) with or without 50 µM H2O2. Oxidation of DCFDA was measured using a FlexStationII384 scanning fluorometer (Molecular Devices) at 488 nm (excitation) and 518 nm (emission) at 37 °C for 20 min with a reading every 30 s. For each treatment the average fluorescence between 4 wells was determined and subtracted from the background auto-fluorescence; then the area under the curve was calculated. Within experiments, independent t-tests were used to determine whether ROS levels in phenol treated myotubes were significantly different from control myotubes with p < 0.05. Data are presented as mean ± SEM and ROS levels are expressed relative to control. | ||
| Fig. 1 Effect of 2 day polyphenol treatment on ROS levels in myotubes following H2O2 treatment. In response to increased oxidative stress, resveratrol, gallic acid and rosmarinic acid, but not o-coumaric acid, flavone and cinnamic acid, reduced ROS levels compared to control myotubes (p-value <0.05). | ||
Results and discussion
Several key bioactives were selected for this study, including simple organic acids (gallic acid, cinnamic acid and o-coumaric acid), some more complex polyphenols (resveratrol and rosmarinic acid) and a structurally related compound: flavone. The relative chemiluminescent signals produced by these analytes are presented in Fig. 2.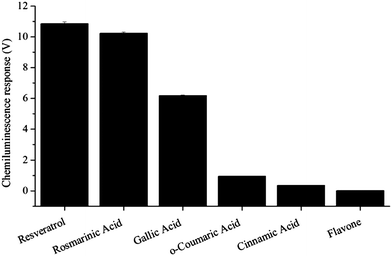 | ||
| Fig. 2 Chemiluminescence response (1 × 10−5 M standards). | ||
Strong chemiluminescence intensities were observed for gallic acid, resveratrol and rosmarinic acid. o-Coumaric acid afforded a more moderate response, whilst cinnamic acid and flavone elicited a relatively small emission intensity. Similar observations were made using other analyte concentrations, from 1 × 10−3 M to 1 × 10−5 M.
To assess the relationship between chemiluminescence signal intensity and in vitro cell culture antioxidant assays, ROS levels were measured in human primary skeletal muscle cell (myotube) cultures. Skeletal muscle is a highly metabolically active tissue constituting ∼40% of body weight and muscle cells are quite responsive to cell stress and antioxidants.19 In initial experiments, myotubes were treated acutely with a given compound and cell ROS levels were assessed in the basal state or in response to oxidative stress (50 µM H2O2) by monitoring the intracellular oxidation of DCFDA using a fluorescent plate reader (Fig. 3). Oxidation of DCFDA is a commonly used assay of generalised oxidative stress, rather than assaying specific free radical species.20,21
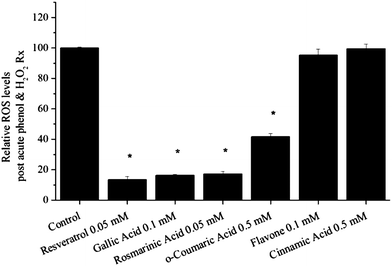 | ||
| Fig. 3 Effect of acute polyphenol treatment on ROS levels in myotubes following H2O2 treatment. In response to increased oxidative stress, resveratrol, gallic acid, rosmarinic acid and o-coumaric acid, but not flavone and cinnamic acid, reduced ROS levels compared to control myotubes (p < 0.05). | ||
In subsequent experiments to determine whether the selected compounds can accumulate in the cell and modulate redox state, myotubes were pre-treated with the compound of interest for 2 days, following a brief washout period (coincident with DCFDA loading), ROS levels were assessed in DMEM without phenols in the basal and oxidative stressed state (Fig. 1). The concentration at which individual phenols were tested was selected from preliminary studies of a wider concentration range where cytotoxicity and antioxidant efficacy were evaluated. At the concentrations used here and as determined by lactate dehydrogenase release (CytoTox 96 Assay, Promega), none of the phenols had unwanted toxic effects (data not shown). From these experiments, the in vitro antioxidant potential was assessed and information about cellular uptake was also obtained. To summarise, resveratrol, gallic acid and rosmarinic acid reduced oxidative stress in the basal state and in response to H2O2 treatment, and data from the 2 days phenol treatment experiments suggest that these compounds accumulate within myotubes. o-Coumaric acid did not accumulate in the cell, but in the acute phenol treatment experiments it was able to quench H2O2 in the cell culture media and thus reduce intracellular stress. Following acute and 2 day treatment, flavone and cinnamic acid did not attenuate ROS levels within myotubes nor in the cell culture media in response to H2O2.
Based on these in vitro cell culture assays, at the concentrations used, resveratrol, gallic acid and rosmarinic acid were the most potent antioxidants, with o-coumaric acid having modest antioxidant effects, and flavone and cinnamic acid had no antioxidant activity.
Conclusions
These observations demonstrate a clear correlation between the acidic potassium permanganate chemiluminescence intensity and cellular antioxidant activity and support our hypothesis that chemiluminescence detection can provide high-throughput data concerning the antioxidant activity of a molecule. This approach has great potential for rapid screening, evaluation and characterisation of the antioxidant capacity of constituents in whole foods (e.g. wine, olive oil) or bioactive fractions.Notes and references
- F. Sofi, F. Cesari, R. Abbate, G. F. Gensini and A. Casini, Br. Med. J., 2008, 337, a1344 CrossRef.
- J. Slavin, Nutr. Res. Rev., 2004, 17, 99 Search PubMed.
- W. C. Willet, F. Sacks, A. Trichopoulou, A. Ferro-Luzzi, E. Helsing and D. Trichopoulos, Am. J. Clin. Nutr., 1995, 61, 1402S.
- J. M. Wu, Z. R. Wang, T. C. Hsieh, J. L. Bruder, J. G. Zou and Y. Z. Huang, Int. J. Mol. Med., 2001, 8, 3 Search PubMed.
- G. K. Beauchamp, R. S. J. Keast, D. Morel, J. Lin, J. Pika, Q. Han, C. Lee, A. B. Smith and P. A. S. Breslin, Nature, 2005, 437, 45 CrossRef CAS.
- L. Dauchet, P. Amouyel and J. Dallongville, Nat. Rev. Cardiol., 2009, 6, 599 Search PubMed.
- M. Singh, M. Arseneault, T. Sanderson, V. Murthy and C. Ramassamy, J. Agric. Food Chem., 2008, 56, 4855 CrossRef CAS.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radical Biol. Med., 1996, 20, 933 CrossRef CAS.
- E. Lund, Br. J. Nutr., 2002, 88, 223 CrossRef CAS.
- M. S. Fernandez-Panchon, D. Villano, A. M. Troncoso and M. C. Garcia-Parrilla, Crit. Rev. Food Sci. Nutr., 2008, 48, 649 CrossRef CAS.
- H. A. G. Niedeländer, T. A. van Beck, A. Bartasiute and I. I. Koleva, J. Chromatogr., A, 2008, 1210, 460.
- S. Shi, H. Zhou, Y. Zhang, X. Jiang, X. Chen and K. Huang, TrAC, Trends Anal. Chem., 2009, 28, 865 CrossRef CAS.
- N. Hermans, P. Cos, L. Maes, T. De Bruyne, D. Vanden Berghe, A. J. Vlietinck and L. Pieters, Curr. Med. Chem., 2007, 14, 417 CrossRef CAS.
- B. Halliwell, R. Aeschbach, J. Loliger and O. I. Aruoma, Food Chem. Toxicol., 1995, 33, 601 CrossRef CAS.
- J. W. Costin, N. W. Barnett, S. W. Lewis and D. J. McGillvery, Anal. Chim. Acta, 2003, 499, 47 CrossRef CAS.
- P. S. Francis, J. W. Costin, X. A. Conlan, S. A. Bellomarino, J. A. Barnett and N. W. Barnett. Submitted for publication.
- J. L. Adcock, P. S. Francis, T. A. Smith and N. W. Barnett, Analyst, 2008, 133, 49 RSC.
- J. L. Adcock, P. S. Francis and N. W. Barnett, Anal. Chim. Acta, 2007, 601, 36 CrossRef CAS.
- J. F. Young, J. Hansen-Moller and N. Oksbjerg, J. Agric. Food Chem., 2004, 52, 7158 CrossRef CAS.
- B. Halliwell and M. Whiteman, Br. J. Pharmacol., 2004, 142, 231 CrossRef CAS.
- H. Wang and J. A. Joseph, Free Radical Biol. Med., 1999, 27, 612 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
