Simultaneous determination of β-sitosterol, campesterol, stigmasterol, and brassicasterol in serum by high-performance liquid chromatography with electrochemical detection†
Nanako
Ito
,
Hideki
Hakamata
* and
Fumiyo
Kusu
Department of Analytical Chemistry, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences, Horinouchi 1432-1, Hachioji, Tokyo 192-0392, Japan. E-mail: hakaman@toyaku.ac.jp
First published on 23rd November 2009
Abstract
A simple method for the determination of phytosterols such as β-sitosterol, campesterol, stigmasterol, and brassicasterol has been developed using high-performance liquid chromatography with electrochemical detection (HPLC-ECD). Under optimized conditions, the current peak height was linearly related to the amount of phytosterol injected, ranging from 10 to 200 μmol L−1 (r = 0.999). The relative standard deviation (RSD) was less than 3.1% (100 μmol L−1, n = 6). The detection limit (S/N = 3) of phytosterol was less than 3.4 μmol L−1 (17 pmol). Phytosterols spiked into the control human and rat sera were determined by the present method with recovery rates of more than 80% and RSDs (n = 3) of less than 4.2%. This method has been successfully applied to the monitoring of experimental phytosterolemia in rats induced by a high-phytosterol diet. Taken together, we found this method, which does not require a derivatization step, is both simple and useful for the simultaneous determination of serum phytosterols, providing an alternative tool for the diagnosis of phytosterolemia.
1. Introduction
Phytosterolemia (β-sitosterolemia) is an autosomal recessive disease caused by the mutation of the ATP-binding cassette transporter G5 (ABCG5) or ABCG8 gene.1 Because ABCG5/ABCG8 proteins play important roles in the intestinal absorption and the biliary excretion of phytosterols, a defect in the protein leads to both increased absorption and decreased excretion of phytosterols. As a result, an accumulation of food-derived phytosterols occurs in serum and tissues except for the brain, thereby inducing tendon and tuberous xanthomas and premature coronary atherosclerosis.2 Thus, it is necessary to determine serum phytosterols, such as β-sitosterol and campesterol, for the proper diagnosis of phytosterolemia.3Various methods for the determination of phytosterols have been reported. For diagnostic purposes, gas chromatography (GC) has been the standard method for the determination of serum phytosterols to date.4,5 The detection modes of the GC methods are flame ionization6,7 and mass spectrometry,8 enabling for sensitive determination. In addition to these GC methods, several methods by high-performance liquid chromatography (HPLC) for the determination of phytosterols in biological samples have been developed. Such methods include HPLC with ultraviolet detection (HPLC-UV),9–11HPLC with fluorescence detection (HPLC-FL),12 and liquid chromatography with tandem mass spectrometric detection (LC-MS/MS).13,14 HPLC-UV is a simple and widely available method, but derivatization is necessary for the diagnosis of phytosterolemia to achieve enough sensitivity. Benzoyl chloride is often used as a labeling reagent for clinical samples.9–11 HPLC-FL is characterized by its high sensitivity after derivatization with a fluorophore, but its application to the determination of serum phytosterols has not yet been demonstrated.
In general, derivatization is an effective method for sensitive and selective detection in HPLC. However, derivatization sometimes requires time-consuming clean-up steps and the reaction efficiency is affected by sample impurities. Thus, to avoid these problems, it would be desirable to develop a simple method without derivatization. At present, LC-MS/MS, a sensitive and informative method, is the only method that can be applied to the diagnosis of phytosterolemia without derivatization.13 However, in LC-MS, the matrix effects are known to affect peak signals15,16 and not every laboratory is equipped with the necessary apparatus. In addition, for accurate determination by LC-MS or LC-MS/MS, it has been recommended that stable isotope-labeled standard compounds of all analytes as internal standards (IS) be prepared.17,18
As an alternative approach, HPLC with electrochemical detection (HPLC-ECD) is a candidate method applicable to the determination of serum phytosterols. Until recently, cholesterol has been regarded as an electrochemically inactive compound.19 However, it has been reported that cholesterol can be electrochemically oxidized and this reaction is applicable to the determination of serum cholesterol by HPLC-ECD.20 Subsequently, it has been shown that the electrochemical oxidation of cholesterol leads to the formation of cholesta-4,6-dien-3-one,21 indicating that a hydroxy group at the 3β-position of cholesterol is a target of electrochemical oxidation. These results suggest that several phytosterols are electrochemically oxidizable under appropriate conditions, because a hydroxy group is present at the 3β-position of the steroid ring. Thus, the present study has been undertaken to develop a simple method for the simultaneous determination of representative phytosterols such as β-sitosterol, campesterol, stigmasterol, and brassicasterol (Fig. 1) in serum by HPLC-ECD. Moreover, the present HPLC-ECD method has been applied to monitor the concentration of serum phytosterols in rats fed a high phytosterol diet, an animal model of phytosterolemia.
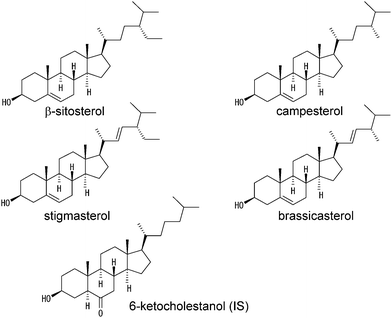 | ||
| Fig. 1 Structures of β-sitosterol, campesterol, stigmasterol, brassicasterol, and 6-ketocholestanol used as an internal standard (IS). | ||
2. Experimental
2.1. Materials
β-Sitosterol, campesterol, stigmasterol, and brassicasterol were obtained from Tama Biochemical Co., Ltd. (Tokyo, Japan). Cholesterol was obtained from Sigma Chemical Co. (St. Louis, MO, USA). 6-Ketocholestanol was obtained from Steraloids Inc. (Newport, RI, USA). Acetonitrile (HPLC grade) and LiClO4 (reagent grade) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Human serum-based reference material (SRM909b) was from NIST (Gaithersburg, MD, USA). A phytosterol mixture for the high-phytosterol diet was kindly provided by Eisai Food & Chemical Co., Ltd. (Tokyo, Japan). Safflower oil was obtained from Nisshin OilliO Group, Ltd. (Tokyo, Japan). All other reagents were of reagent grade available from commercial sources.2.2. HPLC
The HPLC-ECD system consisted of a DP-8020 pump (Tosoh Co., Tokyo, Japan), a model 7125 injector fitted with a 5 μL injection loop (Rheodyne, Cotati, CA, USA), a Develosil C30-UG-3 conventional column (150 × 4.6 mm i.d., 3 μm, Nomura Chemical, Aichi, Japan), a CTO-10AS column oven (Shimadzu, Kyoto, Japan), an HECS 311B 15-01 potentiostat (Fuso ElectroChemical System, Kanagawa, Japan), and a mobile phase, acetonitrile containing 10 mmol L−1 LiClO4. The electrochemical cell (radial flow cell, BAS Inc., Tokyo, Japan) was constructed from a glassy carbonworking electrode, an Ag/AgCl reference electrode, and a stainless steel auxiliary electrode. The column was maintained at 30 °C in the column oven. The flow rate was set at 1.5 mL min−1. An internal standard (IS) method was used for the determination of phytosterol concentration in the sample solution, and 6-ketocholestanol was used as an IS (Fig. 1).2.3. Sample preparation
Human serum (SRM909b) was reconstituted by adding autoclaved distilled water according to the instructions of the manufacturer. Reconstituted SRM909b was stored at −18 °C in a small aliquot of polyethylene tubes unless it was used immediately. Rat serum was obtained by centrifugation of the rat whole blood at 25 °C for 10 min at 3000 rpm (Model 3740, Kubota, Tokyo, Japan).Both human and rat sera (10 μL) was transferred to a screw-capped vial, and 0.05 mL of freshly prepared 1 mol L−1potassium hydroxide in ethanol was added. Alkaline hydrolysis was conducted by vortex for 1 min and sonication (Branson 2200, Yamato Scientific, Tokyo, Japan) for 10 min. This procedure (vortex and sonication) was further repeated four times. To extract lipids, 0.2 mL of distilled water was added to the sample, vortex-mixed with 0.8 mL of hexane for 1 min, and then centrifuged for 5 min at 3000 rpm (Model 3740, Kubota). The supernatant (hexane layer) was transferred to a new tube and further extracted once with 0.2 mL of water. Separately, the water layer was further extracted twice with 0.8 mL of hexane. The hexane extracts were combined and dried at 90 °C in the water bath. The residue was dissolved in 0.2 mL (non-spiked and 10 mg/dL phytosterol spiked serum), 0.4 mL (20 mg/dL phytosterol spiked serum), or 0.5 mL (30 mg/dL phytosterol spiked serum) of the mobile phase containing 50 μmol L−1 of 6-ketocholestanol and passed through a 0.45-μmmembrane filter before injection into the HPLC system.
2.4. Animal experiments
Male rats (SHRSP; stroke-prone spontaneously hypertensive, 9 weeks) were obtained from Japan SLC, Inc. (Shizuoka, Japan). They were maintained under controlled temperature (21–23 °C) and light (lights on, 7:00 a.m. to 7:00 p.m.) conditions with ad libitum access to chow and water. To induce phytosterolemia, SHRSP rats were fed a standard chow (CE-2, Clea Japan, Inc., Tokyo, Japan) supplemented with 10% safflower oil and 0.5% phytosterol mixture for two weeks, as previously described by Ikeda and colleagues.22 The phytosterol mixture contained 95.9% phytosterol, in which the phytosterol composition was: β-sitosterol, 45.8%; campesterol, 27.3%; stigmasterol, 19.4%; brassicasterol, 7.5%. At the indicated time (see Fig. 6), the animals were anesthetized with diethyl ether and around 100 μL of blood were taken from tail vein to prepare the serum samples. The animal experiments were approved by the institutional ethics committee of the Tokyo University of Pharmacy and Life Sciences, and performed under the guidelines of the committee.3. Results and discussion
3.1. HPLC-ECD conditions
HPLC-ECD conditions were optimized in terms of the columns, column temperature, mobile phase composition, flow rate, and detection potential. For the HPLC columns, an ODS column and a C30 column were compared. Our preliminary experiment showed that the resolution (Rs) of campesterol and stigmasterol by the ODS column (Inertsil®ODS-3 conventional column, 150 × 4.6 mm i.d., 5 μm, GL Sciences Inc, Tokyo, Japan) was less than 1.5, suggesting insufficient separation. In contrast, the Rs of campesterol and stigmasterol by the C30 column was nearly equal to 1.5. Therefore, the C30 column was used for the determination of phytosterols in this study.The column temperature was tested from 15 to 40 °C. When the temperature was higher than 30 °C, the Rs of campesterol and stigmasterol was less than 1.5. However, the Rs of stigmasterol and β-sitosterol was less than 1.5, when the column temperature was lower than 30 °C. Thus, we selected 30 °C as the column temperature.
In the early stages of this study, we used an acetonitrile/2-propanol mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) containing 50 mmol L−1 LiClO4 as a mobile phase, because this solution was applicable to the serum cholesterol determination.20 However, the baseline noise levels became higher when 2-propanol was added to the acetonitrile, changing the mobile phase to acetonitrile containing 50 mmol L−1 LiClO4.
1, v/v) containing 50 mmol L−1 LiClO4 as a mobile phase, because this solution was applicable to the serum cholesterol determination.20 However, the baseline noise levels became higher when 2-propanol was added to the acetonitrile, changing the mobile phase to acetonitrile containing 50 mmol L−1 LiClO4.
The flow rate of the mobile phase was examined from 1 to 2 mL min−1. Contrary to our expectations, the current peak height of phytosterol was not so affected by the flow rate. Thinking about the analytical time and the amount of mobile phase required, 1.5 mL min−1 was selected as the flow rate.
To determine the detection potential of phytosterols, hydrodynamic voltammograms were measured (Fig. 2). The oxidation current appeared at more positive than 1.8 V vs.Ag/AgCl and the oxidation peak was around 2.9 V vs.Ag/AgCl. However, an applied potential of 2.8 V vs.Ag/AgCl was selected because of the best signal-to-noise ratios of the phytosterols.
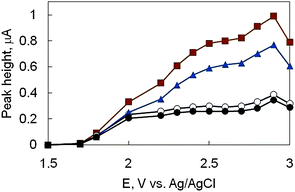 | ||
| Fig. 2 Hydrodynamic voltammogram of phytosterols. β-Sitosterol (●), campesterol (○), stigmasterol (▲), and brassicasterol (■) dissolved in the mobile phase (100 μmol L−1 each) were injected into HPLC-ECD. HPLC conditions; mobile phase, acetonitrile containing 10 mM LiClO4; flow rate, 1.5 mL min−1; column temperature, 30 °C; column, Develosil C30-UG-3 column (250 mm × 4.6 mm i.d., 3 μm). | ||
3.2. Determination of phytosterols by HPLC-ECD
Fig. 3 shows a chromatogram of standard β-sitosterol, campesterol, stigmasterol, and brassicasterol under optimized HPLC conditions. As an IS, 5α-cholestane,23stigmastanol,24cholesterol,255β-cholestan-3α-ol,26 and 6-ketocholestanol27 have been used for the determination of phytosterols. Among them, our preliminary experiments showed that 6-ketocholestanol could be detected and separated from phytosterols under HPLC conditions. Therefore, we decided to use this compound as an IS.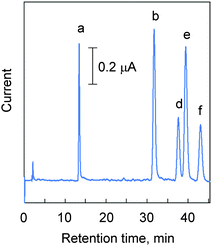 | ||
| Fig. 3 Chromatogram of standard phytosterols. Phytosterols and 6-ketocholestanol (IS) dissolved in the mobile phase (100 μmol L−1 each) were injected into HPLC-ECD. The HPLC conditions; mobile phase, acetonitrile containing 10 mM LiClO4; flow rate, 1.5 mL min−1; column, Develosil C30-UG-3 column (250 mm × 4.6 mm i.d., 3 μm); column temperature, 30 °C; applied potential, +2.8 V vs.Ag/AgCl; injection volume, 5 μL. Peaks: a, 6-ketocholestanol (IS); b, brassicasterol; d, campesterol; e, stigmasterol; f, β-sitosterol. | ||
To assess the performance of the HPLC-ECD for the determination of phytosterols, seven-point calibration curves of β-sitosterol, campesterol, stigmasterol, and brassicasterol were made. The linear ranges, correlation coefficients, detection limits, and RSDs (inter-day and intra-day precision) are listed in Table 1. Each phytosterol at 50 pmol was detected with an RSD of less than 3.1% (n = 6), indicating that the present method is precise. The detection limit (S/N = 3) was less than 17.0 pmol, showing that the present method is also sensitive. Although the sensitivity of the present method is not superior to that of GC7,8 or LC-MS/MS,13 it is comparable to that of derivatized HPLC-UV,9 where the detection limits were 9.65 pmol for β-sitosterol and 20.0 pmol for campesterol. These results suggest that the present method is an alternative to derivatized HPLC-UV.
| Phytosterol | Linear range, μmol L−1 (pmol) | Calibration curve | Detection limit, μmol L−1 (pmol) | RSD, % | |||
|---|---|---|---|---|---|---|---|
| y, sample/I.S. = a + bx, μmol L−1 | Intra-day (n = 6) | Inter-day (n = 6) | |||||
| a | b | r | |||||
| a To draw the calibration curve, various concentrations of phytosterol and 50 μmol L−1 of 6-ketocholestanol (IS) were injected into HPLC-ECD. The value of y was calculated on the basis of the ratio of the current peak heights of phytosterol and IS. r indicates the correlation coefficient. RSD indicates the relative standard deviation. To obtain RSD, a mixture of 100 μmol L−1 of each phytosterol was repeatedly injected into HPLC-ECD in a day (Intra-day) or over six different days (Inter-day). | |||||||
| β-Sitosterol | 10–200 (5–100) | 0 | 0.0074 | 0.999 | 3.40 (17.0) | 1.9 | 1.1 |
| Campesterol | 10–200 (5–100) | 0 | 0.0086 | 0.999 | 2.01 (10.0) | 0.93 | 1.0 |
| Stigmasterol | 10–200 (5–100) | 0 | 0.0162 | 0.999 | 1.06 (5.29) | 1.3 | 1.1 |
| Brassicasterol | 10–200 (5–100) | 0 | 0.0223 | 0.999 | 0.816 (4.08) | 3.1 | 1.2 |
3.3. Simultaneous determination of serum phytosterols by HPLC-ECD
Next, the applicability of the present method to biological samples was examined. For these purposes, human serum was spiked with β-sitosterol, campesterol, stigmasterol, and brassicasterol. Fig. 4 shows a representative chromatogram of phytosterols spiked into human serum at 10 mg/dL each, a reported serum phytosterol level (10–65 mg/dL) of phytosterolemic subjects.3 The results of the recovery test are listed in Table 2. The recovery of the spiked phytosterol was more than 81% with RSD values of less than 3.9%, showing precise determination. These results suggest that the present HPLC-ECD method is applicable to the determination of phytosterols in the serum of phytosterolemic subjects.| Phytosterol | Concentration added, mg/dL | Recovery, % | RSD, % (n = 3) |
|---|---|---|---|
| a For the recovery test, each concentration of phytosterol was spiked into the human serum and analyzed as described in the experimental section. | |||
| β-Sitosterol | 10 | 90 | 0.50 |
| 20 | 91 | 1.8 | |
| 30 | 92 | 1.7 | |
| Campesterol | 10 | 99 | 3.4 |
| 20 | 98 | 2.2 | |
| 30 | 93 | 0.51 | |
| Stigmasterol | 10 | 82 | 3.9 |
| 20 | 81 | 0.93 | |
| 30 | 87 | 0.40 | |
| Brassicasterol | 10 | 91 | 3.0 |
| 20 | 86 | 2.4 | |
| 30 | 88 | 0.068 |
 | ||
| Fig. 4 Chromatogram of phytosterols spiked into human serum (SRM909b). Human serum was spiked at 10 mg/dL of each phytosterol and prepared as described in the experimental section. HPLC conditions used are the same as in Fig. 3. | ||
To further examine the applicability of the present method, rat serum was spiked with phytosterols. The results of the recovery test are listed in Table 3. The recovery of the spiked phytosterol was more than 80% with RSD values of less than 4.2%, suggesting that the current method is applicable for monitoring rat serum phytosterol.
| Phytosterol | Concentration added, mg/dL | Recovery, % | RSD, % (n = 3) |
|---|---|---|---|
| a For the recovery test, each concentration of phytosterol was spiked into the rat serum and analyzed as described in the experimental section. | |||
| β-Sitosterol | 10 | 90 | 4.2 |
| 20 | 94 | 3.2 | |
| 30 | 81 | 1.1 | |
| Campesterol | 10 | 98 | 3.0 |
| 20 | 99 | 3.5 | |
| 30 | 91 | 0.96 | |
| Stigmasterol | 10 | 85 | 0.43 |
| 20 | 80 | 0.15 | |
| 30 | 81 | 0.58 | |
| Brassicasterol | 10 | 87 | 2.7 |
| 20 | 86 | 1.1 | |
| 30 | 82 | 1.1 |
3.4. Monitoring of rat phytosterolemia induced by a high-phytosterol diet
It has been reported that several strains of rats could be regarded as an animal model of phytosterolemia, when fed a high phytosterol diet.22 According to Ikeda's paper, SHRSP rats were fed a 0.5% phytosterol diet and serum phytosterols were determined. Fig. 5 shows a representative chromatogram of phytosterols in rat serum fed a high phytosterol diet. The time course changes in serum phytosterol in the SHRSP rats fed a high-phytosterol diet are shown in Fig. 6. Although four types of phytosterols were loaded, increases in campesterol and β-sitosterol were observed. These results are in good agreement with the previous results of Ikeda22 and suggest the presence of a selective absorption mechanism of these phytosterols in the rat intestine. In addition, it is noteworthy that for the determination of serum phytosterols, 100 μL of blood was enough. This means that it is not necessary to euthanize the rats for blood sampling. Therefore, our results indicate that the current method is useful to monitor experimental phytosterolemia in rats, allowing for a proper diagnosis of these animal models of phytosterolemia.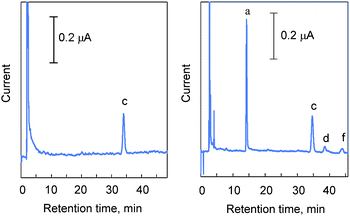 | ||
| Fig. 5 Chromatogram of phytosterols in rat serum fed a 0.5% phytosterol diet. A. Blank (control SHRSP rat serum). B. Serum from an SHRSP rat fed a high-phytosterol diet for three days. The preparation of the serum is described in the experimental section. HPLC conditions used are the same as in Fig. 3. | ||
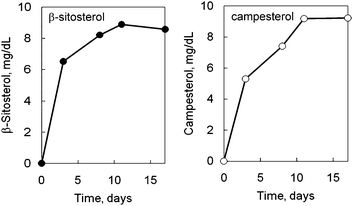 | ||
| Fig. 6 Effect of phytosterol feeding on serum phytosterol concentrations in SHRSP rats. SHRSP rats were fed a 0.5% phytosterol diet or control diet (CE-2) for the indicated periods, and the serum concentrations of the phytosterols were determined by HPLC-ECD. The values are means of rats (n = 2) and the S.D. values are within the symbols. The concentrations of the phytosterols in the control rats were less than the detection limits. The preparation of the serum is described in the experimental section. | ||
4. Conclusions
In the present study, an HPLC-ECD method has been developed for the determination of phytosterols using the direct electrochemical oxidation of phytosterols. It can be noted that the present method does not require any derivatization of phytosterols. This enabled the simple determination of phytosterols without time-consuming clean-up steps after derivatization. In addition, the applicability of the present method to biological samples was shown by the determination of phytosterols in human as well as rat sera by an internal standard method. Moreover, this method was successfully applied to the monitoring of phytosterolemia in SHRSP rats fed a high phytosterol diet. Therefore, the present method provides an alternative for the diagnosis of phytosterolemia and the research fields of phytosterol physiology.Abbreviations used
| HPLC-ECD | high-performance liquid chromatography with electrochemical detection |
| RSD | relative standard deviation |
| ABCG5 | ATP-binding cassette transporter G5 |
| ABCG8 | ATP-binding cassette transporter G8 |
| GC | gas chromatography |
| HPLC-UV | HPLC with ultraviolet detection |
| HPLC-FL | HPLC with fluorescence detection |
| LC-MS/MS | liquid chromatography with tandem mass spectrometric detection. |
Acknowledgements
We are grateful to Eisai Food & Chemical Co., Ltd. (Tokyo, Japan) for providing the phytosterol mixture. We are also grateful to Yu-Ya Hosokawa for his assistance in the early stages of this study.References
- K. E. Berge, H. Tian, G. A. Graf, L. Yu, N. V. Grishin, J. Schultz, P. Kwiterovich, B. Shan, R. Barnes and H. H. Hobbs, Science, 2000, 290, 1771–1775 CrossRef CAS.
- M. D. Patel and P. D. Thompson, Atherosclerosis, 2006, 186, 12–19 CrossRef CAS.
- I. Björkhem, S. Skrede, in: C. R. Scriver, A. L. Beaudet, W. S. Sly, D. Valle (Eds.), The Metabolic Basis of Inherited Disease, 6th Ed., McGraw-Hill, New York, 1989, p. 1283–1302 Search PubMed.
- A. Kuksis, J. Chromatogr., A, 2001, 935, 203–236 CrossRef CAS.
- S. Kidambi and S. B. Patel, J. Clin. Pathol., 2008, 61, 588–594 CrossRef CAS.
- G. Salen, P. O. Kwiterovich Jr., S. Shefer, G. S. Tint, I. Horak, V. Shore, B. Dayal and E. Horak, J. Lipid Res., 1985, 26, 203–209 CAS.
- C. Domeño, B. Ruiz and C. Nerín, Anal. Bioanal. Chem., 2005, 381, 1576–1583 CrossRef CAS.
- H. S. Mohamed Ahmida, P. Bertucci, L. Franzò, R. Massoud, C. Cortese, A. Lala and G. Federici, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2006, 842, 43–47 CrossRef CAS.
- H. Hidaka, T. Nakamura, T. Aoki, H. Kojima, Y. Nakajima, K. Kosugi, I. Hatanaka, M. Harada, M. Kobayashi, A. Tamura, T. Fujii and Y. Shigeta, J. Lipid Res., 1990, 31, 881–888 CAS.
- M. Kuriyama, J. Fujiyama, T. Kasama and M. Osame, J. Lipid Res., 1991, 32, 223–229 CAS.
- S. J. Robins and J. M. Fasulo, J. Clin. Invest., 1997, 99, 380–384 CrossRef CAS.
- Y. T. Lin, S. S. Wu and H. L. Wu, J. Chromatogr., A, 2007, 1156, 280–287 CrossRef CAS.
- J. Lembcke, U. Ceglarek, G. M. Fiedler, S. Baumann, A. Leichtle and J. Thiery, J. Lipid Res., 2005, 46, 21–26 CAS.
- A. Honda, K. Yamashita, H. Miyazaki, M. Shirai, T. Ikegami, G. Xu, M. Numazawa, T. Hara and Y. Matsuzaki, J. Lipid Res., 2008, 49, 2063–2073 CAS.
- B. K. Matuszewski, M. L. Constanzer and C. M. Chavez-Eng, Anal. Chem., 2003, 75, 3019–3030 CrossRef CAS.
- J. L. Little, M. F. Wempe and C. M. Buchanan, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2006, 833, 219–230 CrossRef CAS.
- A. L. Yergey, N. V. Esteban and D. J. Liberato, Biomed. Environ. Mass Spectrom., 1987, 14, 623–625 CAS.
- L. M. Thienpont, K. Van Uytfanghe, S. Blincko, C. S. Ramsay, H. Xie, R. C. Doss, B. G. Keevil, L. J. Owen, A. L. Rockwood, M. M. Kushnir, K. Y. Chun, D. W. Chandler, H. P. Field and P. M. Sluss, Clin. Chem., 2008, 54, 1290–1297 CrossRef CAS.
- S. Görög, Quantitative analysis of steroids, Elsevier Scientific Publishing Company, Inc., Amsterdam, The Netherlands, 1983, p. 255–256 Search PubMed.
- K. Hojo, H. Hakamata, A. Ito, A. Kotani, C. Furukawa, Y. Y. Hosokawa and F. Kusu, J. Chromatogr., A, 2007, 1166, 135–141 CrossRef CAS.
- Y. Y. Hosokawa, H. Hakamata, T. Murakami, S. Aoyagi, M. Kuroda, Y. Mimaki, A. Ito, S. Morosawa and F. Kusu, Electrochim. Acta, 2009, 54, 6412–6416 CrossRef CAS.
- I. Ikeda, H. Nakagiri, M. Sugano, S. Ohara, T. Hamada, M. Nonaka and K. Imaizumi, Metabolism, 2000, 50, 1361–1368.
- P. J. Jones, M. Raeini-Sarjaz, F. Y. Ntanios, C. A. Vanstone, J. Y. Feng and W. E. Parsons, J. Lipid Res., 2000, 41, 697–705 CAS.
- H. R. Davis Jr., L. J. Zhu, L. M. Hoos, G. Tetzloff, M. Maguire, J. Liu, X. Yao, S. P. Iyer, M. H. Lam, E. G. Lund, P. A. Detmers, M. P. Graziano and S. W. Altmann, J. Biol. Chem., 2004, 279, 33586–33592 CrossRef.
- R. E. Ostlund Jr., S. B. Racette, A. Okeke and W. F. Stenson, Am. J. Clin. Nutr., 2002, 75, 1000–1004.
- A. L. Amundsen, F. Ntanios, N. Put and L. Ose, Eur. J. Clin. Nutr., 2004, 58, 1612–1620 CrossRef CAS.
- B. Lu, Y. Zhang, X. Wu and J. Shi, Anal. Chim. Acta, 2007, 588, 50–63 CrossRef CAS.
Footnote |
| † This work was partly presented at the 12th ISEC (International Symposium on Electroanalytical Chemistry) August 12–15, 2009 in Changchun, China. |
| This journal is © The Royal Society of Chemistry 2010 |
