DOI:
10.1039/B9AY00240E
(Paper)
Anal. Methods, 2010,
2, 275-281
HPTLC method for estimation of tazarotene in topical gel formulations and in vitro study
Received
2nd November 2009
, Accepted 3rd December 2009
First published on
4th January 2010
Abstract
A new, eco friendly, simple, and rapid high-performance thin-layer chromatographic method was developed and validated for quantitative determination of tazarotene. The HPTLC separation was achieved on an aluminium-backed layer of silica gel 60F254 using toluene-methanol (9.0 + 1.0 v/v) as mobile phase. Quantitation was achieved by densitometric analysis at 327 nm over the concentration range of 100–500 ng/mL. The method was found to give a compact spot for the drug (Rf = 0.75 ± 0.01). The linear regression analysis data for the calibration plots showed a good linear relationship with r2 = 0.9995. The method was validated for precision, recovery, repeatability, and robustness as per the International Conference on Harmonization guidelines. The minimum detectable amount was found to be 18.59 ng/spot, whereas the limit of quantitation was found to be 56.34 ng/spot. Statistical analysis of the data showed that the method is precise, accurate, reproducible, and selective for the analysis of tazarotene. The method was successfully employed for the estimation of equilibrium solubility, quantification of tazarotene as a bulk drug, in commercially available gel preparation and in-house developed microemulsion based gel formulations.
Introduction
Tazarotene (TZR), 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl] nicotinic acid ethyl ester is a member of a new generation of receptor selective, synthetic retinoids for the topical treatment of mild to moderate plaque psoriasis, acne vulgaris and photoaging. Dermal safety studies also indicated that TZR did not show phototoxic or photoallergic potential.1 However, mild to moderate local cutaneous irritation, with burning, itching, erythema, peeling, and/or dryness, was observed in approximately 25% of treated patients.2 So, it is necessary to improve the topical delivery and reduce the adverse effects of TZR using a carrier with the ability of skin targeting.
Microemulsion (ME) as colloidal carriers is one of the promising systems that have attracted interest in penetration enhancement because of their localized effect. ME is liquid dispersions of water and oil that are made homogenous, transparent (or translucent) and thermodynamically stable by the addition of relatively large amounts of a surfactant and a co-surfactant and having a diameter of droplets in the range of 10–100 nm. Due to their special features, ME offer several advantages for pharmaceutical use, such as ease of manufacturing, long-term stability, high solubilization capacity for hydrophilic and lipophilic drugs, and improved drug delivery.3 Several studies have reported that ME formulations possess improved dermal and transdermal delivery properties, mostly in vitro and in vivo (Table 1).
Table 1 Overview of in vitro and in vivo studies of dermal and transdermal delivery with microemulsions
| Microemulsion formulation components |
Drug |
In vitro/In vivo studies |
Membrane/skin/species used |
Year |
Reference No. |
| Surfactanat/Cosurfactant |
Oil phase |
Aqueous phase |
| Brij 30, ethanol |
Eucalyptus oil |
Water |
Cyproterone Acetate |
In vitro
|
Pig |
2007 |
4
|
| Brij 30, ethanol |
Eucalyptus oil |
Water |
Progesterone |
In vitro
|
Pig |
2007 |
4
|
| Tween 80, ethanol |
Isopropyl myristate |
Buffer pH 7.4 |
Diclofenac |
In vitro
|
Guinea pig |
2007 |
5
|
| Tween 80, span 20, ethanol |
Isopropyl myristate |
Water |
Sodium nonivamide acetate |
In vitro
|
Rat |
2008 |
6
|
| Brij 96V, hexanol |
Jojoba oil |
Water |
Diclofenac sodium |
In vivo
|
Rat |
2008 |
7
|
| Cremophore EL, ethanol |
Oleic acid |
Water |
Penciclovir |
In vitro
|
Mouse skin |
2008 |
8
|
| Cremophore EL, benzyl alcohol, chlorocresol |
Capryol 90 |
Water |
Fluconazole |
In vitro & In vivo
|
Candida albicans, human |
2009 |
9
|
| Labrasol, ethanol |
Lauryl alcohol |
Water |
Fluconazole |
In Vitro
|
Rat |
2009 |
10
|
A literature survey revealed a high performance liquid chromatography (HPLC) method for determination of TZR and its major active metabolite with mass spectroscopy.11 Isocratic RP-HPLC method with UV detection has been described for quantitative and related substance determination of TZR.12 However, HPLC-based separation methods may not be suitable for the determination of the drug from lipid-based delivery systems such as ME formulations. These formulations contain various lipophilic excipients that are not soluble in commonly used organic solvents used in RP-HPLC methods. Further, extraction of the drug from such lipophilic excipients may not be achieved easily, and such excipients may get adsorbed on stationary phase. Hence, analysis of TZR, particularly from lipid based delivery systems, would be difficult with respect to identification of suitable solvents and stationary phases.
In view of this, high-performance thin layer chromatography (HPTLC)-based methods could be considered as a good alternative as they are being explored as an important tool in routine drug analysis. Major advantage of HPTLC is its ability to analyze several samples simultaneously using a small quantity of mobile phase. This reduces time and cost of analysis. In addition, it minimizes exposure risks and significantly reduces disposal problems of toxic organic effluents, thereby reducing possibilities of environment pollution. HPTLC also facilitates repeated detection of chromatogram with same or different parameters. Furthermore, in the case of HPTLC, there are no restrictions on the choice of solvents and mobile phases; drug and lipophilic excipients can be dissolved in a suitable solvent that would evaporate during spotting on TLC plate leaving behind the analyte as a thin band. Therefore, for such methods, extraction procedure is not required always and could be developed for analyzing the drug without any interference from excipients.13 Literature survey revealed that the HPTLC method of analysis has not been explored for TZR until to date. Therefore it was felt necessary to develop a HPTLC method for determination and quantitative estimation of TZR. In view of this, the present study describes the development of a new, simple, rapid, eco-friendly, validated HPTLC method for estimation of TZR from bulk, pharmaceutical dosage forms such as gel and ME based gel developed in-house.
Experimental
Apparatus
The HPTLC system (Camag, Muttenz, Switzerland) consisted of a Limomat V autosprayer connected to a nitrogen cylinder, a twin trough chamber (10 × 10 cm), a derivatization chamber, and a plate heater. Pre-coated silica gel 60 F254 HPTLC plates (10 × 10 cm, layer thickness 0.2 mm (E. Merck KGaA, Darmstadt, Germany) were used as the stationary phase. TLC plates were pre-washed twice with 10 mL of methanol and activated at 80 °C for 5 min prior to sample application. Densitometric analysis was carried out using a TLC scanner III with winCATS software.
Reagents and materials
TZR pure powder was obtained as gratis sample from Dr. Reddy's Laboratory Ltd. (Andhra Pradesh–India) with 99.9% purity. Tazret™ Gel (Glenmark Pharmaceuticals Ltd., India) was obtained commercially with the labeled amounts of 0.05% w/w of TZR. Labrafac CC (caprylic/capric triglycerides, C8–C10 fatty acids), labrasol (caprylocaproyl macrogol-8-glyceride), plurol oleique (polyglyceryl 6-dioleate) (Gattefosse Saint-Priest, France) was procured as gratis sample from Gattefosse Asia Ltd. (Mumbai, India). Corbopol 971P NF was procured as gratis sample from Lubrizol Advance Material India Pvt Ltd. (Mumbai, India). Potassium dihydrogen phosphate, methanol and propylene glycol were purchased from SDfine Chemicals (Ahmedabad, India). Ethanol was purchased from Baroda Chemical Ind. Ltd. (Dabhoi, India). Double distilled water was used throughout the study. All other chemicals and solvents were of analytical reagent grade and used as received without further purification.
HPTLC method and chromatographic conditions
Sample application.
The standard and formulation samples of TZR were spotted on pre-coated TLC plates in the form of narrow bands of lengths 6 mm, with 10 mm from the bottom and left margin and with 9 mm distance between the two bands. Samples were applied under a continuous drying stream of nitrogen gas at a constant application rate of 150 nL/s.
Mobile phase and migration.
Plates were developed using a mobile phase consisting of toluene : methanol (9.0 + 1.0 v/v). Linear ascending development was carried out in a 10 cm × 10 cm twin trough glass chamber equilibrated with the mobile phase. The optimized chamber saturation time for the mobile phase was 20 min at 25 ± 2 °C. Ten milliliters of the mobile phase (5 mL in a trough containing the plate and 5 mL in the other trough) was used for each development and allowed to migrate a distance of 70 mm, which required 10 min. After development, the TLC plates were dried completely.
Densitometric analysis and quantitation procedure.
Densitometric scanning was performed on Camag TLC Scanner III in absorbance mode and operated by winCATS planar chromatography version 1.3.4. The source of radiation utilized was a deuterium lamp. The spots were analyzed at a wavelength of 327 nm. The slit dimensions used in the analysis were length and width of 5 mm and 0.45 mm, respectively, with a scanning rate of 20 mm/s. These are selected as recommended by the CAMAG TLC Scanner III manual. It covers 70–90% of the application band length, which in the present case is 6 mm. The monochromator bandwidth was set at 20 nm. Concentrations of compound chromatographed were determined from the intensity of diffusely reflected light and evaluated as peak areas against concentrations using linear regression equation.
Preparation of TZR standard stock solution.
Stock solution was prepared by weighing TZR (10 mg). Weighed powder was accurately transferred to a volumetric flask of 100 mL and dissolved in and diluted to the mark with methanol to obtain a standard stock solution of TZR (100 µg/mL).
Method validation
Validation of the developed HPTLC method was carried out as per the International Conference on Harmonization (ICH) guidelines Q2 (R1) for specificity, sensitivity, accuracy, precision, repeatability, and robustness.14
Specificity.
The specificity of the developed method was established analyzing the sample solutions containing TZR from ME formulations and marketed tablets in relation to interferences from formulation ingredients. The spot for TZR in the sample was confirmed by comparing retardation factor (Rf) values of the spot with that of the standard.
Sensitivity.
Sensitivity of the method was determined with respect to limit of detection (LOD) and limit of quantification (LOQ). Noise was determined by scanning the blank spot (methanol) six times. Series of concentrations of drug solutions (10–500 ng/spot) were applied on the plate and analyzed to determine LOD and LOQ. LOD was calculated as 3 times the noise level, and LOQ was calculated as 10 times the noise level. LOD and LOQ were experimentally verified by diluting the known concentrations of TZR until the average responses were approximately 3–10 times the standard deviation (SD) of the responses for six replicate determinations.
Linearity and calibration curves.
Linearity of the method was evaluated by constructing calibration curves at six concentration levels. Calibration curves were plotted over a concentration range of 100–500 ng/spot. The calibration curves were developed by plotting peak area vs. concentrations (n = 6) with the help of the win-CATS software.
Accuracy.
Accuracy of the method was evaluated by carrying out the recovery study at three levels. Recovery experiments were performed by adding three different amounts of standard drug, i.e., 80, 100, and 120% of the drug, to the preanalyzed ME based gel formulations and marketed gel formulation, and the resultant was reanalyzed six times.
Precision.
Precision was evaluated in terms of intra-day and inter-day precisions. Intra-day precision was determined by analyzing sample solutions of TZR from ME based gel formulations at three levels covering low, medium, and higher concentrations of calibration curve for five times on the same day. Inter-day precision was determined by analyzing sample solutions of TZR at three levels covering low, medium, and higher concentrations over a period of seven days (n = 5). The peak areas obtained were used to calculate mean and % RSD (relative SD) values.
Repeatability (system precision).
Repeatability of measurement of peak area was determined by analyzing different amount of TZR samples covering low, medium, and higher ranges of the calibration curve seven times without changing the position of plate. Repeatability of sample application was assessed by spotting TZR samples covering similar range of calibration curve seven times and analyzing them once.
Robustness.
By introducing small changes in mobile phase composition, its volume, chamber saturation time, and slight change in the solvent migration distance, the effects on the results were examined. Robustness of the method was determined in triplicate at a concentration level of 300 ng/spot and the mean and % RSD of peak area was calculated.
Application of developed method
Determination of equilibrium solubility.
Solubility of TZR in various excipients was determined by the shake flask method. An excess of TZR was added to 1 g of each of the excipient and vortexed to facilitate the mixing. Mixtures were shaken for 48 h in a reciprocating water bath shaker maintained at room temperature. After 48 h, each tube was centrifuged at 600 × g for 10 min, and the insoluble drug was discarded by filtration using a 0.45-µm membrane filter. The filtrate was suitably diluted with methanol and the concentration of drug was quantified by a developed HPTLC method.
TZR formulations.
Two ME based gel formulations of TZR were developed in-house, one by mixing labrasol and plurol oleique in a weight ratio of 3 : 1, with 12% (wt/wt) Labrafac CC and 0.50% (wt/wt) carbopol 971P NF (formulation F1), and another by mixing labrasol and plurol oleique in a weight ratio of 4 : 1, with 12% (wt/wt) labrafac CC and 0.50% (wt/wt) carbopol 971P NF (formulation F2). Both formulations on dilution with water yield ME with mean particle size less than 40 nm as determined by photon correlation spectroscopy with in-built Zetasizer (Nano ZS, Malvern Instruments, UK) at 633 nm. A helium–neon gas laser having an intensity of 4 mW was the light source. The commercially available gel of TZR, 0.05% w/w was also used in this investigation to verify the suitability of the method for analysis of TZR from conventional dosage forms.
Analysis of TZR in formulations.
About 5 gm gel was weighed in a 25 ml volumetric flask and dissolved in methanol. The solution was sonicated for 15 min and diluted suitably with methanol to obtain a 100 µg/mL concentration of TZR. ME based gel formulations containing 0.05% w/w of TZR were treated in a similar manner as that of marketed gel formulations to obtain a stock solution of 100 µg/mL. On TLC plates, 3 µL of these solutions were spotted and analyzed for TZR content using the proposed method as described earlier. The possibility of interference from other components of the gel formulation in the analysis was studied. Placebo ME based plain gel formulations were analyzed similarly to study the potential interference.
In vitro diffusion profile of TZR formulation.
ME based gel formulations of TZR for topical delivery (formulation F1 and F2) were evaluated for in vitro release using a Franz diffusion cell with a diameter of 10 mm. The temperature of the receiver chamber containing 15 mL of diffusion media (physiological saline + 95% ethanol, 7.0 + 3.0 v/v) was controlled at 37 ± 1 °C under continuous stirring with a Teflon-coated magnetic bar at a constant rate, in a way that the skin surface just flushes the diffusion fluid. During study, 2 mL of aliquots were removed at 15, 30, 60, 90, 120, and 240 min and replaced with fresh buffer. The amount of drug released was determined using the developed HPTLC method.
Results and discussion
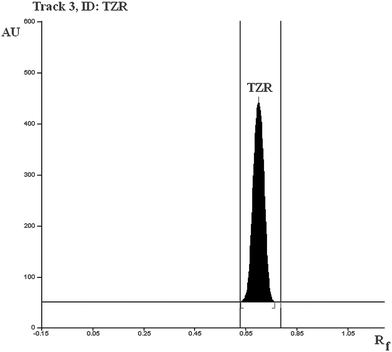
To develop a HPTLC method of analysis for TZR for routine analysis, selection of mobile phase was carried out on the basis of polarity. A solvent system that would give dense and compact spots with appropriate and significantly different Rf value for TZR was desired. Various solvent systems such as acetone–methanol, methanol–chloroform, methanol–toluene, toluene–ethyl acetate, hexane–ethyl acetate, hexane–acetone, toluene–acetonitrile, and toluene–acetonitrile–glacial acetic acid were evaluated in different proportions. Among these, the solvent system comprising of toluene : methanol (9.0 + 1.0, v/v) gave good separation of TZR from its matrix with an Rf value of 0.75. It was also observed that chamber saturation time and solvent migration distance are crucial in chromatographic separation as chamber saturation time of less than 15 min and solvent migration distances greater than 70 mm resulted diffusion of analyte spot. Therefore, the toluene : methanol solvent system in 9.0 + 1.0 (v/v) proportion with chamber saturation time of 20 min at 25 °C and solvent migration distance of 70 mm was used as the mobile phase. These chromatographic conditions produced a well-defined compact spot of TZR with optimum migration at Rf = 0.75 ± 0.01 (Fig. 1). It also gave a good resolution of analyte from excipients used in various ME based gel formulations and marketed gel formulation.
Method validation
Sensitivity.
Under the experimental conditions employed, the lowest amount of drug that could be detected was found to be 18.59 ng/spot and the lowest amount of drug that could be quantified was found to be 56.34 ng/spot, with RSD < 5%.
Specificity.
Specificity is the ability of an analytical method to assess unequivocally the analyte in the presence of sample matrix. TZR was separated from excipients with an Rf of 0.75 ± 0.01. There was no interfering peak at the Rf value of TZR from excipients such as labrasol, plurol oleique, labrafac CC and corbopol 971P NF present in ME based gel formulations. In addition, there was no interference from excipients, present in commercial gel formulation, thereby confirming specificity of method.
Linearity and calibration curves.
Linearity of an analytical method is its ability, within a given range, to obtain test results that are directly, or through a mathematical transformation, proportional to concentration of analyte. The method was found to be linear in a concentration range of 100–500 ng/spot (n = 6), with respect to peak area. The regression data as shown in Table 2 reveal a good linear relationship over the concentration range studied demonstrating its suitability for analysis. No significant difference was observed in the slopes of standard curves (ANOVA, p > 0.05).
Table 2 Linear regression data for the calibration curves (n = 6)
| Range (ng/spot) |
r
2 ± SD |
Slope ± SD |
Intercept ± SD |
| 100–600 |
0.9995 ± 0.001 |
16.741 ± 0.204 |
6506.473 ± 94.327 |
Accuracy.
Accuracy of an analytical method is the closeness of test results to true value. It was determined by the application of analytical procedure to recovery studies, where a known amount of standard is spiked in preanalyzed samples solutions. Results of accuracy studies from excipient matrix were shown in Table 3; recovery values demonstrated the accuracy of the method in the desired range.
Table 3 Recovery Studies (n = 6)
| Formulation |
Amount of drug analyzed (ng) |
Amount of drug added (ng) |
Theoretical concentration (ng) |
Total amount of drug analyzed (ng) |
% Recovery ± SD |
| F1 |
200 |
160 |
360 |
357.00 |
99.17 |
| 200 |
200 |
400 |
399.66 |
99.92 |
| 200 |
240 |
440 |
436.67 |
99.24 |
|
|
|
|
%Average recovery ± SD |
99.44 |
| |
| F2 |
200 |
160 |
360 |
361.66 |
100.46 |
| 200 |
200 |
400 |
395.72 |
98.93 |
| 200 |
240 |
440 |
441.24 |
100.28 |
|
|
|
|
%Average recovery ± SD |
99.89 |
| |
| Marketed gel |
200 |
160 |
360 |
359.41 |
99.84 |
| 200 |
200 |
400 |
400.28 |
100.07 |
| 200 |
240 |
440 |
441.86 |
100.42 |
|
|
|
|
%Average recovery ± SD |
100.11 |
Precision.
The precision of an analytical method expresses the degree of scatter between a series of measurements obtained from multiple sampling of the same homogeneous sample under prescribed conditions. Intra-day precision refers to the use of analytical procedure within a laboratory over a short period of time using the same operator with the same equipment, whereas inter-day precision involves estimation of variations in analysis when a method is used within a laboratory on different days, by different analysts. The results obtained are shown in Table 4. In all instances, % RSD values were less than 5% confirming the precision of the method.
Table 4 Intra and inter-precision studies (n = 5)
| Amount of drug spotted (ng) |
Amount of drug detected (ng, mean ± SD) |
% RSD |
| Intra-day (n = 5) |
| 100 |
99.97 ± 1.31 |
1.21 |
| 300 |
298.11 ± 2.05 |
1.35 |
| 500 |
500.09 ± 1.03 |
1.49 |
| |
| Inter-day (n = 5) |
| 100 |
100.11 ± 1.07 |
1.78 |
| 300 |
299.35 ± 1.75 |
1.66 |
| 500 |
499.71 ± 1.48 |
1.85 |
Repeatability.
Ten-microliter aliquots of samples containing 100, 300, and 500 ng of TZR were analyzed according to proposed method. In order to control scanner parameters, i.e., repeatability of measurement of peak area, one spot was analyzed without changing position of plate (n = 7). By spotting and analyzing the same amount several times (n = 7), precision of automatic spotting device was evaluated. %RSD was consistently less than 5% (Table 5), which was well below the instrumental specifications, ensuring repeatability of developed method as well as proper functioning of the HPTLC system.
Table 5 Repeatability studies (n = 7)
| Parameters |
Amount of drug detected (ng, mean ± SD) |
|
One spot is scanned eight times.
Eight spots scanned once.
|
| Amount of tazarotene spotted (ng) |
100 |
300 |
500 |
| Measurement of peak areaa |
98.72 ± 3.64 |
297.29 ± 4.22 |
495.41 ± 6.57 |
| % RSD |
1.59 |
1.73 |
2.58 |
| Sample applicationb |
99.49 ± 2.57 |
297.44 ± 2.87 |
496.39 ± 5.21 |
| % RSD |
2.69 |
3.46 |
2.28 |
Robustness.
The low values of % RSD (Table 6) obtained after introducing small deliberate changes in the developed HPTLC method confirmed the robustness of the method.
Table 6 Robustness of Method (n = 3)
| Parameters |
Amount of tazarotene spotted (ng) |
Amount of tazarotene detected (ng, mean ± SD) |
% RSD |
| Mobile phase composition: 7.1 : 2.9 |
300 |
299.11 ± 1.04 |
1.67 |
| Mobile phase composition: 6.9 : 3.1 |
300 |
299.02 ± 2.08 |
1.88 |
| Mobile phase volume: 8 mL |
300 |
297.79 ± 3.16 |
1.49 |
| Mobile phase volume: 12 mL |
300 |
298.38 ± 2.56 |
1.29 |
| Chamber saturation time: 15 min |
300 |
296.97 ± 4.88 |
1.43 |
| Chamber saturation time: 25 min |
300 |
300.76 ± 1.59 |
1.71 |
| Solvent migration distance: 68 mm |
300 |
301.21 ± 1.06 |
1.86 |
| Solvent migration distance: 72 mm |
300 |
298.83 ± 2.47 |
1.91 |
Application of developed method
Determination of equilibrium solubility
TZR is practically insoluble in water, which limits the development of new pharmaceutical formulations, aimed to improve its delivery. Therefore, solubility studies were performed to identify suitable oily phases, surfactants, and co-surfactants that are generally employed in commercially available topical, intranasal, parenteral and oral products (Table 7). In spite of being lipophilic in nature, TZR exhibited very low solubility in oily phases such as isopropyl palmitate and miglyols. It was found that only labrafac CC exhibited good solubility for TZR. The drug exhibited good solubility in labrasol, tween 80 (surfactants), plurol oleique, transcutol P, capmul MCM and propylene glycol (co-surfactants). Among the various excipients tried, the oily phases such as isopropyl myristate, captex 200, and labrafac CC exhibited good solubilization potential for TZR. Labrasol and tween 80 amongst surfactants and plurol oleique, transcutol P, cremophor RH 40 and capmul MCM as a co-surfactant were found to solubilize the maximum amount of TZR.
| Excipients |
Solubilitya |
|
Data expressed as mg/g, mean ± SD, n = 3.
Data expressed as µg/mL, mean ± SD, n = 3.
|
|
Oily phases
|
| Labrafil M 1944 (pleoyl polyoxylglycerides) |
9.01 ± 3.11 |
| Labrafac CC (caprylic/capric triglycerides) |
38.66 ± 7.21 |
| Olive oil |
0.85 ± 0.33 |
| Isopropyl myristate |
59.14 ± 7.44 |
| Labrafac lipophile (medium chain triglycerides) |
0.32 ± 0.14 |
| Labrafac PG (propylene glycol dicaprylocaprate) |
0.23 ± 0.21 |
| Maisine 35-1 (glyceryl mono-linoleate) |
0.76 ± 0.12 |
| Miglyol 810 (caprylic/capric triglyceride) |
0.92 ± 0.59 |
| Miglyol 812 (caprylic/capric triglyceride) |
2.01 ± 0.32 |
| Miglyol 840 (propylene glycol dicaprylate/dicaprate) |
1.24 ± 0.55 |
| Lauryl alcohol |
1.06 ± 0.21 |
| Isostearylic isostearate |
0.21 ± 0.13 |
| Isopropyl palmitate |
0.35 ± 0.11 |
| Captex 200 (Propylene glycol dicaprylate/dicaprate) |
20.45 ± 5.32 |
| Captex 355 (Glycerol caprylate caprate) |
1.25 ± 0.51 |
| |
|
Surfactants
|
| Labrasol (caprylocaproyl polyoxylglycerides) |
80.17 ± 8.17 |
| Tween (polysorbate) 80 |
75.47 ± 6.77 |
| Plurol stearique WL (polyglyceryl-6-distearate) |
0.65 ± 0.16 |
| Plurol diisostearique (polyglyceryl diisostearate) |
0.19 ± 0.07 |
| Cremophor RH 40 (polyoxyl 40 hydrogenated castor oil) |
18.49 ± 3.19 |
| |
|
Cosurfactants
|
| Plurol oleique CC (polyglyceryl oleate) |
14.38 ± 4.95 |
| Plurol oleique 5203 (polyglyceryl 6-dioleate) |
0.62 ± 0.47 |
| Lauroglycol 90 (propylene glycol monolaurate) |
8.75 ± 4.09 |
| Capryol 90 (propylene glycol monocaprylate) |
6.55 ± 1.04 |
| Ethanol |
80.57 ± 7.84 |
| Transcutol P (diethylene glycol monoethyl ether) |
16.08 ± 6.02 |
| Capmul MCM (glyceryl mono- & dicaprate) |
11.61 ± 4.57 |
| Propylene glycol |
6.31 ± 2.46 |
| |
|
Aqueous Phases
|
| Physiological saline |
59.54 ± 4.09 |
| Phosphate buffer pH 7.4 |
32.11 ± 2.88 |
Analysis of TZR in formulations
A single spot at Rf = 0.75 was observed in the chromatogram of TZR. No interference from the excipients present in the marketed tablet formulation was observed. Analysis of marketed gel showed a drug content of 0.051 ± 0.035 mg. The applicability of the method was verified by the determination of TZR in two ME based gel formulations (developed in-house), and no interference from the excipients matrix was observed. The TZR content of the developed and the marketed formulations was found to be within the limits (±5% of the theoretical value) and are mentioned in Table 8. The low %RSD value indicated the suitability of this method for routine analysis of TZR in various formulations.
Table 8 Content of tazarotene in various formulations
| Formulation |
Label claim (% wt/wt) |
Amount found (mg, mean ± SD) |
% RSD |
| F1 |
0.05 |
0.056 ± 0.011 |
2.19 |
| F2 |
0.05 |
0.055 ± 0.015 |
2.06 |
| Marketed Gel |
0.05 |
0.051 ± 0.035 |
1.03 |
In vitro diffusion profile of TZR formulation
In vitro diffusion profiles of TZR Formulation F1, F2 and marketed gel are presented in Fig. 2. Formulation F2 was found to exhibit a release of 30% drug within 30 min in diffusion media. It was also evident that release of TZR from ME based gel was consistent with the results of solubility profile study.
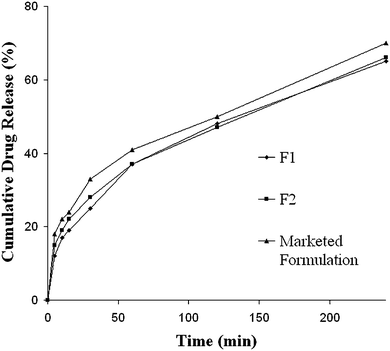 |
| | Fig. 2
In vitro diffusion profiles of tazarotene from various formulations, data expressed as mean ± SD, n = 3. | |
Conclusion
A new HPTLC method has been developed for the identification and quantification of TZR. Low cost, environment friendly, faster speed, and satisfactory precision and accuracy are the main features of this method. The method was successfully validated as per ICH guidelines and statistical analysis proves that the method is sensitive, specific, and repeatable. It can be conveniently employed for routine quality control analysis of TZR as bulk drug in marketed gel formulations; ME based gel formulations without any interference from excipients. The method was also applied for the estimation of equilibrium solubility of TZR in various excipients and diffusion studies.
Acknowledgements
Authors are thankful to Astron Research Ltd. for the gift sample of TZR pure powder, Sophisticated Instrumentation Center for Applied Research and Testing (SICART) (Vallabh Vidyanagar, India) for providing facilities for carrying out analytical work, Gattefosse (Saint-Priest, France), Colorcon (Asia) Pvt. Ltd. (Mumbai, India), Abitec Corporation (Janesville, USA), BASF (Mumbai, India), Sasol (Witten, Germany), Lubrizol Advance Material India Pvt. Ltd. (Mumbai, India), Noveon (Cleveland, USA) for providing gratis samples of excipients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- S. Bershad, G. S. Kranjac, J. E. Parente, M. Tan, D. W. Sherer, A. N. Persaud and M. Lebwohl, Arch. Dermatol., 2002, 138, 481–489 CAS.
- R. Marks, Br. J. Dermatol., 1996, 135, 26–31 CrossRef CAS.
- M. J. Lawrence and G. D. Rees, Adv. Drug Delivery Rev., 2000, 45, 89–121 CrossRef CAS.
- B. Biruss, H. Kahlig and C. Valenta, Int. J. Pharm., 2007, 328, 142–151 CrossRef CAS.
- M. A. H. M. Kamal, N. Iimura, T. Nabekura and S. Kitagawa, Chem. Pharm. Bull., 2007, 55, 368–371 CrossRef CAS.
- Y. B. Huang, Y. H. Lin, T. M. Lu, R. J. Wang, Y. H. Tsai and P. C. Wu, Int. J. Pharm., 2008, 349, 206–211 CrossRef CAS.
- M. Shevachman, N. Garti, A. Shani and A. C. Sintov, Drug Dev. Ind. Pharm., 2008, 34, 403–412 CrossRef CAS.
- W. Zhu, A. Yu, W. Wang, R. Dong, J. Wu and G. Zhai, Int. J. Pharm., 2008, 360, 184–190 CrossRef CAS.
- Y. G. Bachhav and V. B. Patravale, Int. J. Pharm., 2009, 365, 175–179 CrossRef CAS.
- R. B. Patel, M. R. Patel, J. R. Parikh, A. B. Solanki and B. G. Patel, AAPS PharmSciTech, 2009, 10, 917–923 CrossRef.
- M. Attar, D. Yu, J. Ni, Z. Yu, K. H. Ling and D. D. Tang-Liu, J. Pharm. Sci., 2005, 94, 2246–2255 CrossRef CAS.
- D. B. Pathare, A. S. Jadhav and M. S. Shingare, Chromatographia, 2007, 66, 247–250 CrossRef CAS.
- R. B. Patel, A. B. Patel, M. R. Patel, M. B. Shankar and K. K. Bhatt, Anal. Lett., 2009, 42, 1588–1602 CrossRef CAS.
- Validation of Analytical Procedure: Methodology Q2 (R1) 1996. International Conference on Harmonization (ICH), International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here.