Comparative analysis of plant essential oils by GC-MS coupled with integrated chemometric resolution methods
Xiaona
Xu
ab,
Zhonghai
Tang
c and
Yizeng
Liang
*a
aResearch Center of Modernization of Chinese Traditional and Herbal Drug Modernization, College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, P. R. China. E-mail: yizeng_liang@263.net; Fax: +86-731-882-5637
bCollege of Chemistry and Chemical Engineering, University of South China, Hengyang, Hunan, P. R. China
cBio-science and Bio-technology College of Hunan Agriculture University, Changsha, Hunan 410128, China
First published on 28th January 2010
Abstract
The volatile components obtained from drug pair (DP) Pogostemon cablin (P. cablin)–Perilla frutescens (P. frutescens) and its single herbs were analyzed for the first time by gas chromatography–mass spectrometry (GC-MS) combined with three chemometric resolution methods, namely alternative moving window factor analysis (AMWFA), heuristic evolving latent projections (HELP) and selective ion analysis (SIA). Temperature-programmed retention indices (PTRIs) were also used together with mass spectra for tentative identification of the essential oil constituents. A total of 66, 75 and 84 volatile compounds in the essential oils of the studied samples were qualitatively and quantitatively determined, representing 84.17%, 96.19% and 93.44% of the total content, respectively. Comparative analysis between the DP and its single herbs was done, which showed that the number of essential components of the DP is a little less than the sum of the number of its two single herbs, and the major components of the volatile oil of the DP, except the compound of Patchouli alcohol coming from P. cablin's essential oil, are mainly from that of P. frutescens. The results obtained may provide a useful chemical basis for future research on the correlation between the pharmacodynamic action and chemical constituents of the DP and its single herbs. Our work demonstrated that chemometric resolution techniques and PTRIs could provide a complementary and convenient method for accurate analysis of complex systems once again.
1. Introduction
Traditional Chinese medicines (TCMs), due to their long time clinic test and reliable therapeutic efficacy, has been widely used for thousands of years. In some circumstances, it is no doubt that a single herb has an effect on a disease, but it is very difficult to adapt complicating pathogenetic conditions only with one single herb to achieve the goal of helping patients recover completely. Compound recipes are the biggest characteristic of TCM and the theories of compatibility are the essence of its basic theories. Among the numberless complex prescriptions, drug pair (DP), namely the mating partner of two single herb medicines, is the smallest fixative unit. It is commonly used in clinics. When two single herbs are paired, their drug action may be enhanced, or their toxicity may be lowered.1 Thus, it is of great significance to conduct the research on the relationship among the chemical components of DP and those in its single herbs. When the changes of the chemical compositions after pairing of TCMs are clarified better in a way, can the compatibility mechanism of TCMs be better elucidated and the application of TCMs will be extensively expanded.P. cablin, originating in Malaysia and India, is a widely used TCMs. It has the effects of removing dampness, relieving summer-heat and exterior syndrome, stopping coughing and vomiting, eliminating sputum, and stimulating the appetite and others.2–5P. frutescens, which belongs to the family Lamiaceae, is an edible plant frequently used as one of the most popular garnishes and food colorants in some Asian countries such as China and Japan. It can be used as an antitussive, an antibiotic, an antipyretic, an anti-inflammatory and for the treatment of intestinal disorders and allergies.6–8 Medically these two herbal medicines are often used together to relieve exterior syndromes, remove dampness, cure the common cold caused by summer hygrosis, abate headache, heavy body, stomach ache and chest distress, treat nephrasthenia asthma and febricity, and cherish stomach.9 The essential oil components of the two single herbs have been reported respectively,10–14 but those of the DP have not been reported up to now, as well as the comparison of essential oil constituents between the DP and its single herbs. Therefore, it is very necessary to conduct the research on the chemical components of essential oils of DP and its single herbs, and the relationship between the DP and its single herbs. Such investigation will do good to the apprehension of the reason and mechanism why herbal medicines pair and the expansion of recipes' applications.
This investigation aims to explore some relationship among the studied samples from the point of view of chemistry and to acquire some knowledge about the biological activity ingredients. Attention was focused on comparative analysis of their volatile compositions of the DP and its single herbs to find out the similarities and differences between their essential oil compositions by GC–MS with the help of chemometric resolution methods, say alternative moving window factor analysis15 (AMWFA), heuristic evolving latent projections16,17 (HELP) and selective ion analysis18 (SIA), and temperature-programmed retention indices (PTRIs).19 The three chemometric resolution methods forementioned were precisely employed according to GC-MS data. The results obtained appear quite interesting, and provide a useful chemical basis for future research on the correlation between the pharmacodynamic action and chemical constituents of the DP and its single herbs.
2. Materials and methods
2.1 Plant material and alkane standard solution
The materials of P. cablin and P. frutescens were collected by ourselves in Qingping medical material market county (Guangdong Province, China) and Yulin medical material market county (Guangxi Province, China), respectively. The botanical origins of the materials were identified morphologically and microscopically by Mr. Xiangqian Liu, working at Central South University, Changsha, Hunan, P. R. China. The voucher specimens of samples were deposited at our lab. Alkane standard solutions of C8–C20 (mixture no. 04070) and C21–C40 (mixture no. 04071) were purchased from Fluka Chemika (Buchs, Switzerland).2.2 Extraction of essential oil
After the samples were dried for 4 h at 40 °C and smashed, 50 g of sample was swollen with 500 ml of distilled water in advance at room temperature for 2 h, using a standard apparatus for extracting volatile oil according to the standard extracting method described in the Chinese pharmacopoeia.20 Essential oil samples were prepared by water distillation for approximately 5 h until the oil quantity in the extractor did not increase. The obtained essential oil was recovered with n-hexane, dried over anhydrous sodium sulfate until the last traces of water were removed and finally stored in the dark glass bottle at 4 °C prior to GC-MS analysis.Percent yield of the essential oils (w/w) were as follows: 1.42% for P. cablin, 0.36% for P. frutescens, 0.78% for DP P. cablin–P. frutescens.
2.3 GC-MS analysis of volatile oil
GC-MS was performed with a Shimadzu GC-2010 gas chromatography instrument coupled to a Shimadzu QP2010 mass spectrometer. Compounds were separated on a fused silica capillary column DB-1 (100% polymethylsiloxane, 30 m × 0.25 mm i.d., 0.25 μm film thickness). The following oven temperature program was initiated at 50 °C, held for 2 min, then increased at the rate of 4 °C min−1 to 220 °C, held for 2 min. The spectrometers were operated in electron-impact (EI) mode, the scan range was 35–500 amu, the ionization energy was 70 eV and the scan rate was 0.20 s per scan. Injector, interface and ion-source were kept at 250, 250 and 200 °C, respectively. Split injection (1 μl) was conducted with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and helium was used as carrier gas of 1.0 ml min−1 flow-rate.
10 and helium was used as carrier gas of 1.0 ml min−1 flow-rate.
2.4 Calculation of retention indices
Van Den Dool and Krutz21 proposed a quasi-linear equation for PTRIs, namely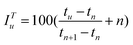 , where ITu is the temperature-programmed retention index of interest; tn, tn+1, and tu are the retention times (in minutes) of the two standard n-alkanes containing n and n + 1 carbons and the interest, respectively. Apparently, the PTRIs are determined in relation to a homologous series of n-alkanes (C8–C24 and C21–C40) under the same operating conditions.
, where ITu is the temperature-programmed retention index of interest; tn, tn+1, and tu are the retention times (in minutes) of the two standard n-alkanes containing n and n + 1 carbons and the interest, respectively. Apparently, the PTRIs are determined in relation to a homologous series of n-alkanes (C8–C24 and C21–C40) under the same operating conditions.
In the present work, the equation above-mentioned was used to calculate retention indices, linear temperature-programmed GC operating conditions.
2.5 Identification of essential oil components
The identification of the components was based on comparison of their mass spectra with NIST05 database through G1701DA mass spectrum ChemStation or with mass spectra reported in the literature,22 in addition to the comparison of their PTRIs with the data web-available.23 At the same time three chemometric resolution methods are adopted to resolve the studied complex systems. The identification of common compounds existing in the drug pair and its single herbs, AMWFA method is used. Regarding those partially overlapped peaks which have their own selective regions or two chromatographic segments with poor relation which perhaps have not got common components, the HELP method is utilized. Concerning those seriously overlapped or embedded peaks which cannot be resolved by the two methods above-mentioned, SIA method is applied.All the compounds identified (match quality higher than 0.90) were adopted and the retention index thresholds for compound matching were set to 50. The identification results of qualitative analysis, listed in order of elution on a DB-1 column, are given in Table 1, together with and the PTRIs calculated and retention indices web-available.23 Those compounds with bold chemical names are identified with chemometric resolution methods. As expected, there are still some components cannot be identified because of their low signal-to-noise ratios or limitation of the mass spectral database.
| RI a | RI b | Name of compound | Relative content (%) in the samples | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| a Note: 1. P. cablin; 2. Perilla; 3. DP P. cablin–Perilla. b RI a and RI b denote programmed-temperature retention index calculated in this paper and literature reported, respectively. c Compound's retention index not found in the literature. —: not identified. tr: Trace (<0.01%). bold chemical name: identified with chemometric resolution methods. | |||||
| 750 | 3-methyl-2-Pentanone | 0.02 | — | 0.01 | |
| 3-Methyl-2-hexene | — | — | tr | ||
| 771 | Hexanal | 0.01 | 0.01 | 0.01 | |
| 819 | 804 | 4-Methyl-3-hexanone | 0.01 | — | tr |
| 823 | 827 | (E)-2-Hexenal | — | 0.01 | 0.01 |
| 832 | 831 | 5-methyl-2-Hexanone | 0.01 | tr | 0.01 |
| 833 | 5,5-dimethyl-2-ethyl-1,3-Cyclopentadiene | tr | — | — | |
| 924 | 961 | Benzaldehyde | 0.01 | 0.01 | 0.01 |
| 927 | 931 | .alpha.-Pinene | 0.01 | 0.01 | 0.06 |
| 939 | 959 | (+)-Camphene | tr | 0.02 | — |
| 954 | 980 | 1-Octen-3-one | 0.01 | 0.02 | 0.01 |
| 960 | 980 | 2,5-Octanedione | 0.01 | 0.01 | 0.01 |
| 961 | 5-Hepten-2-one,6-methyl- | — | 0.02 | 0.01 | |
| 962 | 983 | 1-Octen-3-ol | 0.02 | — | 0.05 |
| 964 | 988 | 3-Octanone | — | — | 0.01 |
| 967 | 961 | (−)-.beta.-Pinene | 0.43 | 0.01 | 0.17 |
| 977 | 993 | Furan, 2-pentyl- | 0.01 | 0.01 | 0.01 |
| 980 | 995 | 3-Octanol | — | 0.04 | 0.03 |
| 982 | 990 | .beta.-Myrcene | — | 0.03 | 0.01 |
| 985 | 1003 | trans-2-(2-Pentenyl)furan | — | — | tr |
| 1004 | 3,3,6-trimethyl-1,5-heptadien-4-ol | 0.01 | — | tr | |
| 1005 | 1045 | Benzeneacetaldehyde | 0.01 | — | 0.01 |
| 1009 | 1022 | Benzene,1-methyl-2-(1-methylethyl)- | — | — | 0.01 |
| 1011 | 1035 | Cyclohexanone,2,2,6-trimethyl- | — | — | tr |
| 1017 | 1022 | 4-Carene | — | — | tr |
| 1018 | 1035 | D-Limonene | 0.03 | 0.03 | 0.03 |
| 1083 | 1101 | β-Linalool | 0.01 | 0.71 | 0.04 |
| 1086 | 1111 | Furan,3-(4-methyl-3-pentenyl)- | — | 0.12 | 0.52 |
| 1114 | 1139 | (−)-Camphor | — | 0.01 | 0.01 |
| 1124 | 1140 | o-Hydroxyacetophenone | 0.21 | 0.05 | 0.12 |
| 1126 | Cyclopentane, 2-methyl-1-methylene-3-(1-methylethenyl)- | — | — | 0.02 | |
| 1128 | 1166 | Cyclohexanone,5-methyl-2-(1-methylethyl)- | 0.01 | 0.01 | 0.01 |
| 1133 | 1162 | 2(10)-Pinen-3-one, (. ±.)- | 0.01 | — | — |
| 1154 | 1150 | L-(−)-Menthol | 0.01 | — | 0.01 |
| 1157 | 1175 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 0.01 | — | 0.01 |
| 1163 | 1193 | (1R)-(−)-Myrtenal | 0.01 | — | — |
| 1168 | 1187 | α-Terpineol | 0.02 | — | — |
| 1174 | 1204 | l-Verbenone | 0.01 | — | — |
| 1178 | 1175 | Elsholtzia retone | — | 0.01 | — |
| 1235 | 1-Heptanone, 1-(2-furanyl)- | — | 10.96 | 7.81 | |
| 1240 | 1254 | 4-Hydroxy-3-methylacetophenone | 0.02 | — | 0.01 |
| 1241 | 1246 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (E)- | — | — | 0.02 |
| 1246 | 1262 | trans-Citral | — | 0.01 | — |
| 1247 | 1216 | 2,6-Octadienal, 3,7-dimethyl- | — | — | 0.02 |
| 1248 | 1285 | Ethanone, 1-(2-hydroxy-5-methylphenyl)- | — | 0.06 | 0.06 |
| 1257 | 1283 | Benzene, 1-methoxy-4-(1-propenyl)- | 0.16 | 0.26 | 0.27 |
| 1269 | 1278 | Phenol, 2-methyl-5-(1-methylethyl)- | 0.01 | — | — |
| 1302 | 1298 | 2,6-Octadienoic acid, 3,7-dimethyl-, methyl ester, (Z)- | — | 0.02 | 0.01 |
| 1326 | 1357 | Eugenol | 0.03 | 0.56 | 0.05 |
| 1332 | 1344 | δ-Elemene | 0.17 | 0.04 | 0.10 |
| 1345 | 1346 | Ylangene | — | — | 0.01 |
| 1372 | 1375 | Copaene | — | 0.11 | 0.06 |
| 1374 | 1375 | β-Patchoulene | 3.31 | — | 1.14 |
| 1378 | 1362 | β-bourbonene | — | 0.25 | — |
| 1386 | 1398 | (−)-β-elemene | 1.50 | 0.27 | 0.76 |
| 1402 | 1423 | Ethanone, 1-(2-hydroxy-6-methoxyphenyl)- | 0.38 | 0.20 | 0.48 |
| 1415 | 1423 | Caryophyllene | 3.49 | 14.03 | 7.48 |
| 1427 | 1390 | 4-Isopropyl-7-methyl-3-methyleneoctahydro-1H-cyclope | — | 0.09 | 0.04 |
| 1431 | 1445 | cis-Geranylacetone | — | 0.16 | — |
| 1433 | 1442 | α-Guaiene | 12.32 | 0.06 | 5.13 |
| 1437 | 1396 | (+)-Sativene | — | 0.06 | — |
| 1450 | 1456 | α-Caryophyllene | — | 2.25 | 2.13 |
| 1452 | 1458 | (Z)-β-Farnesene | — | 2.12 | 0.71 |
| 1454 | 1470 | (+)-Epi-bicyclosesquiphellandrene | — | 0.05 | — |
| 1459 | 1481 | Germacrene D | — | 0.05 | — |
| 1461 | 1464 | α-Patchoulene | 5.99 | — | 1.86 |
| 1462 | 1486 | β-Ionone | — | 0.07 | — |
| 1464 | 1416 | β-cis-caryophyllene | 0.62 | 0.33 | 0.28 |
| 1470 | 1466 | γ-Muurolene | 0.72 | 0.04 | — |
| 1475 | 1481 | D-Germacrene | 0.12 | 1.07 | 0.88 |
| 1476 | cis-4,11,11-Trimethyl-8-methylenebicyclo(7.2.0)undeca-4-ene | — | 0.04 | — | |
| 1479 | 1487 | β-Eudesmene | 0.23 | — | 0.13 |
| 1480 | 1470 | (+)-Epi-bicyclosesquiphellandrene | — | 0.01 | — |
| 1481 | 1454 | β-Humulene | 0.49 | — | 0.23 |
| 1485 | 1467 | Patchoulene | 0.61 | — | 0.27 |
| 1487 | 1474 | .beta.-Chamigrene | 0.20 | — | — |
| 1488 | 1492 | o-Menth-8-ene, 4-isopropylidene-1-vinyl- | — | 2.97 | 0.32 |
| 1491 | 1484 | (Z,E)-.alpha.-Farnesene | — | 2.50 | 1.24 |
| 1500 | 1526 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | — | 4.80 | 4.10 |
| 1504 | Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene- | — | 0.33 | — | |
| 1510 | 1524 | (+)-δ-Cadinene | — | 0.39 | 1.31 |
| 1513 | 1505 | δ-Guaiene | 15.90 | — | 6.57 |
| 1515 | Butyric acid,3-methyl-3-[2-isopropylphenyl]- | 0.23 | — | — | |
| 1518 | 1542 | Eudesma-3,7(11)-diene | 0.52 | — | 0.32 |
| 1548 | 1556 | Elemicine | — | 16.81 | 9.82 |
| 1552 | 1556 | 1,5-Cyclodecadiene, 1,5-dimethyl-8-(1-methylethylidene)-, (E,E)- | — | — | 0.08 |
| 1560 | 1569 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | — | 0.71 | 0.51 |
| 1567 | 1551 | Ledol | — | — | 0.59 |
| 1574 | 1591 | Benzene, 1,2,3,4-tetramethoxy-5-(2-propenyl)- | — | 6.87 | 4.79 |
| 1576 | 1582 | Caryophyllene oxide | 0.52 | 2.41 | 1.21 |
| 1580 | 1572 | (−)-Spathulenol | 0.35 | — | — |
| 1590 | Tricyclo[3.1.0.0(2,4)]hexane,3,6-diethyl-dimethyl-,trans- | — | 0.54 | — | |
| 1632 | 1682 | Parsley camphor | — | 21.32 | 11.74 |
| 1634 | 1659 | Ageratochromene | — | 0.18 | — |
| 1648 | 1653 | α-Cadinol | — | 0.20 | — |
| 1665 | 1661 | Patchouli alcohol | 23.27 | — | 14.63 |
| 1668 | 1631 | Longifolenaldehyde | 0.39 | — | 0.23 |
| 1681 | Tetrahydrosmilagenin | 0.17 | — | — | |
| 1693 | 3-Hydroxy-4-methoxybenzoic acid | 8.90 | — | 2.54 | |
| 1698 | 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | 0.21 | — | 0.34 | |
| 1703 | 1700 | n-Heptadecane | 0.42 | 0.01 | — |
| 1707 | 1740 | Farnesyl alcohol | 0.57 | — | — |
| 1718 | 1735 | 2,6,10-Dodecatrienal, 3,7,11-trimethyl-,(E,E)- | 0.01 | — | — |
| 1735 | Cyclopentanone,3-[3,5-decadienyl]-,(E,E)- | — | — | 0.08 | |
| 1746 | 1772 | Tetradecanoic acid | — | 0.02 | — |
| 1779 | 1738 | Solavetivone | — | — | 0.02 |
| 1817 | 1826 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, acetate, (E,E)- | 0.01 | — | — |
| 1825 | Phthalic acid, butyl undecyl ester | — | — | 0.02 | |
| 1830 | 1842 | 2-Pentadecanone, 6,10,14-trimethyl- | 0.05 | 0.15 | 0.08 |
| 1843 | 1857 | Pentadecanoic acid | 0.02 | — | 0.01 |
| 1864 | 1871 | 1-Hexadecanol | — | 0.02 | — |
| 1892 | 1914 | 5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl-, (E,E)- | — | 0.02 | 0.01 |
| 1910 | 1926 | Hexadecanoic acid, methyl ester | — | 0.01 | 0.01 |
| 1914 | 1900 | 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester | — | — | 0.01 |
| 1938 | 1939 | Isophytol | 0.01 | 0.01 | tr |
| 1953 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 0.89 | 1.05 | 0.99 | |
| 2008 | 2020 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-, (e,e)- | — | 0.03 | 0.01 |
| 2070 | 2082 | Linoleic acid, methyl ester | — | 0.01 | 0.01 |
| 2080 | 2107 | 8-Octadecenoic acid, methyl ester | — | 0.01 | — |
| 2098 | 2138 | Phytol | 0.14 | 0.32 | 0.16 |
| 2109 | 2130 | 9,12-Octadecadienoic acid (Z,Z)- | 0.13 | — | 0.27 |
| 2112 | 2058 | 9,12,15-Octadecatrien-1-ol, (Z,Z,Z)- | 0.08 | — | 0.17 |
| 2116 | 2152 | Oleic Acid | 0.06 | 0.08 | 0.08 |
| 2122 | Cyclopentanone, 2-(2-octenyl)- | 0.02 | 0.04 | — | |
| 2143 | 2187 | Octadecanoic acid | 0.01 | 0.02 | 0.02 |
| Total | 84.15 | 96.14 | 93.44 | ||
All the program writing and calculations were performed with Matlab 6.5.
3 Results and discussion
According to the procedures described above, the essential oils of drug pair P. cablin–P. frutescens and its single herbs were separated and analyzed by GC-MS and the TICs of the studied samples are shown in Fig. 1.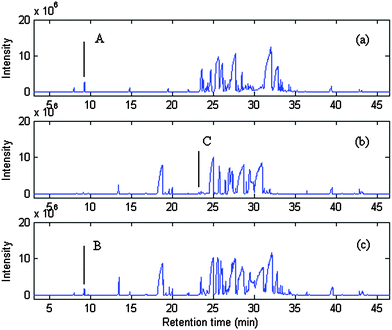 | ||
| Fig. 1 The TICs of the essential oils of drug pair P. cablin–P. frutescens and its single herbs. (a) P. cablin; (b) P. frutescens; (c) the DP. | ||
From Fig. 1, it can be seen that there are a great number of peaks and their contents vary greatly. Some heights of peaks are very high, while others are very low. The plots clearly demonstrate that the volatile oil systems to be studied are very complex analytical systems. Overlapped peaks and/or embedded ones extensively exist. If these peaks could not be resolved by chemometric resolution methods, the identification of these complex compounds would become very difficult by PTRIs or mass similarity search. Recently chemometric resolution techniques combined with a GC-MS method have displayed strong capabilities in the analysis of complex systems and have provided many satisfactory results.24–26 Here, in addition to PTRIs, three chemometric methods, namely AMWFA, HELP and SIA, were used respectively to inspect specific GC-MS data fragments.
The background of all the initial GC-MS data is deducted and smoothing is done before the chemometric resolution methods forementioned are applied.
3.1 Identification of common components by AMWFA15
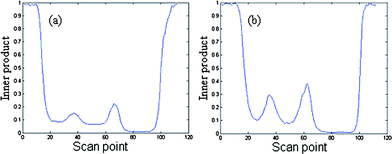
Common components of samples were identified by AMWFA, which is an extensive and conjoint version of multicomponent spectral correlative chromatography (MSCC)27 and sub-window factor analysis (SFA).28,29 The good quality of AMWFA lies in its full use of the cross-information hidden in two systems to determine the number of common components in different samples and then to identify their corresponding spectra of common components automatically. So it is very suitable for comparative analysis of components present in different but related complex systems. Firstly, common rank map obtained by this method is applied to decide whether the two compared peak clusters have the same components and the number of common components. Then, pure spectra and chromatograms of common compounds could be resolved by the AMWFA method for qualitative and quantitative analysis. Details can be seen in Ref. 15.Peak clusters A and B are taken as an example to illustrate how our resolution procedure works. The two chromatographic segments are both in the range of 8.930–9.300, taken from the TIC of P. cablin essential oil and that of the DP, respectively. The TICs of the two peak clusters above mentioned are shown in Fig. 2 (a) and (b) respectively, they look like to be a two-component peak cluster and a three-component peak cluster, respectively. Here we take Fig. 2 (a) as the base matrix, named X, and Fig. 2 (b) as the target matrix, named Y, then MSCC and IP-MSSC can be performed as shown in Fig. 3(a) and (b), respectively. It can easily be seen that the components existing in target matrix Y are highly correlated with those in base matrix X. In order to confirm the conclusion of rank estimation and detect peak purity of the two-dimensional data, fixed size moving window evolving factor analysis (FSMWEFA)30 was applied. The logarithm of the eigenvalue curves indicated that (a) and (b) in Fig. 2 were a four-component and a five-component system, respectively. Then moving window searching was conducted on Y with a fixed window size 3. The results obtained by common rank analysis clearly show that the number of common components in the two peak clusters is 3 (see Fig. 4(1)). It is to say that there are three common components between X and Y. Further, the spectral auto-correlative curve and the common rank map were procured by AMWFA (see Fig. 4(2) and (3) respectively). If the number of common components is equal to 1, a pure spectrum can be acquired from the corresponding region with a correlation coefficient close to 1 in the spectral-auto-correlative curve. In Fig. 4(2), three flat parts marked by R1, R2, and R3 show the regions in which the three identified spectra were picked out. Their correlation coefficients are all close to 1. By matching search from NIST 05 mass library, the resolution result of AMWFA shows that the three common components in peak clusters A and B are 2,5-Octanedione, 1-Octen-3-ol, and (−)-.beta.-Pinene, respectively, with the match quality (MQ) of 0.98, 0.97 and 0.98. The corresponding obtained pure chromatographic profiles are shown in Fig. 5. Likewise, other common components existing in the DP and its single herbs could be treated in the same way.
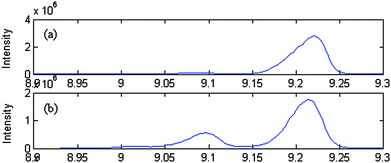 | ||
| Fig. 2 The TICs of the peak clusters A and B from the TICs of P. cablin essential oil and the DP, denoted by (a) and (b), respectively. | ||
 | ||
| Fig. 3 The results of MSCC of peak cluster A and IP-MSC C of peak cluster B, represented by (1) and (2) respectively. | ||
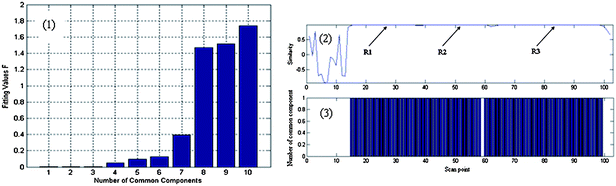 | ||
| Fig. 4 The results of AWFMA of peak cluster A and B: (1) is the result from common rank analysis by AWFMA; (2) is the spectral auto-correlative curves from AWFMA between the data (a) and (b), respectively; (3) is the common rank maps from AWFMA between the data (a) and (b), respectively. | ||
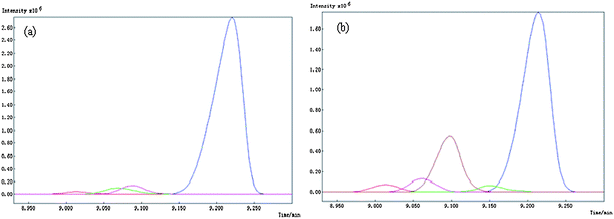 | ||
| Fig. 5 Resolved chromatographic curves of peak cluster A and B of Fig. 1, denoted by (a) and (b) in this plot, respectively. | ||
3.2 Resolution of partial overlapped by HELP16,17
The AWFMA method forementioned in section 3.1 is effective and useful in fast analysis between two different complicated systems only when they have one common composition at least. Still, about those partial overlapped peaks or two chromatographic segments with poor relation which perhaps do not have common components, now HELP method can be used. The basic idea of this method is to obtain a pure chromatogram and spectrum by means of a so-called full rank resolution technique after the determination of zero-component and selective regions of the target components. Details can be found in the Ref. 16 and 17.Here peak clusters C (see Fig. 1), selected from the TIC of P. frutescens essential oil with the range of 23.030–24.032 min, serves to show how it was identified. Seen from the TIC of this peak cluster, it looks like a three-component segment (see Fig.6 (a)). However, identification is difficult because of the low match quality (MQ) in direct mass spectrum library searches and different mass spectra were obtained at the adjacent retention time points. Fig. 6(b) shows all the chromatograms at different mass/charge points, indicating that there are at least four components. In fact, on resolution by HELP, we found that five pure chromatograms are actually involved as shown in Fig. 6(c), marked as component 1–5 according to elution sequence, respectively. Combined with the character and structure of compounds and PTRIs, components 1, 3 and 5 were provisionally identified as copaene (MQ 0.99), β-bourbenene (MQ 0.99), and (−)-β-elemene (MQ 0.98) respectively, while components 2 and 4 were not determined because of the limitation of the mass spectral database or because of their low signal-to-noise ratios. Other partial overlapped peaks or two chromatographic segments with poor relation between the DP and its single herbs could be resolved in the same way.
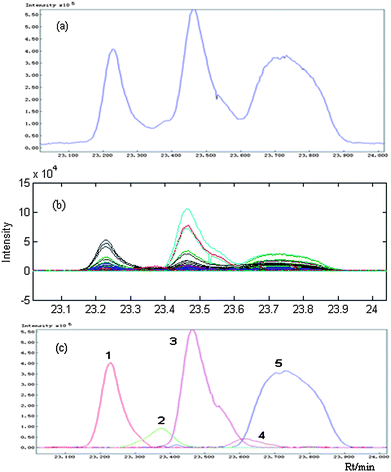 | ||
| Fig. 6 Original peak cluster C and the pure peaks after resolution by chemometrical method HELP: (a) the TIC of peak cluster C from P. frutescens essential oil; (b) the corresponding two-dimension plots; (c) the corresponding pure peaks after resolution. | ||
3.3 Resolution of the seriously overlapped peaks and embedded ones by SIA18
A succinct summary to section 3.1 and 3.2, alternative moving window factor analysis is often employed to do fast comparison analysis in two complicated fingerprints which at least contain a common component, and heuristic evolving latent projections is usually used to resolve the partial overlapping peak only when it has its own selective region in the chromatographic direction. About the seriously overlapping peak which does not have its own selective region or the embedded peak which locates inside into another peak along the time coordinate direction, both AWFMA and HELP seem incapable of action. In such circumstances, a new chemometric resolution method proposed by Tan,18 say SIA, is used to deal with these formidable problems.The SIA method is based on the theory of a different response at certain m/z points which might be found as long as a small difference in structure between the mass spectra of two analytes, where only one analyte gives a signal. Such points are called selective points. Obviously, the major idea of SIA lies in it efficiently using the selectivity of mass spectra. The procedure of SIA works principally in the following steps. (1) Search the selective ion of each component. (2) Extract the chromatographic profile of each component from its corresponding selective ion. (3) Resolve the pure mass spectrum of each component by means of the least squares technique. (4) Authenticate the reliability of the resolved result. It is worth mentioning that multivariate curve resolution-alternating least squares (MCR-ALS)31–36 can solve the same problem as SIA. Compared between the two chemometrics resolution methods, the algorithm of SIA is simple and easy since it is non-iterative and MCR-ALS is iterative.
The chromatographic segment in the range of 5.212–5.334 min of P. cablin is taken as an example to demonstrate how the algorithm of SIA works. Its original peak cluster and corresponding two-dimensional plot are shown in Fig. 7 (1) and (2), respectively. The two-dimensional plot presents a seriously overlapped situation for this cluster peak under study. In the selective ion detection plot (Fig. 7 (3)) the selective ions 43 and 107 of the two constituents respectively are considered to be the most suitable ones. The resolution procedure continued and completed, the corresponding pure peaks after resolution are shown in Fig. 7 (4). Finally, the identification of chemical compounds can be performed directly by similarity searches in the Class5000 database coupled with the PTRIs. The results show that components 5 and 6 can be tentatively identified as 5-methyl-2-Hexanone and 5,5-dimethyl-2-ethyl-1,3-Cyclopentadiene respectively, with the match qualities 0.98 and 0.97. Other seriously overlapping peaks and embedded peaks in the studied samples are determined qualitatively in the same way as described above.
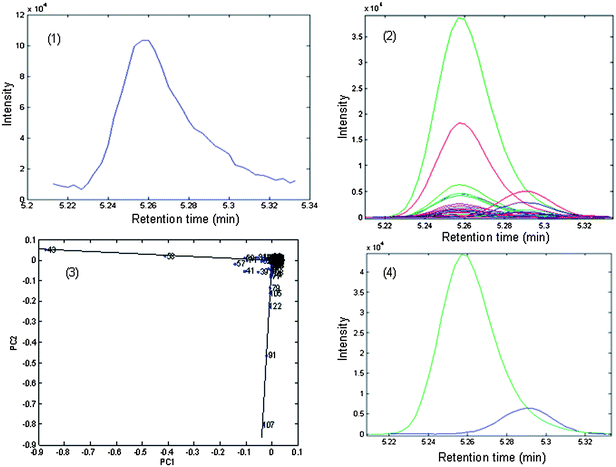 | ||
| Fig. 7 Original peak cluster D and the pure peaks after resolution by chemometrical method SIA: (1) the TIC of peak cluster D from P. cablin essential oil; (2) the corresponding two-dimension plot; (3) the selective ion detecting plot (SIDP); (4) the corresponding pure peaks after resolution. | ||
3.4 Quantitative analysis and comparing analysis
According to the resolved chromatogram and mass spectra, the quantitative analysis of each component can be directly calculated by the overall volume integration (OVI).37–39 They are proportional to the content of the peak as integration based on TIC. The quantitative results for all components are listed in Table 1. By and large, 66, 74 and 91 volatile components of P. cablin, Perilla and DP P. cablin–P. frutescens were determined qualitatively and quantitatively respectively, accounting for 84.15%, 96.14% and 93.44% total contents of volatile oil of the three samples above mentioned, respectively.From Table 1, some chemical components were disappeared in the essential oil of the DP P. cablin–P. frutescens, such as (−)-Spathulenol and Farnesyl alcohol of P. cablin, and β-Bourbonene and trans-3,6-diethyl-dimethyl-Tricyclo[3.1.0.0(2,4)]hexane of P. frutescens. At the same time there were some new chemical components emerging in the essential oil of the DP, such as Ledol, (E)-3,7-dimethyl-2,6-Octadien-1-ol. These phenomena may be caused by chemical reactions, usually including oxidation, reduction, condensation and hydrolysis, and physical changes like solubilization and co-dissolution in the process of decoction40–42 when heat is applied to distillate essential oil compositions, or maybe something else.
Comparison of the essential oil compositions of the studied samples showed that there are 49 common essential chemical components between the DP and the single herb P. cablin, 51 common essential chemical components between the DP and the single herb P. frutescens and 28 common essential chemical components among the three systems. The main volatile constituents of P. cablin are Patchouli alcohol, δ-Guaiene, α-Patchoulene, α-Guaiene, Caryophyllene, β-Patchoulene and so on. Pogostone reported by Hu10 has not been found in our studied sample, which maybe indicate that our plant sample of P. cablin belongs to the chemotype of patchouli alcohol. The primal volatile components of P. frutescens are Caryophyllene, 1-(2-furanyl)-1-Heptanone, 4-methoxy-6-(2-propenyl)-1,3-Benzodioxole, 1,2,3,4-tetramethoxy-5-(2-propenyl)-Benzene, Elemicine and Parsley camphor. Perillaldehyde previously reported to be one of the main volatile extracts in Perilla leaves was not detected in this study.13,14 Patchouli alcohol, Parsley camphor, Elemicine, 1-(2-furanyl)-1-Heptanone and Caryophyllene are composed of the principal substances of the DP, with the relative contents of 14.63%, 11.74%, 9.82%, 7.81% and 7.48%, respectively. Except the compound of Patchouli alcohol coming from P. cablin's essential oil, the latter four ones forementioned are from that of P. frutescens. It is well-known that the main volatile components of the DP are mostly from P. frutescens.
4 Conclusion
This work represents systematic research on DP P. cablin–P. frutescens and its two single herbal medicines. Qualitative and quantitative analysis of their volatile compounds was performed by mass spectrometry library searching and retention indices with the help of integrated chemometric resolution methods. Comparing analysis was also employed among the studied samples, and considerable similarities and differences were found. The results obtained may help to find the possible active ingredients and provide a useful chemical basis for future research on the correlation between the pharmacodynamic action and chemical constituents of these herbs. Meanwhile, the strength of the chemometric resolution techniques assisted with PTRIs in obtaining more accurate information from multi-dimension data is demonstrated once again.Acknowledgements
This work is financially supported by the international cooperation project on traditional Chinese medicines of ministry of science and technology of China (No. 2007DFA40680) and China Postdoctoral Science Foundation (No. 20080440181). Thanks goes to all employees of this institute for their encouragement and support of this research.References
- Q. H. Xu, L. Y. Liu, R. H. Zhao, et al, Collection of Drug Pairs in Traditional Chinese Medicine [M], Beijing: Publishing House of Traditional Chinese Medicine, 1996:1–4 Search PubMed.
- Pharmacopeia Commission of PRC, Pharmacopoeia of The People's Republic of China, Chemical Industry Press, Beijing, 2005: 30 Search PubMed.
- X. L. Xiao and Y. X. Long, J. Chin. Med. Mater., 2004, 27, 456–459 Search PubMed.
- Y. M. Du, L. Z. Chen and B. R. Hu, Trad. Chin. Drug Res. Clin. Pharmacol., 1998, 9, 238–241 Search PubMed.
- J. P. Luo, Y. P. Liu, Y. F. Feng, X. L. Guo and H. Cao, Acta Pharmacol. Sin., 2003, 38, 307–310 Search PubMed.
- W. J. Gu, C. Y. Zhu and J. M. Zhang, Heilongjiang Ani. Sci. Vet. Med., 2006, 8, 26–28 Search PubMed.
- K. Kasahara, K. Nishibori, N. S. Gakkaishi, 1988, 54:p. 315 (in Japanese).
- Pharmacopeia Commission of PRC, Pharmacopoeia of The People's Republic of China, Chemical Industry Press, Beijing, 2000: 279–280 Search PubMed.
- Q. H. Xu, L. Y. Liu, R. H. Zhao, et. al, Collection of Drug Pairs in Traditional Chinese Medicine [M], Beijing: Publishing House of Traditional Chinese Medicine, 1996:50–51 Search PubMed.
- L. F. Hu, S. P. Li, H. Cao, J. J. Liu, J. L. Gao, F. Q. Yang and Y. T. Wang, J. Pharm. Biomed. Anal., 2006, 42, 200–206 CrossRef CAS.
- A. Donelian, L. H. C. Carlson, T. J. Lopes and R. A. F. Machado, J. Supercrit. Fluids, 2009, 48, 15–20 CrossRef CAS.
- J. F. Wu, X. Lu, W. Y. Tang, H. W. Kong, S. F. Zhou and G. W. Xu, J. Chromatogr., A, 2004, 1034, 199–205 CrossRef CAS.
- M. Ito, M. Toyoda and G. Honda, Nat. Med., 1999, 53, 32–36 CAS.
- M. Nitta, H. Kobayashi, M. Ohnishi-Kameyama, T. Nagamine and M. Yoshida, Biochem. Syst. Ecol., 2006, 34, 25–37 CrossRef CAS.
- Z. D. Zeng, Y. Z. Liang, Y. L. Wang and et al, J. Chromatogr., A, 2006, 1107, 273–285 CrossRef CAS.
- O. M. Kvalheim and Y. Z. Liang, Anal. Chem., 1992, 64, 936–946 CrossRef CAS.
- Y. Z. Liang, O. M. Kvalheim, H. R. Keller, D. L. Massart, P. Kiechle and F. Erni, Anal. Chem., 1992, 64, 946–953 CrossRef.
- B. B. Tan, Y. Z. Liang, L. Z. Yiet al. Identification of free fatty acids profiling of type 2 diabetes mellitus and exploring possible biomarkers by GC–MS coupled with chemometrics. Metabolomics DOI:10.1007/s11306-009-0189-8.
- C. X. Zhao, T. Zhang, Y. Z. Liang and et al, J. Chromatogr., A, 2007, 1144, 245–254 CrossRef CAS.
- Chinese Pharmacopoeia Committee, Chinese Pharmacopoeia, Appendix 57, Publishing House of Chemical Industry, Beijing 2005 Search PubMed.
- H. van den Dool and P. D. Kratz, J. Chromatogr., A, 1963, 11, 463–471 CrossRef CAS.
- K. Varmuza and W. Werther, J. Chem. Inf. Comput. Sci., 1996, 36, 323–333 CrossRef CAS.
- NIST standard reference database 69, US National Institute for Science and Technology (NIST) MS data Central, Gaithersburg, MD, 2005. http://www.webbook.nist.gov Search PubMed.
- C. X. Zhao, Y. X. Zeng, M. Z. Wan and et al, J. Sep. Sci., 2009, 32, 660–670 CrossRef CAS.
- L. Z. Yi, D. L. Yuan, Y. Z. Liang, P. S. Xie and Y. Zhao, Anal. Chim. Acta, 2009, 649, 43–51 CrossRef CAS.
- L. Z. Yi, D. L. Yuan, Y. Z. Liang, P. S. Xie and Y. Zhao, Anal. Chim. Acta, 2007, 588, 207–215 CrossRef CAS.
- Y. Hu, Y. Z. Liang, B. Y. Li, X. N. Li and Y. P. Du, J. Agric. Food Chem., 2004, 52, 7771–7776 CAS.
- R. Manne, H. L. Shen and Y. Z. Liang, Chemom. Intell. Lab. Syst., 1999, 45, 171–176 CrossRef CAS.
- H. L. Shen, R. Manne, Q. S. Xu, D. Z. Chen and Y. Z. Liang, Chemom. Intell. Lab. Syst., 1999, 45, 323–328 CrossRef CAS.
- H. R. Keller and D. L. Massart, Anal. Chim. Acta, 1991, 246, 379–390 CrossRef CAS.
- R. Tauler, Chemom. Intell. Lab. Syst., 1995, 30, 133–146 CrossRef CAS.
- R. Tauler, A. K. Smilde and B. J. Kowalski, J. Chemom., 1995, 9, 31–58 CAS.
- R. Tauler, J. Chemom., 2001, 15, 627–646 CrossRef CAS.
- M. Amrhein, B. Srinivasan, D. Bonvin and M. M. Schumacher, Chemom. Intell. Lab. Syst., 1996, 33, 17–33 CrossRef CAS.
- J. Saurina, S. Hernández-Cassou, R. Tauler and A. Izquierdo-Ridorsa, J. Chemom., 1998, 12, 183–203 CrossRef CAS.
- A de Juan and R. Tauler, Anal. Chim. Acta, 2003, 500, 195–210 CrossRef CAS.
- F. Gong, Y. Q. Peng, H. Cui, Y. Z. Liang, A. K. M. Leung and F. T. Chau, Acta Pharm. Sin., 1999, 34, 214–217 Search PubMed.
- F. Gong, Y. G. Peng, H. Cui, Y. Z. Liang, A. K. M. Leung and F. T. Chau, Chem. J. Chin. Univ., 1999, 20, 199–203 CAS.
- H. L. Shen, Y. Z. Liang, R. Q. Yu, X. C. Li and X. X. Sun, Sci. China, Ser. B: Chem., 1998, 41, 21–29 CrossRef CAS.
- X. Huang and P. Ren, Chin. Trad. Herbal Drugs, 2001, 32, 411–413 Search PubMed.
- P. X. Cao and G. X. Liang, Chin. Trad. Herbal Drugs, 2001, 32, 981–983 Search PubMed.
- R. Rong and J. R. Yuan, Chin. Trad. Herbal Drugs, 2001, 32, 114–115 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
