Determination of free acid in rare earth solution by a fixed pH method
Zhaowu
Zhu
*a,
Zhenyu
Bian
b and
Zhiqi
Long
a
aBeijing General Research Institute for Nonferrous Metals, Beijing, China. E-mail: zwuzh@263.net
bSouthwest University for Nationalities, Chengdu, China
First published on 17th November 2009
Abstract
A fixed pH method was developed for free acid determination in solutions containing large amounts of rare earths, especially Ce(IV). With Ca–EDTA as the masking regent at a fixed pH of 4.5, free acid was determined in a solution containing about 100 g/L of rare earths including about 50 g/L Ce(IV) with a relative standard deviation (RSD) of less than 1%. The deviation error for 50 measurements in 5 groups for a known amount of H2SO4 solution was −1.44%. Compared with other reported methods, the newly developed method has the advantages of higher precision, higher accuracy, simpler procedure and no need for an indicator.
1. Introduction
Free acid is one of the essential factors in solvent extraction processes for metallic separation and purification. At present, the separation and purification of rare earths (RE) are commonly conducted by solvent extraction with organophosphorus extractants such as di-2-ethylhexyl phosphoric acid (D2EHPA) and 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (EHEHPA). Free acid is a key process control factor for the distribution of rare earths between aqueous and organic phase. Therefore, accurate determination of free acid in rare earth solutions is critical for process control and new process design. Traditionally, free acid determination is carried out by standard alkali titration methods with indicator(s) to identify the titration end point. However, in the presence of large amounts of rare earth ions, the color change near the titration point becomes difficult to distinguish and can readily lead to analysis error. In the processes for rare earth bastnasite ore,1,2 cerium in the pregnant leach solution was present as Ce(IV) which has a high oxidation potential.3 The determination of free acid in solutions containing Ce(IV) is even more difficult because Ce(IV) can precipitate under very low pH values of about 1 and it can oxidise the organic indicator. Thus, direct titration methods with indicators to identify the end point for solutions containing Ce(IV) are often not possible. Over the years, many methods have been developed for free acid determination in the presence of large amounts of metal ions4–7 but few methods are reported for solutions containing Ce(IV). Methods reported for free acid determination in the presence of Ce(IV) included procedures to remove Ce from the solution or to reduce Ce(IV) to Ce(III).8 These additional procedures complicate the free acid determination and can result in high analysis error. In the present paper, a fixed pH method was developed for free acid determination of solutions containing Ce(IV). Optimum conditions of fixed pH and amount of Ca–EDTA required were discussed.2. Experimental
2.1 Reagents and instrument
Cerium sulfate (Ce(SO4)2·4H2O) with CeO/TREO (Total Rare Earth Oxides) (w/w) >99.9% from commercial supplier was used to prepare Ce(IV) solution. Analysis results showed that Ce(III) content was less than 1%. La2O3 with La2O3/TREO (w/w) >99.9% was used to prepare the RE(III) solution. De-ionised water was used in the preparations. Any other chemicals used were in analytical grade.A digital pH meter (Shanghai Analytical Instrumental Corporation) with standard buffer solutions of pH 4.00 and 7.00 were used to calibrate the pH.
2.2 Method description
The Ca–EDTA solution was prepared by mixing 0.2 mol/L CaCl2 and 0.1 mol/L EDTA solutions. The pH of prepared Ca–EDTA solution was adjusted to 7.00 with 0.01 mol/L NaOH and 0.01 mol/L HCl solutions. Ca–EDTA solution (20mL) was added as a masking reagent into a 100 mL glass beaker and the pH was adjusted to a fixed value with 0.01 mol/L HCl and 0.01 mol/L NaOH solutions. A certain volume of sample solution was added into the beaker. The pH of the system decreased due to the free acid contained in the added sample. The system was stirred with magnetic stirrer and titrated with a standard NaOH solution until the pH returned to the originally set value. Free acid in the samples was calculated based on the volume of the NaOH solution used and the volume of the sample.3 Results and discussion
3.1 Selection of fixed pH
Rare earths exist in solution normally in the form of RE(III). After high temperature (400–500 °C) roasting at an oxidation condition and sulfuric acid leaching, however, rare earth bastenite ore can give Ce(IV) in the leach solutions.3 Direct free acid determinations with indicator colour change can not be employed for these solutions because precipitation readily occurs as the pH is increased. Precipitation starts at pH of 5–7 for RE(III)9 but at only 0.7–1 for Ce(IV).10Ca–EDTA and Mg–EDTA are commonly used to mask high ionic potential metal ions.7 In the present method, Ca–EDTA was selected to mask RE(III) and Ce(IV). As Ca–EDTA reacts with RE(III) and Ce(IV) in the following equations (1) and (2), no precipitation was observed during the titration process.
| CaY2− + RE3+ = REY− + Ca2+ | (1) |
| CaY2− + Ce4+ = CeY + Ca2+ | (2) |
Free acid was determined in a known solution containing 50 g/L Ce(IV) and 50 g/L RE(III) sulfate solutions. Free acid was determined for a set of fixed pH values. The results are shown in Table 1. A reported method8 which used KI as Ce(IV) reductant and Na2S2O3 as the masking reagent for generated I2, as the reference method was also conducted. The results are also shown in Table 1.
For the three fixed pH values of 4.00, 4.50 and 5.00, the free acid concentrations determined varied from each other. The smallest value was obtained at the fixed pH of 4.50. A slight increase was observed at the fixed pH of 4.00 and 5.00. At lower pH incomplete masking metal ions by Ca–EDTA is possible, whilst at higher pH metal precipitation may occur. Both conditions can result in a higher free acid titration value and hence the smallest result obtained at the fixed pH of 4.50 was considered more accurate. On the other hand, relative standard deviation (RSD) for 6 replicate measurements was found to be the minimum of 0.81% at the fixed pH of 4.50 comparing with 2.48 and 1.92 obtained at pH 4.00 and 5.00, respectively (Table 1).
Determined value at pH 4.50 was 4.93% more than determined by the reference method (Table 1). This difference most possibly is caused by the negative error of reference method considering many operation procedures conducted in the reference method.
3.2 Precision of fixed pH method
For a given solution containing 50 g/L Ce(IV) and 50 g/L RE(III), the amount of free acid was determined at a fixed pH of 4.50 for different volumes of the solution (Table 2). Comparatively, the reference method was also conducted at the same time with the results given in Table 2. The RSD of 6 measurements for each solution volume was less than 1% for the fixed pH method and greater than 1% at solution volume of 0.1 mL and 0.2 mL for the reference method. Good linear relationship of the amount of free acid and solution volume was obtained with R2 value of 0.9999 and 0.9996 for the fixed pH method and the reference method, respectively (Fig. 1). Free acid concentration of the given rare earth contained solution was calculated based on the solution volumes as shown in Fig. 2. The RSD of the fixed pH method for 4 solution volumes was 0.95% which was better than that of the reference method of 1.84% (Fig. 2).| Solution volume (mL) | Ce(IV) (mmol) | RE(III) (mmol) | Average free acid in the solution (mmol) | RSD of 6 times determination (%) | ||
|---|---|---|---|---|---|---|
| Fixed pH method | Reference method | Fixed pH method | Reference method | |||
| 0.1 | 0.0328 | 0.0325 | 0.294 | 0.272 | 0.24 | 1.78 |
| 0.2 | 0.0656 | 0.0650 | 0.589 | 0.557 | 0.55 | 1.24 |
| 0.3 | 0.0984 | 0.0975 | 0.900 | 0.831 | 0.95 | 0.34 |
| 0.4 | 0.1312 | 0.1300 | 1.194 | 1.135 | 0.95 | 0.30 |
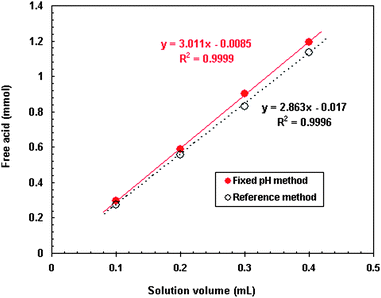 | ||
| Fig. 1 Relationship of measured free acid amount and solution volume. | ||
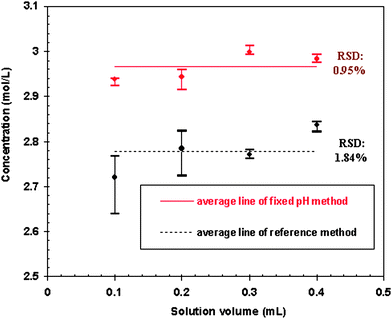 | ||
| Fig. 2 Comparison of precision of the fixed pH method and the reference method (Bars on the top and bottom are maximum and minimum, respectively, and dots in the middle are average value of repeat measurements). | ||
For different solution volumes, the free acid and the rare earth content in the analysis system were different. From Table 2, it is obvious that over a large range of free acid amount and rare earth content in the analysis system, the fixed pH method showed higher precision with relative standard deviation less than 1%. Relatively lower precision for the reference method may result from more operational procedures required compared to the newly developed fixed pH method.
3.3 Accuracy of fixed pH method
The fixed pH method returned value around 5% higher than that of reference method. The accuracy of the newly developed method, however, can not be evaluated by direct comparison to the reference method. As a large amount of rare earths exist in the tested solution, multiple procedures are required to obtain a result via the referred method. The color change at the end point normally is difficult to detect and the systematic error is possibly high.To determine the accuracy of newly developed method, a known amount of H2SO4 was added to the test solutions (0.2 mL samples containing 50 g/L Ce(IV) and 50 g/L RE(III) solution) to standardise its accuracy. Each amount of H2SO4 was measured over 10 replicates. The determined values of free acid amount by the fixed pH method versus that of known added amounts are shown in Fig. 3. Within the range of added H2SO4 from 0.1 to 0.5 mmol, the determined values were very close to unit gradient line (y = x). The RSD of total 50 measurements for 5 known H2SO4 amount additions was found to be −1.44%, suggesting the high accuracy of the fixed pH method.
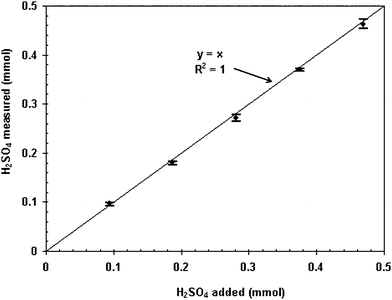 | ||
| Fig. 3 Measured amount of acid versus the known amount of added acid (Bars on the top and bottom are maximum and minimum, respectively, and dots in the middle are average value of over 10 replicates). | ||
3.4 Effect of Ca–EDTA amount
In the outlined procedure in experimental section, 20 mL of Ca–EDTA solution was used to mask rare earth ions in the test solution. The amount of Ca–EDTA required however, will vary with the rare earth content. Thus a sensitivity test was carried out on the 50 g/L Ce(IV) and 50 g/L RE(III) solution. Results are shown in Table 3.The results show that within a wide range of Ca–EDTA to rare earth molar ratio from 14 to 35, the RSD was found to be 0.86%, indicating that Ca–EDTA can mask rare earths well and there was no significant effect of added amount on the free acid determination.
As both Ce(IV) and RE(III) form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with EDTA, Ca–EDTA using amount could not depend on the variance of Ce(IV) to RE(III) ratio.
1 complex with EDTA, Ca–EDTA using amount could not depend on the variance of Ce(IV) to RE(III) ratio.
3.5 Suitability assessment for the fixed pH method
Although the tests were conducted with La(III) representing RE(III), as no appreciable difference of complexation among RE(III) with EDTA, any RE(III) containing solutions could be suitable to this method.The stability constants of EDTA with some tri-valence metals such as Fe(III), Al(III) are greater than that with RE(III), and tetra-valence metals, such as Th(IV), U(IV), can form very stable complex with EDTA.11 It is therefore reasonable to assume that the fixed-pH method can also be equally applied in these ions contained solutions.
4. Conclusions
The fixed pH method was developed for free acid determination in the solutions containing high concentrations of rare earths, especially in Ce(IV) contained solutions. With Ca–EDTA as the masking reagent, at a fixed pH of 4.50, the free acid determination gave a small relative standard deviation compared to the reported method. The developed method provides high precision and enhances reliability. The method is also likely to be suitable to solutions containing Fe(III), Al(III), Th(IV) and U(IV).Acknowledgements
The authors would like to thank Dr Chu Yong Cheng and Mr Ron Pleysier of CSIRO, Minerals, Australia for reviewing the paper and providing the valuable suggestions.References
- Z. Zhaowu, Z. Na, L. Zhiqi, L. Dedong, C. Dali and Z. Guocheng, Journal of Rare Earths, 2005, 23(2), 178.
- L. Jun, W. Zhenggui, L. Deqian, M. Gengxiang and J. Zucheng, Hydrometallurgy, 1998, 5077.
- L. Dedong, L. Zhiqi, L. Hongwei and Z. Guocheng, Chinese Journal of Rare Metals, 2004, 28(2), 452 Search PubMed.
- M. Anwar and D. Mohammad, J. Radioanal. Nucl. Chem., 1989, 134(1), 45 CAS.
- U. Sundar, P. C. Sivadasan, R. B. Yadav, B. Gopalan and S. Syamsundar, Analyst, 1998, 123, 1875 RSC.
- A. Diana, G. Vincenzo, R. Vincenzo and Z. Roberto, Polyhedron, 2002, 21, 1369 CrossRef.
- E. Rolia and J. E. Dutrizac, Canadian Metallurgical Quarterly, 1984, 23(2), 159 CAS.
- l. Yuqing and S. Yongming, Journal of Southwest Nationalities College. Natural Science Edition, 1995, 21(1), 80 Search PubMed.
- Unknown, Constants of Rare Earth Physicochemistry, 1978, Metallurgical Industry Press, Beijing, Chnia Search PubMed.
- Z. Xi, Handbook of Application Chemistry, 2003, Science Press, Beijing, China Search PubMed.
- L. G. Sillen, A. E. Martell, E. Hogfeldt and R. M. Smith, Stability Constants Supplement No. 1, Special Publication 25. 1971, The Chemical Society, London Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
