Method for cadmium and lead longitudinal profiles determination in hair by solid sampling graphite furnace atomic absorption spectrometry
Cassiana Seimi
Nomura
*a and
Pedro Vitoriano
Oliveira
b
aUniversidade Federal do ABC, Centro de Ciências Naturais e Humanas, Rua Santa Adélia, 166, CEP 09210-170, Santo André, SP, Brazil. E-mail: cassiana.nomura@ufabc.edu.br; Tel: 55(11) 4996-0184
bInstituto de Química, Universidade de São Paulo, P.O Box. 26077, 05513-970, São Paulo, SP, Brazil. E-mail: pvolivei@iq.usp.br; Tel: 55(11)3091-8516
First published on 10th November 2009
Abstract
This paper describes methods for the direct determination of Cd and Pb in hair segments (c.a. 5 mm, ∼80 μg) by solid sampling graphite furnace atomic absorption spectrometry, becoming possible longitudinal profiles in a single strand of hair. To distinguish endogenous and exogenous content, strands of hair were washed by using two different procedures: IAEA protocol (acetone + water + acetone) and the combination of IAEA protocol with HCl washing (acetone + water + acetone + 0.1 mol l−1 HCl). The concentration of Cd and Pb increased from the root until the tip of hair washed according to IAEA protocol. However, when the strand of hair was washed using the combination of IAEA protocol and 0.1 mol l−1 HCl, Cd concentrations decreased in all segments, and Pb concentrations decreased drastically near to the root (5 to 12 mm) and was systematically higher in the end. The proposed method showed to be useful to assess the temporal variation to Cd and Pb exposure and can be used for toxicological and environmental investigations. The limits of detection were 2.8 ng g−1 for Cd and 40 ng g−1 for Pb. The characteristic masses based on integrated absorbance were 2.4 pg for Cd and 22 pg for Pb.
Introduction
Human hair has the capacity to store and to retain trace elements over an extended period of time from either endogenous or exogenous sources, and consequently can be used as an indicator for environmental and occupational exposure.1 Although the use of elemental hair analysis is still controversial, the determination of at least 40 elements has been already reported2 for medical,3 environmental4,5 and toxicological6 investigation. The biological significance is generally inferred by assuming that the incorporation of an element in the hair is correlated with its concentration in the blood at the time of hair formation. This has been to some extent confirmed for mercury,7 selenium8 and lead.9,10 Thus, each hair stores chronological information concerning exposure to an element during extended periods.11 Due to this characteristic, hair analysis is an important key that allows one to retrieve information about past health issues, even if its cause has ceased.When compared with other biological tissues, hair has some advantages to be used in monitoring studies: (i) the less invasive character of collection procedures, (ii) the stability to transport and storage, and (iii) the higher concentrations of residues usually found in hair samples when compared with those on blood and urine.12 Besides all these positive characteristics, there are limitations associated with hair analysis, e.g., the differentiation between exogenous and endogenous contaminations. Many factors contribute to the results: gender, ethnicity, age, hair color, dietary habits, use of cosmetics such as shampoos, soaps, hair sprays and perfums, geographic origin of the individual, hair morphologic characteristics, and hair treatments such as dying, bleaching and permanent waving.13 For all these advantages and limitation, hair analysis was sometimes glorified, sometimes condemned, sometimes accepted and sometimes disdained.14
Several techniques can be used for the determination of trace elements in hair, including neutron activation analysis (NAA),15 X-ray fluorescence (XRF),16 and inductively coupled plasma atomic emission spectrometry (ICP OES).17 However, the main disadvantage of these techniques is that the measurements reflect the average composition of trace elements in the hair sample and several strands of hair are required for each sample. Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS),18,19 electrothermal vaporization inductively coupled plasma mass spectrometry (ETV-ICP-MS)20 and time-of-flight secondary ion mass spectrometry (ToF-SIMS),21 due to the high sensitivity and the possibility to analyze low sample mass size, have been used to analyze one single hair, obtaining spatially resolved information about the temporal changes in the intake of the analyte.
Compared with other spectrochemical techniques, graphite furnace atomic absorption spectrometry is well suited for the direct analysis of solids. Solid sampling graphite furnace atomic absorption spectrometry (SS-GF AAS) combines a lot of advantageous characteristics, such as high sensitivity, selectivity and the relative facility for sample introduction. The heating program is one of the most important attributes of SS-GF AAS, during the drying and pyrolysis steps it is possible to eliminate matrix components and to carry out in situ sample pretreatment. Additionally, it has provided good analytical results with relatively inexpensiveness, robustness and prevention of the use of dangerous and corrosive reagents. The only disadvantage is the relatively high imprecision with relative standard deviation (RSD) values around 10%, which are, however typical for all direct solid sampling techniques.22–24
Using “tape sandwich” as a sample introduction system, which is a kind of graphite cup put in the inner of a graphite tube, Cd and Pb profile in biological samples25 and Pb longitudinal profile in hair26 were investigated. However, in this case several segments of hair had to be used. More recent instrumentation involves a pair of tweezers for the reproducible introduction of a graphite “boat-type” platform, in which analysis of a little segment of a single strand of hair is possible.
Considering the capacity of hair in storing and retaining trace elements in its structure, it is believed that the analysis of hair segments is an important key to present chronological information of the exposure. In this way, the aim of this study is to present methods for direct determination of Cd and Pb in segments of one strand of hair by solid sampling graphite furnace atomic absorption spectrometry. Using the developed methods, it was possible to assess the longitudinal profiles.
Experimental
Apparatus
Analysis of hair was carried out using a ZEEnit® 60 model atomic absorption spectrometer (Analytik Jena, Jena, Germany) equipped with a transversely heated graphite tube atomizer, an inverse and transversal 2- and 3-field mode Zeeman-effect background corrector, and a manual solid sampling accessory (SSA-6 Z). Pyrolytically coated graphite tube atomizer and boat-type platform were used throughout. Cadmium and Pb hollow cathode lamps were used. The instrumental parameters adopted are summarized in Table 1.| Wavelength/nm | Bandpass/nm | Lamp current/mA | Chemical modifiera | |
|---|---|---|---|---|
| Cd | 228.8 | 0.8 | 4.0 | yes |
| Pb | 217.0 | 0.5 | 4.0 | yes |
Hair samples were weighed in a microbalance, Auto Balance AD-4 (Perkin-Elmer, Norwalk, USA) with a sensitivity of 0.001 mg. All measurements were based on integrated absorbance values, controlled by Windows NT® software. Argon 99.998% (v/v) (Air Liquide Brazil, São Paulo, Brazil) was used as protective and purge gas.
Reagents and solutions
High purity deionized water was obtained from a Milli-Q water purification system (Millipore, Billerica, USA). Nitric acid (Synth, São Paulo, Brazil) was distilled in quartz sub-boiling stills (Marconi, Piracicaba, Brazil). Analytical calibration solutions of Cd(II) and Pb(II) were prepared by suitable dilution of 1000 mg l−1 stock standard solutions (Merck, Darmstadt, Germany).The universal chemical modifier containing 500 mg l−1 of Pd(NO3)2 + 300 mg l−1 of Mg(NO3)2 was obtained after appropriate dilution of the high purity solutions of 10,000 mg l−1 of Pd(NO3)2 and 10,000 mg l−1 of Mg(NO3)2 (Suprapur, Merck). This modifier was prepared in presence of 0.05% m/v Triton X-100 (Merck, Darmstadt, Germany) to decrease the surface tension of solution, facilitating the interaction between hair sample and chemical modifier.
Samples
Certified reference material (CRM) of human hair, GBW 09101 and BCR 397, produced respectively by the National Research Center for Certified Reference Materials (NRCCRM) and by the Community Bureau of Reference (CBR) were used to check the reliability of the methods.Scalp hair of a healthy woman (approximately 18 cm of length) was collected using stainless steel scissors.
Procedure
All glassware and polypropylene bottles (Nalge Company, Rochester, NY, USA) used to prepare and store solutions and samples were cleaned with detergent solution, soaked in 10% (m/v) HNO3 for 24 h, rinsed with deionized water, dried in a class 100 laminar flow hood and stored in a closed polypropylene container.For SS-GF AAS heating program optimization, pyrolysis and atomization temperature curves were obtained for the elements using an aqueous solution (0.5 μg l−1 of Cd and 20 μg l−1 Pb in 0.1% v/v of HNO3) and a solid sample (GBW 09101), with and without addition of 10 μl of 500 mg l−1 of Pd(NO3)2 + 300 mg l−1 of Mg(NO3)2 in 0.05% m/v Triton X-100. For the heating program optimizations in the presence of hair, aliquots around 80 μg were weighted directly onto the boat-type platform with and without addition of 10 μl of the chemical modifier.
Calibration curves were obtained using 10 μl of aqueous reference solutions containing 0.5 to 5 μg l−1 of Cd and 5 to 60 μg l−1 of Pb in 0.1% v/v HNO3, added with a micropipette onto the boat-type platform. Over the analytical solutions 10 μl of the chemical modifier was injected.
To evaluate the longitudinal distribution and to distinguish between the endogenous and exogenous content of Cd and Pb, stand of hairs were submitted to two different washing procedures. The first was based on the International Atomic Energy Agency (IAEA) protocol27 and the second was based on the IAEA protocol followed by washing with 0.1 mol l−1 HCl. To dry the washed hair sample, we wrapped them up with filter paper for approximately 24 h in a stove (45 °C). After that, one strand of hair was cut in 36 segments of 5 mm (masses around 80 μg) and each one was directly analyzed by SS-GF AAS. The segments of three different strands of hair were analyzed for each washing procedure.
Aliquots of 80 μg of the certified reference materials were weighed onto the platform and l0 μl of the chemical modifier were added to execute the analysis. Five replicates of the certified materials were carried out for each determination and the median was taken as representative value in order to minimize the possible influence of outliers.28
Results and discussion
Pyrolysis and atomization temperatures
In the absence of chemical modifier, Cd stability in aqueous solution was 400 °C and in CRM of hair was 600 °C, showing that, in this particular case of this sample, the matrix itself could stabilize the analyte, probably due to the protein fraction made of cysteine.14 When the mixture of Pd + Mg was used, the Cd pyrolysis temperature increased to 800 °C and to 700 °C in the aqueous solution and in the CRM of hair, respectively. The thermal stability of Pb in the aqueous solution increased from 600 °C to 800 °C, when Pd + Mg was used. In the presence of hair sample the thermal stability was very close to that observed for aqueous solution, 700 °C and 800 °C with and without chemical modifier.Pyrolysis holding time was also optimized and 40 s (Cd) and 30 s (Pb) were sufficient to ensure effectiveness of background correction. Peak shapes and background absorption were also considered when choosing the furnace conditions. The heating program described in Table 1 was used for the determination of Cd and Pb in hair.
Calibration curves
The calibration curves for Cd and Pb in the aqueous solution and in the certified reference material (GBW 09101) are shown in Fig. 1 and 2, respectively.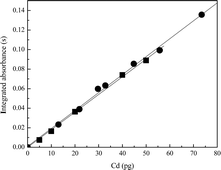 | ||
| Fig. 1 Calibration curves for Cd: (■) aqueous solutions (y = 0.00180x; R2 = 0.9994), and (●) BCR 397 - hair reference material (y = 0.00186x; R2 = 0.9980). | ||
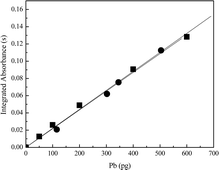 | ||
| Fig. 2 Calibration curves for Pb: (■) aqueous solutions (y = 0.000221x; R2 = 0.9985), and (●) GBW 09101 – hair reference material (y = 0.000217x; R2 = 0.9982). | ||
The comparison of the slopes (b) observed for calibration graphs obtained from aqueous solution with those in presence of hair sample can be use to estimate the effect caused by the matrix. In absence of matrix effect, the ratio between the slopes obtained from aqueous solutions and sample must be, approximately, 1, and this condition ensures the adequacy of using aqueous reference solution for instrument calibration. The regression coefficients (r) and slopes (b) obtained from aqueous solution were very close to those obtained from hair sample. The ratio between the slopes obtained from aqueous and hair samples for Cd (0.00180/0.00186 = 0.97) and Pb (0.000221/0.000217 = 1.0) showed no matrix effect. In all cases the calibration curves regression coefficients and slopes are in good agreement (according to t-Student at a 95% confidence level).
The good agreement between the two calibration curves procedures can be credited to the similar atomization mechanism of the analytes in aqueous solution and in the sample. The peak shapes of Cd and Pb in aqueous solution are similar to that obtained in matrix. This fact can be attributed to the heating program optimization and to the use of appropriate chemical modifier.
Figures of merits and determination of Cd and Pb
The found concentrations of Cd and Pb in the certified hair using solid sampling and calibration against aqueous solutions are showed in Table 2. The results are in good agreement (according to t-Student at a 95% confidence level) with the certified values.| GBW 09101 (mg kg−1) | BCR 397 (mg kg−1) | |||
|---|---|---|---|---|
| Certified values | Found values | Certified values | Found values | |
| Cd | 0.095 ± 0.012 | 0.101 ± 0.009 | 0.521 ± 0.024 | 0.540 ± 0.030 |
| Pb | 7.2 ± 0.7 | 7.4 ± 0.6 | 33.0 ± 1.2 | 30.2 ± 1.7 |
The limits of detection were calculated based on the zero mass response, which is the ratio between three times the standard deviation of ten readings of an empty platform and the slope of the calibration curve, adjusted to a sample mass of 1 mg.22,29 The concentrations of 2.8 μg kg−1 and 40 μg kg−1 were obtained for Cd and Pb, respectively. The characteristic masses based on integrated absorbance were 2.4 pg for Cd and 22 pg for Pb.
Distribution of Cd and Pb in the human hair
The washing procedure is an important step in differentiation between endogenous and exogenous contamination in hair. A number of studies have used the IAEA method to wash sample hair prior to analysis. It is known that this washing procedure removes only dust and dirt, but there is no evidence that it removes exogenously bounded trace elements of the hair.30 In this way, through the analysis of hair washed by the IAEA protocol, it will not be possible to differentiate between trace elements bound exogenously and endogenously. On the other hand, washing using 0.1 mol l−1 HCl removes Cr, Cd and Pb bounded exogenously in hair structure.31 Based on these two washing procedures, we are proposing the combination of IAEA and 0.1 mol l−1 HCl to differentiate between Cd and Pb endogenously and exogenously bounded. The analysis of the hair washed by the IAEA procedure attested the total concentration of Cd and Pb (exogenous plus endogenous), while the analysis of hair washed using IAEA and 0.1 mol l−1 HCl attested only endogenously bounded elements.The distribution of Cd and Pb through the extent of the hair is showed in Fig. 3 and 4.
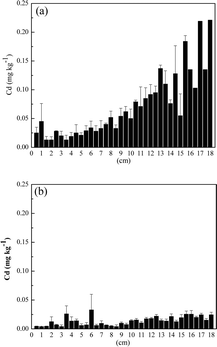 | ||
| Fig. 3 Cadmium concentration in each 0.5 cm through the extent of the hair: (a) washing with IAEA method and (b) washing with IAEA +0.1 mol l−1 HCl. | ||
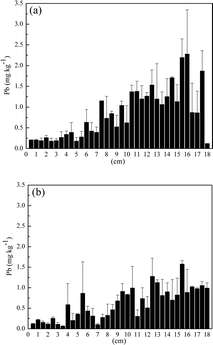 | ||
| Fig. 4 Lead concentration in each 0.5 cm through the extent of the hair: (a) washing with IAEA method and (b) washing with IAEA +0.1 mol l−1 HCl. | ||
The concentration of Cd (Fig. 3a) and Pb (Fig. 4a) increased from the root until the tip of hair washed according to IAEA protocol. However, when the hair was washed using the combination of IAEA protocol associated with 0.1 mol l−1 HCl, Cd concentration diminished drastically in all segments of the hair (Fig. 3b). Even considering the exogenous contamination, Cd and Pb level in this particular hair sample is normal. Cadmium and Pb concentration in hair can vary from 0.04 to 5.3 and 0.004 to 95 mg kg−1.14
The capacity of hair in storing and retaining trace elements in its structure made it an important key to evaluate the exposure to toxic element such as Cd and Pb. Besides hair analysis is commonly performed in total hair, the analysis of segments showed to be more realistic, allowing one to obtain chronological information of the exposure. As human hair grows at a rate of approximately 1 cm per month, it is possible to infer that the elements level in hair reflects its level in the body medium from with it was formed and provides a historical record of elements assimilated from the environment.32
Conclusions
The main worth of hair as a bioindicator is its capacity to store and retain trace elements over an extended period of time. Considering this characteristic, analysis of hair segments is an important key to reveal a possible temporal exposure to toxic elements. SS-GF AAS is a good alternative for this kind of application. Among the advantages, it excuses the step of sample digestion, minimizing the sample manipulation and consequently contamination or analyte loss. Moreover, the heating program optimization and the use of appropriate chemical modifier become possible the use of aqueous calibration. The most important characteristics of this technique is the possibility to analyze sample mass size lower than 1 mg, allowing the determination of trace elements in little hair segments.Associating the capacity of hair in storing trace elements and the characteristic of SS-GF AAS in analyzing low sample mass size, the proposed method showed to be useful to assess the temporal variation to Cd and Pb exposure, showing that this kind of analysis allows one to retrieve information about the past health issues and provides information about occupational and environmental status.
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support to CSN and PVO and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support and researchship to PVO.Notes and references
- A. Chatt, S. A. Katz, Hair Analysis: Applications in the Biomedical and Environmental Sciences, VCH Publishers, New York, NY, 1988 Search PubMed.
- K. Bencze, Fresenius J. Anal. Chem., 1990, 337, 867 CrossRef CAS.
- A. Fido and S. Al-Saad, Autism, 2005, 9, 290 CrossRef.
- S. Diez, S. Delgado, I. Aguilera, J. Astray, B. Perez-Gomez, M. Torrent, J. Sunyer and J. M. Bayona, Arch. Environ. Contam. Toxicol., 2009, 56, 615 CrossRef CAS.
- J. Stupar and F. Dolinsek, Spectrochim. Acta, Part B, 1996, 51, 665 CrossRef.
- P. Kintz, M. Ginet, N. Marques and V. Cirimele, Forensic Sci. Int., 2007, 170, 204 CrossRef CAS.
- J. C. Hansen, H. C. Wulf, N. Kromann and K. Aloge, Sci. Total Environ., 1983, 26, 233 CrossRef CAS.
- X. Chen, G. Yang, J. Chen, X. Chen, Z. Wen and K. Ge, Biol. Trace Elem. Res., 1980, 2, 91 CAS.
- J. Chlopicka, Z. Zachwieja, P. Zagrodzki, J. Frydrych, P. Slota and M. Krosniak, Biol. Trace Elem. Res., 1998, 62, 229 CrossRef.
- E. Esteban, C. H. Rubin, R. L. Jones and G. Moonan, Arch. Environ. Health, 1999, 54, 436 CAS.
- F. Barbosa Jr., J. E. Tanus-Santos, R. F. Gerlach and P. J. Parsons, Environ. Health Perspect., 2005, 113, 1669 CAS.
- R. Pereira, R. Ribeiro and F. Gonçalves, Sci. Total Environ., 2004, 327, 81 CrossRef CAS.
- N. Miekeley, M. T. W. C. Dias and C. L. S. Porto, Sci. Total Environ., 1998, 218, 9 CrossRef CAS.
- D. Pozebon, V. L. Dressler and A. J. Curtius, Quim. Nova, 1999, 22, 838 CAS.
- M. Saiki, E. R. Alves, O. Jaluul, N. M. Sumita and W. Jacob Filho, J. Radioanal. Nucl. Chem., 2008, 276, 53 CrossRef CAS.
- Y. Kolmogorov, V. Kovaleva and A. Gonchar, Nucl. Instrum. Methods Phys. Res., Sect. A, 2000, 448, 457 CrossRef CAS.
- G. Forte, A. Alimonti, N. Violante, M. Di Gregorio, O. Senofonte, F. Petrucci, G. Sancesario and B. Bocca, J. Trace Elem. Med. Biol., 2005, 19, 195 CrossRef CAS.
- H. Sela, Z. Karpas, M. Zoriy, C. Pickhardt and J. S. Becker, Int. J. Mass Spectrom., 2007, 261, 199 Search PubMed.
- S. Steely, D. Amarasiriwardena, J. Jones and J. Yanez, Microchem. J., 2007, 86, 235 CrossRef CAS.
- J. P. Lafleur, R. Lam, H. M. Chan and E. D. Salin, J. Anal. At. Spectrom., 2005, 20, 1315 RSC.
- I. M. Kempson and W. M. Skinner, Sci. Total Environ., 2005, 338, 213 CrossRef CAS.
- D. L. G. Borges, A. F. Silva, B. Welz, A. J. Curtius and U. Heitmann, J. Anal. At. Spectrom., 2006, 21, 763 RSC.
- C. S. Nomura, C. S. da Silva and P. V. Oliveira, Quim. Nova, 2008, 31, 104 CAS.
- B. Welz, D. L. G. Borges, M. G. R. Vale and U. Heitmann, Anal. Bioanal. Chem., 2007, 389, 2085 CrossRef CAS.
- J. Stupar and F. Dolinsek, Acta Chim. Slov., 2004, 51, 641 CAS.
- J. Stupar, F. Dolinsek and I. Erzen, Ecotoxicol. Environ. Saf., 2007, 68, 134 CrossRef CAS.
- P. Bermejo-Barrera, M. J. A. Lorenzo, A. Bermejo-Barrera, J. A. Cocho de Juan and J. M. B. Fraga, Forensic Sci. Int., 2000, 107, 149 CrossRef.
- Y. S. Ryabukin, 1978. Environmental Trace Element Pollutants. IAEA ReportIAEA/RL/50. Vienna: International Atomic Energy Agency Search PubMed.
- M. A. Belarra, M. Resano and J. R. Castillo, J. Anal. At. Spectrom., 1999, 14, 547 RSC.
- U. Kurfürst, Solid Sample Analysis – Direct and Slurry Sampling, Springer-Verlag, Berlin, Germany, 1998 Search PubMed.
- J. Morton, V. A. Carolan and P. H. E. Gardiner, Anal. Chim. Acta, 2002, 455, 23 CrossRef CAS.
- V. Valkovic, Human hair, Vols. I and II, CRC Press, FL, 1988 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
