A novel reversible relative-humidity indicator ink based on methylene blue and urea
Andrew
Mills
*,
Pauline
Grosshans
and
David
Hazafy
Department of Pure & Applied Chemistry, University of Strathclyde, Glasgow, UK G1 1XL. E-mail: a.mills@strath.ac.uk; Fax: +44 (0) 141 548 4822; Tel: +44 (0) 141 548 2458
First published on 19th November 2009
Abstract
A new relative-humidity sensitive ink based on methylene blue and urea is described which can utilise the deliquescent nature of urea.
Humidity sensors are used extensively in industry as well as for environmental monitoring. Their widespread applications cover a broad range of domestic, medical and industrial applications. For example, in food packaging, excess moisture in meat packaging can accelerate food spoilage, and as a consequence desiccants are often included in packaging to extend the shelf life.1 At low relative humidities (RHs) some dry grain products can undergo rapid free-radical oxidation and become rancid.1 Most fruit and vegetables are composed largely of water, consequently their optimum storage conditions are typically 90–95% RH, T = 0 °C,2 whereas products such as sugar and raisins lose their desirable texture if the RH is this high. Thus, most food stuffs require a degree of moisture management and humidity measurement to obtain optimum relative-humidity (RH) conditions.1 Humidity control is also important for the preservation of artefacts such as books and paintings and to prevent bacterial and mould growth in certain manufactured products. Another application is in the packaging and transportation of sensitive electrical appliances/components which are adversely affected by high relative humidity and therefore require accurate monitoring to ensure efficient functionality.3
There are a wide range of commercial products currently available for monitoring relative humidity. These are, more often than not, electronic hygrometers based on capacitive or resistive systems which measure the change in conductivity of a polymer or ceramic film as a function of relative humidity.4 Such devices have limitations in their operating conditions and can be expensive and bulky to use.5,6
There have been a number of proposed colorimetric relative-humidity indicators, the majority of which are based on the use of inorganic salts7–17 such as cobalt(II) chloride, CoCl2, which at a defined RH level (typically RH 40%) convert from their anhydrous form to their hydrated form, which is usually marked by a colour change, in the case of CoCl2, blue (anhydrous) to pink (hydrated). The sensitivity of this type of indicator can be tailored to the desired application by one of three methods: changing the concentration of the inorganic salt,18 adding a deliquescent synergic salt19–21 or, for systems with a silica support, altering the drying and activation temperatures of the CoCl2-doped silica.7 Such relative-humidity indicators have been proposed for a variety of applications including: refrigerated systems,12,13 clothes dryers,14 shoe storage,16 desiccant absorbent capacity indicators,18,20,21 electronic device storage17 and are often reversed by heating the hydrated salt to regenerate the anhydrous starting material. A typical commercial form, based on CoCl2, is the Humitector® Humidity Indicator Card (Süd-Chemie Inc., USA).22
Recently, Matsushima et al. have proposed using thiazine and flavylium salts in gels as simple colorimetric humidity and temperature sensors.23–25 These salts exhibit reversible colour changes from blue (dry) to purple (humid), as a result of a change in relative humidity. This has been attributed to the absorption of water vapour by the gel in humid conditions which encourages the dye to form dimers and so leads to a shift in λmax absorbance (ca. 10–20 nm) to a lower wavelength.
Whilst looking at this system, we have found that when the thiazine dye, methylene blue (MB), is encapsulated within a polymer, such as hydroxy ethyl cellulose (HEC), with a notable excess of urea, (20 times w/w more than MB), the product ink is blue, but casts as a thin, opaque pink film, under ambient (RH = 60%, T = 20 °C) or dry conditions, and rapidly and reversibly is rendered blue coloured and clear when exposed to RH values >85%. Note that the observed colour changes for MB/urea/HEC films are the opposite of those observed by Matsushima et al.23–25 for their MB/gel films so the explanation for the effect is very different. It is also unusual to find a relative-humidity indicator which gives such a sharp, reversible colour change at high relative humidities. Consequently, this novel and promising relative-humidity indicator is the subject of this communication.
In this work, all relative humidities were measured at 20 °C unless otherwise stated. A typical relative-humidity sensor was made by spin coating an ink comprising 5 mg MB, 100 mg urea in 2 g of a 5% w/v HEC aqueous solution, for 30 seconds at 3500 rpm on to a 25 mm glass disc. Following the drying of the film at 70 °C for a few minutes, the final product is an opaque pink film (ca. 1.7 µm thick) under ambient conditions (40–60% RH) with a λmax at 570 nm, which upon exposure to high relative-humidity conditions (>85% RH) turns rapidly blue (λmax = 600 nm) and clear as illustrated in Fig. 1 and Fig. 2.
 | ||
| Fig. 1 Photographs of a typical MB/urea/HEC relative-humidity indicator changing colour from pink (left) to blue (right) upon exposure to 100% RH air, from the bottom right. | ||
 | ||
| Fig. 2 Spectral changes of a typical MB/urea/HEC relative-humidity indicator film before (—) and after (■) exposure to 100% RH air. Inset diagram is a plot of the change in absorbance MB λmax humid (600 nm) for indicator film on exposure to 3 cycles of 1 min 100% RH air and 1 min dry air. Abs600 recorded every 6 s. | ||
This colour change process occurs rapidly (response and recovery times: 10 s and 60 s respectively) and is reversible as illustrated by the data in the inset of Fig. 2 which show the response and recovery of the absorbance (at λmax for blue form, 600 nm) for a typical MB/urea/HEC relative-humidity indicator film upon exposure to repeated cycles of humid (100% RH) and dry air.
The initial spectrum of the dry film (see Fig. 2) is very different from that observed for the monomer and dimer, thus, it is not instantly apparent what the colour change is due to.26–28 Under ambient conditions the film is pink and the absorption spectrum of the film (λmax = 570 nm) is, if anything, like that of the MB trimer (λmax = 578 nm).26,28
When exposed to high relative humidities the resulting blue film has a spectrum more characteristic of a mixture of the MB monomer and dimer.26–28 The monomer is attributed to the smaller peak at 665 nm while the larger peak at 600 nm is attributed to the MB dimer. An inspection of films containing no dye reveals a concomitant change in the optical clarity of the urea/HEC film in the absence (opaque) and presence (clear) of a stream of air containing a high (100%) RH, as illustrated in Fig. 3.
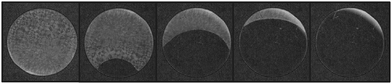 | ||
| Fig. 3 Photographs of a urea/HEC with no dye changing from an opaque film (left) to a clear film (right) upon exposure to 100% RH air. | ||
Further work shows that urea forms highly crystalline, optically opaque films with a characteristic XRD peak at 2θ = 22.25° at moderate and low RH. These crystallites rapidly dissolve when exposed to relative humidities above 85%, rendering a clear film. The urea XRD peak reappears when the opacity change is reversed by blowing dry air over the film or placing it in oven (70 °C for few minutes). Thus, it appears that the colour change associated with the MB in the urea film (pink (dry) ↔ humid (blue)) is linked to the change in crystallinity of the urea when exposed to high relative humidities. Indeed a dye-free relative-humidity indicator based on optical clarity can be simply created using just urea in a polymer, such as HEC, since Fig. 3 shows that such a film is opaque at medium and low (<85%) RH levels, but clear at RH values >85%; the process is entirely reversible. The polymer used, in this case HEC, does not appear to have any effect on the colour or opacity change, but merely acts as an encapsulation agent. This was confirmed by observing a similar effect in different polymers (such as polyvinyl alcohol, PVA and polyethylene oxide, PEO). Also in a polymer-free environment, achieved by grinding up a sample of urea with MB, the resulting pink powder exhibits a similar reversible colour change to blue when exposed to RH >85%.
The simplest explanation is that under ambient conditions (RH < 85%) MB is encapsulated in urea crystals as the pink trimer and when exposed to high RHs the urea crystals dissolve, thereby releasing the MB into an environment in which its more stable form is the blue coloured MB dimer and monomer. In support of this, it is well known that urea is a hygroscopic compound which deliquesces under high RH conditions of >80% RH at 18 °C.29–31
The notable features of this type of relative-humidity indicator are not only that it can be used exclusively for monitoring high (>85%) relative humidities, but it is quick to respond, highly reversible and has a good long-term stability. As it stands, such a >85% RH indicator has a potential application ensuring the correct RH conditions for the storage and ripening of fruit,32 for example. Further work is in progress developing this system using a series of hygroscopic, urea and non-urea-related, compounds which undergo deliquescence at different relative humidities (e.g.N,N′-dimethylurea deliquesces at RH >63% at 18 °C) to generate a set of relative-humidity indicators for providing a sharp register at different, defined relative-humidity levels that span the RH scale.
Notes and references
- R. Esse and A. Saari, in Smart Packaging Technologies, ed. J. Kerry and P. Butler, John Wiley & Sons, West-Sussex, 2008, pp. 130–149 Search PubMed.
- J. E. Robinson, K. M. Browne and W. G. Burton, Ann. Appl. Biol., 1975, 81, 399–408 Search PubMed.
- R. H. Feinzig and D. Sullivan, Commissioning Humidity Control Systems in Critical Environments, in National Conference on Building Commissioning, held at Chicago, IL, 2–4 May 2007 Search PubMed.
- N. Yamazoe and Y. Shimizu, Sens. Actuators, 1986, 10, 379–398 CrossRef CAS.
- http://www.hannainst.com/usa/prods2.cfm%3Fid%20%3D%20012001, last accessed November 2009.
- http://www.michell.co.uk/products/classification/browse/portables, last accessed November 2009.
- P. B. Davis, US Pat. 2460065–2460070, 1949 Search PubMed.
- P. B. Davis, US Pat. 2580737, 1949 Search PubMed.
- D. L. Fuller, US Pat. 4034609, 1977 Search PubMed.
- D. L. Fuller, US Pat. 4150570, 1979 Search PubMed.
- J. S. Haswell, US Pat. 5520041, 1996 Search PubMed.
- W. O. Krause, US Pat. 3533277, 1970 Search PubMed.
- W. O. Krause, US Pat. 3499616, 1970 Search PubMed.
- J. F. McBride, US Pat. 4909179, 1990 Search PubMed.
- S. D. Price, US Pat. 3121615, 1964 Search PubMed.
- E. Wroth and K. Sheraw, US Pat. 5950323, 1999 Search PubMed.
- Y. Yamakawa, US Pat. 7316198 B2, 2008 Search PubMed.
- M. Hamada, US Pat. Appl. 2007/0157702 A1, 2007 Search PubMed.
- P. B. Davis and J. N. Pryor, US Pat. 2526938, 1950 Search PubMed.
- M. Gattiglia, US Pat. 6655315 B1, 2003 Search PubMed.
- M. Gattiglia and E. Gandolfo, WO 02/44712 A1, 2002.
- http://www.sud-chemie.com/scmcms/web/page_en_6254.htm, last accessed November 2009.
- R. Matsushima, N. Nishimura, K. Goto and Y. Kohno, Bull. Chem. Soc. Jpn., 2003, 76, 1279–1283 CrossRef CAS.
- R. Matsushima, A. Ogiue and S. Fujimoto, Chem. Lett., 2000, 590–591 CrossRef CAS.
- R. Matsushima, A. Ogiue and Y. Kohno, Chem. Lett., 2002, 436–437 CrossRef CAS.
- E. Braswell, J. Phys. Chem., 1968, 72, 2477–2483 CrossRef CAS.
- A. Ghanadzadeh, A. Zeini, A. Kashef and M. Moghadam, J. Mol. Liq., 2008, 138, 100–106 CrossRef CAS.
- Z. Zhao and E. R. Malinawski, Appl. Spectrosc., 1999, 53, 1567–1574 CrossRef CAS.
- J. R. Adams and A. R. Merz, Ind. Eng. Chem., 1929, 21, 305–307 CrossRef CAS.
- A. Clow, Nature, 1940, 146, 26 CrossRef CAS.
- E. A. Werner, Nature, 1937, 139, 512 CrossRef CAS.
- C. Vazquez-Salinas and S. Lakshminarayana, J. Food Sci., 1985, 50, 1646–1648 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
