Molecular imaging of adrenal gland by desorption electrospray ionization mass spectrometry†
Chunping
Wu
,
Demian R.
Ifa
,
Nicholas E.
Manicke
and
R. Graham
Cooks
*
Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: cooks@purdue.edu
First published on 17th November 2009
Abstract
Subtle differences in the spatial distributions of closely related compounds including norepinephrine and epinephrine as well multiple lipids are easily distinguished in adult porcine (17 × 8 mm) and rabbit (7 × 4 mm) adrenal glands in a DESI-MS imaging experiment at atmospheric pressure with a spatial resolution of ∼200 µm. Sensitive and specific detection in the course of DESI imaging discloses details of catecholamine distribution in porcine adrenal medulla and cortex; the average mass of epinephrine interrogated in each pixel is estimated to be about 150 pg. The distribution of ascorbic acid was revealed in the negative ion mode. In addition, the distribution of cholesterol, which cannot be observed using conventional DESI, was obtained using in situ reaction with betaine aldehyde added to the DESI spray while imaging the porcine adrenal gland tissue. Four characteristic types of distributions were observed, with major amounts of the components in the medulla, the cortex, the reticular zone or in the fourth case, being homogeneously distributed. The results agree with and extend information available from histological studies.
Introduction
In many mammalian species, adrenal glands regulate stress response through the synthesis, storage and delivery of the catecholamine neurotransmitters and hormones (such as norepinephrine, epinephrine and dopamine) by chromaffin cells in the adrenal medulla. Chromaffin cells consist of epinephrine-storing (E) cells and norepinephrine-storing (N) cells.1 The distinct physiological and pharmacological properties of the different catecholamines have encouraged histological studies of chromaffin cells in adrenal glands. Traditional histochemical2 and immunohistochemical3 studies of adrenal glands, coupled to light or electron microscopy, allow the distribution of E and N cells to be determined. However, these traditional methods are time-consuming, labor-intensive, and often involve indirect analysis of the target compounds. For example, in some immunohistochemical studies of adrenal glands,3,4 determination of epinephrine was achieved by an immunoassay for phenylethanolamine N-methyltransferase (PNMT), the enzyme that converts norepinephrine to epinephrine. Only one endogenous compound (or one class of endogenous compounds) can be analyzed at a time using traditional histochemical methods. In addition, targeted endogenous compound dislocation can occur during histochemical sample treatment procedures, leading to inaccurate molecular distributions.5Mass spectrometric (MS) imaging techniques provide information on the spatial distribution of particular biomolecules in tissues.6–10 Specifically, matrix-assisted laser desorption ionization (MALDI) is noteworthy for allowing imaging large molecules like proteins with spatial resolution on the low micron scale, and secondary-ion mass spectrometry (SIMS)11 has the advantage of very high spatial resolution (∼100 nm), although it is best at targeted inorganic compounds or biomolecules with low molecular weight. Desorption electrospray ionization (DESI) is an ionization technique which can be applied to tissue samples in the ambient environment with minimum sample preparation (no matrix added, no staining, or vacuum used).12 Currently, DESI imaging is often used to analyze compounds with molecular weights less than 2000 Da, at a spatial resolution ranging from tens to hundreds of microns.13 Ionization by DESI is soft, occurring with minimal fragmentation.
This communication reports the direct and specific determination of the distribution of the small hormones epinephrine and norepinephrine, along with many phospholipids, in porcine adrenal gland in a single acquisition using DESI imaging (positive ion mode) without staining or labeling the tissue sections. The mapping of multiple biomolecules in the course of a single acquisition is desirable not only in terms of analysis time, but because it allows better correlations between biomolecules and with the substructures of the tissue in histological, physiological and pharmacological studies. In particular, the problem of target endogenous compound diffusion during histochemical procedures5 can be avoided using sample-preparation-free DESI imaging. Ascorbic acid, which yields one of the major peaks in the negative ion mode, shows the highest signal intensity in the reticular zone, approximately two times greater than that in the fasciculate zone. In addition, the distribution of cholesterol, which is inefficiently ionized in conventional DESI, was obtained using reactive DESI.14,15 Tandem MS and exact mass measurements on an Orbitrap mass spectrometer were used to identify endogenous compounds (epinephrine, norepinephrine, fatty acids, phospholipids, cholesterol and ascorbic acid) in adrenal tissues.
Experimental
Chemicals and biological samples
Healthy adult porcine and rabbit adrenal glands were purchased from Pel-Freez Inc. They were cut into 20 µm sections at −20 °C using a Shandon SME Cryotome Cryostat and thaw-mounted onto microscope glass slides (Gold Seal Products Inc., Portsmouth, NH). The tissue sections were stored at −80 °C until analysis. Prior to analysis the sections were dried in a vacuum desiccator for at least 2 hours. Epinephrine, norepinephrine, L-ascorbic acid standards and betaine aldehyde chloride were purchased from Sigma-Aldrich Inc. (Milwaukee, WI).DESI-imaging
DESI imaging analysis was performed using a Thermo-Fisher Scientific LTQ linear ion trap mass spectrometer (San Jose, CA) equipped with a custom-built, automated DESI ion source and a surface moving stage. The surface moving stage included an XYZ integrated linear stage (Newport, Richmond, CA) and a rotary stage (Parker Automation, Irwin, PA). Detailed information about the experimental setup and instrumentation can be found elsewhere.14 Briefly, mass spectra were acquired with automatic gain control (AGC) turned off. Imaging experiments were performed by continuously scanning the surface in the x-direction and stepping in the y-direction (moving opposite to the direction of the spray) at the end of each line. A spray impact angle of 52° was used. Solvent MeOH/H2O (1:1) was sprayed at a flow rate of 1.5 µL/min in conventional DESI imaging. The time required to record the whole image depended on the size of the tissue and the pixel size selected. Typically, a porcine adrenal gland slice (17 × 8 mm) was imaged using 60 lines and a step size of 200 µm in the y-direction. The lines were scanned at a constant velocity of 330 µm/s, while collecting one mass spectrum every 0.6 seconds over the range m/z 150–1200. The number of spectra per line was 175. This procedure resulted in arrays of 175 × 60 pixels, each pixel covering an area of 200 × 200 µm and corresponding to a single mass spectrum. The total time to record this image was ∼105 minutes (0.6 sec × 175 × 60). For the smaller size rabbit adrenal gland (7 × 4 mm), the total time to record an image at the same spatial resolution was ∼ 60 minutes. Under these conditions, a lateral spatial resolution of ∼200 µm can normally be achieved in DESI-MS imaging.16 Reactive DESI imaging of adrenal glands was performed using the same experimental setup, except that the spray solvent was changed to acetonitrile/chloroform (1:2) containing 50 ppm betaine aldehyde chloride.BioMap (freeware, http://www.maldi-msi.org/) was used to process the mass spectral data to generate two-dimensional ion images. A rainbow color scale and appropriate contrast in the color bar were selected independently for each ion (see more details in the Supporting Information†).
Tandem mass spectrometry and exact mass measurement
Tandem MS product ion scans, using collision-induced dissociation (CID), were carried out on tissue samples and compared to that of authentic standards to confirm the identity of the endogenous compounds. For the MS/MS experiments, an isolation window of 1.2 mass/charge units, a normalized collision energy of 20–40% (manufacturer's units) and a Mathieu parameter qz value of 0.25 during collisional activation were used.Exact mass measurements of epinephrine and norepinephrine in the adrenal medulla and cortex extracts were performed using a commercial Thermo Scientific LTQ-Orbitrap XL mass spectrometer (Bremen, Germany), with the resolution set to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The lock mass function was used with arginine (m/z 175.11895) as the internal standard. To prepare porcine adrenal medulla and cortex extracts for exact mass measurements, ∼200 mg medulla or cortex sections were cut from porcine adrenal glands immediately after taking the samples from a −80 °C freezer. The medulla or cortex sections were collected in 1.5 mL Eppendorf vials and 1 mL of deionized water was added to each vial. The samples were centrifuged in an Eppendrof centrifuge (Westbury, NY) at 9800 RPM (revolutions per minute) for 3 minutes. Then the upper clear solutions were diluted 10 times before analysis with electrospray ionization (ESI) on the LTQ-Orbitrap mass spectrometer. The same procedures were used for preparing the rabbit adrenal medulla or cortex extracts.
000. The lock mass function was used with arginine (m/z 175.11895) as the internal standard. To prepare porcine adrenal medulla and cortex extracts for exact mass measurements, ∼200 mg medulla or cortex sections were cut from porcine adrenal glands immediately after taking the samples from a −80 °C freezer. The medulla or cortex sections were collected in 1.5 mL Eppendorf vials and 1 mL of deionized water was added to each vial. The samples were centrifuged in an Eppendrof centrifuge (Westbury, NY) at 9800 RPM (revolutions per minute) for 3 minutes. Then the upper clear solutions were diluted 10 times before analysis with electrospray ionization (ESI) on the LTQ-Orbitrap mass spectrometer. The same procedures were used for preparing the rabbit adrenal medulla or cortex extracts.
Results and discussion
Fig. 1 shows the 2-D distributions of catecholamines and lipids obtained by directly imaging the porcine adrenal gland tissue slice using DESI-MS. All the data are recorded in a single acquisition. The adrenal gland is separated into two distinct structures, medulla and cortex, with veins in the medulla (Fig. 1a). As shown in Fig. 1b and 1c, norepinephrine (m/z 170.1) and epinephrine (m/z 184.1) are clearly observed in the porcine medulla, which is consistent with the known location of catecholamine synthesis. The concentrations of epinephrine and norepinephrine vary in different mammals, normally being at the level of a few hundred ng/mg in the adrenal gland.17 Given the ∼2 mg mass of the porcine adrenal tissue section (20 µm thickness) and the pixel number of 175 × 60 (note only around half of these pixels correspond to the tissue section, the others cover the tissue borders and glass substrate), and assuming the concentration of epinephrine of ∼400 ng/mg,17 the average mass of epinephrine interrogated in each pixel is about 150 pg (400 ng/mg × 2 mg × 2/(175 × 60) = 150 pg). The fact that the distribution of theses catecholamines can be mapped is an indication of the sensitivity of this analytical technique. To rule out the possibility that m/z 184 and m/z 170 are from fragmentation of diacyl-glycerophosphocholines (PCs), as can occur in SIMS, adrenal tissues were analyzed by DESI-Orbitrap combination. The results show that peak m/z 184.0970 and m/z 170.0812 (present in the cortex and medulla) can be accurately assigned to epinephrine and norepinephrine with errors less than 1.5 ppm, and there are no significant neighboring peaks within ±1 Da of each of these two peaks. Also, tandem MS of m/z 184 and m/z 170 (Fig. S1, Supporting Information†) were performed while the spray rasters the tissue surface, which were consistent with that of the authentic epinephrine and norepinephrine standards. This indicates ionization with DESI is soft enough, so the distributions of epinephrine and norepinephrine can be accurately mapped without the interference of the fragmentation from phospholipids.![(a) Optical image of the porcine adrenal gland tissue (17 × 8 mm). (b–i) Selected ion images of norepinephrine; epinephrine; m/z 226.2 ([C12H20O3N]+), assigned tentatively as trimethylamino-hydroxymethylphenylethane-diol; PC (16:0/16:0, sodium adduct); PC (16:0/16:0, potassium adduct); PC (36:0); PC (36:4, sodium adduct); and PC (44:4, sodium adduct). PC stands for diacyl-glycerophosphocholines; (X:Y) represents the total number of carbon atoms and the total number of double bonds in the fatty acid chains; (X1:Y1/X2:Y2) indicates the number of carbon atoms and double bonds in each of two fatty acid chains. These images were obtained using normal DESI in the positive mode, with MeOH/H2O (1:1) as the spray solvent.](/image/article/2010/AN/b919816d/b919816d-f1.gif) | ||
| Fig. 1 (a) Optical image of the porcine adrenal gland tissue (17 × 8 mm). (b–i) Selected ion images of norepinephrine; epinephrine; m/z 226.2 ([C12H20O3N]+), assigned tentatively as trimethylamino-hydroxymethylphenylethane-diol; PC (16:0/16:0, sodium adduct); PC (16:0/16:0, potassium adduct); PC (36:0); PC (36:4, sodium adduct); and PC (44:4, sodium adduct). PC stands for diacyl-glycerophosphocholines; (X:Y) represents the total number of carbon atoms and the total number of double bonds in the fatty acid chains; (X1:Y1/X2:Y2) indicates the number of carbon atoms and double bonds in each of two fatty acid chains. These images were obtained using normal DESI in the positive mode, with MeOH/H2O (1:1) as the spray solvent. | ||
Reproducible observations on the distribution of catecholamines were obtained from four adult porcine adrenal glands. First, the overall peak intensity of epinephrine is higher than norepinephrine in the porcine medulla, and the intensity ratio varies at different locations in the tissue (Fig. 2). Note that the peak intensity in the mass spectrum does not exactly represent molecular concentrations in tissue. However, for the same molecule in similar tissues or closely related compounds in the same tissue, relative peak intensities should approximately reflect differences in concentrations. Evidence for this is found in the fact that a peak intensity ratio of 2:3 is measured for a mixture of equal amounts of authentic norepinephrine (5 × 10−11 mol) and epinephrine (5 × 10−11 mol) analyzed using DESI with spray solvent MeOH/H2O (1:1) (Fig. S2, Supporting Information†). Dopamine and the metabolites of catecholamines were not observed in DESI imaging experiments due to their relatively low concentrations in adrenal glands; they were also not detectable using liquid chromatography tandem mass spectrometry (LC-MS/MS).17 Second, norepinephrine is most abundant in the central area close to the vein (Fig. 1b). Conversely, epinephrine shows a complementary distribution as compared to norepinephrine. The epinephrine signal is greater at the top edge of the adrenal medulla (Fig. 1c). Our results provide more details on catecholamine distribution compared with the known distribution of the chromaffin cells as reported by immunohistochemical studies,3 owing to the specific and sensitive detection of catecholamine using DESI-MS. Another new finding is that catecholamines are present not only in the porcine medulla, but also in the porcine cortex, albeit with much lower intensity. The overall peak intensity of epinephrine is more than 60 times less in the cortex compared to the medulla. To rule out the possibility that the catecholamines present in the cortex were due to carryover from the medulla, by the spray solvent or by the blade during the tissue sectioning, exact mass measurements of the medulla extract and cortex extract (see the Experimental section) were performed on a high resolution Orbitrap mass spectrometer. Epinephrine and norepinephrine were observed and accurately assigned (errors < 1.5 ppm) in the porcine cortex extract (Fig. S3a, Supporting Information†) with peak intensities much lower than those in the porcine medulla extract (Fig. S3b, Supporting Information†). According to immunohistochemical studies,4 PNMT immunoreactivity was not found in porcine adrenocortical cells, so the low concentration of epinephrine detected in the porcine cortex is not synthesized in adrenocortical cells, but mostly like diffuses from the medulla.
 | ||
| Fig. 2 Peak intensity comparison of norepinephrine (NEP) and epinephrine (EP) at different positions in the porcine adrenal gland. | ||
Besides catecholamines, 2-D distributions of multiple phospholipids in the mass range of m/z 750–1000 were obtained in the same single acquisition DESI imaging experiment (see Fig. S4a† for the DESI mass spectrum of the porcine adrenal gland tissue). The individual lipids, in the form of sodium adducts or potassium adducts, show characteristic and different spatial distributions (Fig. 1e–1i). For example, the diacyl-glycerophosphocholines (PCs) 16:0/16:0 and 44:4 are more intense in the medulla. In contrast, PC 36:4 show a complementary location, focused in the cortex. Some lipids, such as PC 36:0, show a homogenous distribution across the entire tissue section except where the vein is located. This result implies that intrinsic concentration is the major determinant of signal strength, and the relative chemical distributions are not artifacts due to changes in tissue texture, composition or surface topology. More ion images obtained in the same acquisition can be found on Fig. S5 (Supporting Information†). The peak assignments of lipids were confirmed by tandem MS (Fig. S1, Supporting Information†) and comparisons to literature data.18
For the detection of lipids such as fatty acids, dihydroceramide (DHCer), plasmenyl ethanolamines (PlsEtns), phosphatidylethanolamines (PEs), phosphatidylserines (PSs) and phosphatidylinositols (PIs) which cannot be observed in the positive ion mode, negative ion mode DESI was implemented. Distributions of these lipids in the porcine adrenal tissue are shown in Fig. 3 and Fig. S6 (Supporting Information†). Of particular note is the abundant peak at m/z 175.1 in the negative ion mode mass spectrum (Fig. S4c†). This signal shows higher signal intensity at the reticular zone (innermost layer of the adrenal cortex and superficial to the adrenal medulla), approximately two times higher than that of the fasciculate zone (the middle zone of the adrenal cortex). Using tandem MS and exact mass measurements (Fig. S7, Supporting Information†), m/z 175.1 was identified as ascorbic acid and confirmed with data for the authentic compound; its distribution is consistent with histochemical data.5 Similarly, the peak at m/z 226.2 in the positive ion mode also shows a higher signal intensity in the reticular zone (Fig. 1d), and its elemental composition was determined as [C12H20O3N]+ using exact mass measurements on the Orbitrap instrument. The structure of m/z 226.2 is proposed using tandem MS data (Fig. S8, Supporting Information†), to be trimethylamino-hydroxymethylphenylethane-diol, although this remains to be confirmed due to limited literature information.
 | ||
| Fig. 3 (a–f) Selected ion images of ascorbic acid, arachidonic acid, PlsEtn (38:4), PlsEtn (40:4), PE (40:4) and PS (40:4). These images were obtained using normal DESI in the negative mode, with MeOH/H2O (1:1) as the spray solvent. | ||
The relative proportions and distribution of epinephrine and norepinephrine in vertebrates is known to be species specific.3 DESI imaging of a rabbit adrenal gland (∼5 times smaller in area than porcine adrenal gland) suggests that the overall intensity of catecholamines in the rabbit adrenal gland is lower than in the porcine adrenal gland. Unlike the porcine adrenal, epinephrine in the rabbit adrenal is uniformly distributed in the medulla (Fig. 4c), with a diameter of ∼2 mm. On the other hand, the intensity of norepinephrine signal is more than 100 times lower than that of epinephrine (Fig. 4b). This result is consistent with data obtained from immunohistochemical studies,2,3 which demonstrate that the majority of adrenal chromaffin cells are epinephrine-storing in rabbit. A low concentration of epinephrine, but no norepinephrine, was observed in the rabbit adrenal cortex. Using exact mass measurement of the medulla extract and cortex extract, we confirmed the existence of a low level of norepinephrine in the adult rabbit adrenal medulla, but not in the adrenal cortex (Fig. S3, Supporting Information†). The distributions of lipids were obtained analogously for the rabbit adrenal gland (Fig. 4d–4f).
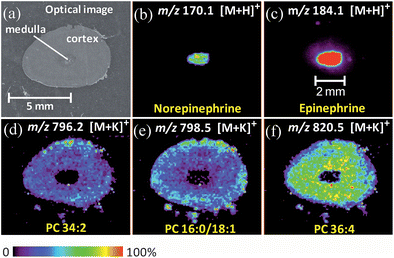 | ||
| Fig. 4 (a) Optical image of the rabbit adrenal gland tissue (7 × 4 mm). (b–f) Selected ion images of norepinephrine, epinephrine, PC (34:2, potassium adduct), PC (16:0/18:1, potassium adduct), PC (36:4, potassium adduct). These images were obtained using normal DESI in the positive mode, with MeOH/H2O (1:1) as the spray solvent. | ||
Another feature of DESI is that by simply doping reagent into the spray solvent, detection of some compounds, which cannot be observed using conventional DESI, can be greatly improved. One example of this reactive DESI imaging was to map endogenous free cholesterol in rat brain tissues using betaine aldehyde chloride as the reagent.14 The mass spectrum (Fig. S4b, Supporting Information†) obtained in reactive DESI imaging (positive ion mode) of the porcine adrenal gland shows an intense peak at m/z 488, corresponding to the reaction product of endogenous cholesterol with betaine aldehyde. Two-dimensional imaging shows that the cholesterol signal is less intense in the area close to the vein in the porcine medulla compared to the cortex (Fig. S9†). Most of the other biological compounds observed in conventional DESI (Fig. S4a, Supporting Information†), for example epinephrine and norepinephrine, did not react with betaine aldehyde, either because the aromatic system and the preferential protonation on the nitrogen moiety lower the reactivity, or the concentrations of catecholamines are relatively lower than cholesterol in adrenal glands,19 so peaks corresponding to unreacted molecular ions can still be observed in reactive DESI.
Conclusion
By using conventional DESI (positive ion and negative ion mode) as well as reactive DESI, we are able to obtain more complete MS images of biological molecules, including catecholamines, phospholipids, fatty acids, ascorbic acid and cholesterol in adrenal glands. The 2-D distributions of different classes of biological molecules were obtained in a single experiment on each sample under atmospheric pressure without any sample preparation after tissue sectioning. Details on catecholamine distribution in the porcine adrenal medulla and low levels of catecholamines in the porcine adrenal cortex were uncovered. With a high mass resolution Orbitrap, endogenous compounds (epinephrine, norepinephrine, and ascorbic acid) were accurately identified. Compared to other MS imaging techniques, DESI-imaging shows great potential for imaging of endogenous small molecules and drugs because it is soft (with minimum fragmentation), ambient, and matrix-free (no matrix interference in the low mass range). In contrast to traditional histochemical and immunohistochemical studies, DESI-MS imaging is a direct, specific, label-free and easy-to-implement technique that provides accurate molecular distributions in tissue.Acknowledgements
This work was supported by US National Institutes of Health (NIH grant 1R21EB009459-01), the Office of Naval Research (N00014-05-1-0405), and in part by a fellowship from Merck Research Laboratories (to N. E. M.).Notes and references
- J. G. Wood, Am. J. Anat., 1963, 112, 285–303 Search PubMed.
- R. E. Coupland and D. Hopwood, Nature, 1966, 209, 590–591 CrossRef CAS.
- T. Suzuki and T. Kachi, Anat. Rec., 1996, 244, 358–365 CrossRef CAS.
- T. Suzuki and T. Kachi, Neurosci. Lett., 1994, 176, 217–220 CrossRef CAS.
- O. Eränkö, J. Histochem. Cytochem., 1954, 2, 167–177 CAS.
- J. T. Shelley, S. J. Ray and G. M. Hieftje, Anal. Chem., 2008, 80, 8308–8313 CrossRef CAS.
- T. R. Northen, O. Yanes, M. T. Northen, D. Marrinucci, W. Uritboonthai, J. Apon, S. L. Golledge, A. Nordstrom and G. Siuzdak, Nature, 2007, 449, 1033–1037 CrossRef CAS.
- L. Zheng, C. M. McQuaw, A. G. Ewing and N. Winograd, J. Am. Chem. Soc., 2007, 129, 15730–15731 CrossRef CAS.
- P. Nemes, A. A. Barton, Y. Li and A. Vertes, Anal. Chem., 2008, 80, 4575–4582 CrossRef CAS.
- E. B. Monroe, S. R. Annangudi, N. G. Hatcher, H. B. Gutstein, S. S. Rubakhin and J. V. Sweedler, Proteomics, 2008, 8, 3746–3754 CrossRef CAS.
- A. Carado, M. K. Passarelli, J. Kozole, J. E. Wingate, N. Winograd and A. V. Loboda, Anal. Chem., 2008, 80, 7921–7929 CrossRef CAS.
- J. M. Wiseman, D. R. Ifa, Q. Y. Song and R. G. Cooks, Angew. Chem., Int. Ed., 2006, 45, 7188–7192 CrossRef CAS.
- V. Kertesz and G. J. Van Berkel, Rapid Commun. Mass Spectrom., 2008, 22, 2639–2644 CrossRef CAS.
- C. Wu, D. R. Ifa, N. E. Manicke and R. G. Cooks, Anal. Chem., 2009, 81, 7618–7624 CrossRef CAS.
- C. Wu, K. Qian, M. Nefliu and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2009 DOI:10.1016/j.jasms.2009.10.006.
- D. R. Ifa, J. M. Wiseman, Q. Y. Song and R. G. Cooks, Int. J. Mass Spectrom., 2007, 259, 8–15 Search PubMed.
- Q. Gu, X. Z. Shi, P. Y. Yin, P. Gao, X. Lu and G. W. Xu, Anal. Chim. Acta, 2008, 609, 192–200 CrossRef CAS.
- N. E. Manicke, J. M. Wiseman, D. R. Ifa and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2008, 19, 531–543 CrossRef CAS.
- C. L. Xie, J. A. Richardson, S. D. Turley and J. M. Dietschy, J. Lipid Res., 2006, 47, 953–963 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: rainbow color scale details, additional mass spectra and ion images of compounds in adrenal glands. See DOI: 10.1039/b919816d |
| This journal is © The Royal Society of Chemistry 2010 |
