Potassium-sensitive G-quadruplex DNA for sensitive visible potassium detection†
Xuan
Yang
ab,
Tao
Li
ab,
Bingling
Li
ab and
Erkang
Wang
*ab
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, P. R. China. E-mail: ekwang@ciac.jl.cn; Fax: +86-431-85689711; Tel: +86-431-85262003
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, P. R. China
First published on 10th November 2009
Abstract
A novel visual method for K+ detection is developed utilizing a K+-sensitive G-quadruplex DNA named PS5.M as the sensing element. In the absence of K+, PS5.M is in a random coil state and does not bind hemin. Upon addition of K+, PS5.M folds into the quadruplex structure stabilized by K+. Such quadruplex structure is able to bind hemin to form the hemin-G-quadruplex DNAzyme that catalyzes the H2O2-mediated oxidation of colorless 3,3′,5,5′-tetramethylbenzidine to the blue product. Under the optimal conditions, the color change can be clearly observed with the naked eye in the concentration range from 2 to 1000 μM. Through this facile approach, K+ can be detected down to 2 μM, with high selectivity against other cations. In view of its simplicity, sensitivity and specificity, our developed visual method for K+ detection would potentially be utilized for bioassays and K+-sensitive test paper.
Introduction
Potassium ion (K+) plays an important role in biological systems, and therefore the development of a simple and sensitive method to detect K+1–9 is very important. However, owing to the presence of sodium ion (Na+) and other cations, it is always a challenge to determine the concentration of K+ in physiological conditions. Towards this goal, several techniques have been developed for the detection of K+.1–11 Lately, there is increasing interest in the utilization of G-quadruplex DNAs as the sensing elements for K+ detection.2–11 G-quadruplexes are four-stranded structures formed by G-rich sequences.12 Such structures are generally stabilized by alkali metal cations, especially K+.11,13 This feature confers the selectivity of some G-quadruplex DNAs for K+.2,3,11,14 A number of K+ sensors have been developed utilizing G-quadruplex DNAs labeled with fluorescent or electrochemical indicators due to the selectivity.2–6,8,9 But most of these sensing processes required signal probe labeled or modified on the G-quadruplex DNAs, which makes the experiments relatively complex and expensive.DNAzymes are functional nucleic acid enzymes with wide applications in biocatalysis.15,16 Under certain conditions, some G-quadruplexes can bind hemin to form a kind of DNAzyme which will mimic catalytic activity like horseradish peroxidase (HRP).11,14,17,18 Until now, such DNAzymes have been widely applied in catalyzing reactions such as the H2O2-mediated oxidation of 2,2-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS)11,17–20 and luminol.21, 22 And various label-free sensors were fabricated based on these two reactions, using UV-visible spectrum and chemiluminescence, respectively.14,19,20,22,23–26 Just recently, 3,3′,5,5′-tetramethylbenzidine (TMB) was also investigated as the substrate of the hemin-G-quadruplex DNAzymes. It is proven that in the presence of the DNAzymes, H2O2-mediated oxidation of TMB could be sharply accelerated compared to the conditions without DNAzymes or with only hemin. Two oxidated products are continuously formed, one is a middle product with blue color and the other is an ultimately yellow product formed after using strong acids to end the reaction. The color change is very sensitive and easy to identify, which thus provides TMB the high potential in both DNAzyme based UV-visible spectrum and colorimetric sensors.27,28 Lately, using the TMB-H2O2 reaction system, our group successfully realized sensitive and selective colorimetric sensing for Hg2+.28 It was a good example importing TMB into DNAzyme based colorimetric sensors. However, such application of TMB is still in its initiative stage.
Herein, a visual DNAzyme based sensing system is further developed for K+, employing TMB as the signal indicator. Because DNA strands should fold into G-quadruplexes before binding with hemin, in several cases, the catalytic activity of DNAzymes may display a K+ dependent character.2,3,11–13 The design is based on this principle. As shown in Fig. 1, only in the presence of K+, effective DNAzymes would be formed and the TMB could be catalytically oxidated into the colored product. Just through this phenomenon, recognition of K+ is realized directly with the naked eye. To get a more sensitive and selective result, several affecting factors such as DNA strands, working solution pH, and concentrations of hemin and H2O2 are optimized, after which the color change can be clearly observed with the naked eye in the concentration range from 2 to 1000 μM. The lowest detection limit is 2μM. The sensing strategy is very simple. It avoids the commonly used experimental steps such as labeling and separation. It also provides a colorimetric sensing process in which complex instrumental measurements are released. Meanwhile, the applications of DNAzyme catalyzed TMB oxidation is further extended.
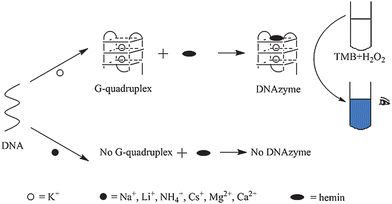 | ||
| Fig. 1 The facile colorimetric detection of K+ based on hemin-G-quadruplex DNAzyme. | ||
Experimental
Chemicals and solutions
The G-quadruplex DNAs, hemin, tris(hydroxymethyl)aminomethane (Tris) and 2-(N-morpholino)ethanesulfonic acid (MES) were obtained from Sangon Biotechnology Co., Ltd (Shanghai, China). Dimethylsulfoxide (DMSO) and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich Co (America). Hydrogen peroxide (H2O2) was from Shanghai Reagent (Shanghai, China). DNA stock solutions were prepared by dissolving DNA in TE buffer (10 mM Tris-HCl, 100 μM EDTA, pH = 8.00), and diluted to required concentrations before use. The buffer solution used for colorimetric assay was Tris-MES buffer (25 mM MES, pH = 5.10). The stock solutions of hemin (5 mM) and TMB (40 mM) were prepared in DMSO, stored in the dark at −20 °C, and diluted to the required concentration with aqueous buffer.Apparatus
Cary 500 UV/Vis Spectrophotometer (Varian, USA) was used to quantify the oligonucleotides.Sequences and pretreatment of DNAs
Six G-quadruplex DNAs (AG4: 5′-TGGGTAGGGCGGGTTGGGAAA-3′, PS5.M: 5′-GTGGGTCATTGTGGGTGGGTGTGG-3′, PS2.M: 5′-GTGGGTAGGGCGGGTTGG-3′, AGRO100: 5′-GGTGGTGGTGGTTGTGGTGGTGGTGG-3, T30695: 5′-GGGTGGGTGGGTGGGT-3′, HT-DNA: 5′-GGGTTAGGGTTAGGGTTAGGG-3′) were synthesized by Sangon Biotechnology Co., Ltd (Shanghai, China). Before use, these oligonucleotides were dissolved in the TE buffer (10 mM Tris-HCl, 100 μM EDTA, pH = 8.00) and quantified by using UV/Vis absorption spectroscopy in Cary 500 (Varian, USA) with the following extinction coefficients (ε260nm, M−1cm−1): A = 15400, G = 11500, C = 7400, T = 8700. The DNA solutions were then heated at 88 °C for 10 min, and gradually cooled to room temperature.Formation of DNAzymes
An equal volume of KCl solution (in water) was added to the DNA solutions (20 μM DNA, 10 mM Tris-HCl, 100 μM EDTA, pH = 8.00), allowing the DNA strands to form the G-quadruplex structure in 40 min. Then an equal volume of hemin (in DMSO) were dissolved to the above G-quadruplex solutions and kept for 2 h in room temperature to form the DNAzymes.Colorimetric assay protocol
The colorimetric assay protocol based on DNAzyme is shown in Fig. 1. In a typical experiment after optimization, the working solution of colorimetric assay is Tris-MES buffer (25 mM MES, pH = 5.10) which contains 296 μM TMB and 1.76 mM H2O2. Then, 180 μL working solution is added to 20 μL DNAzyme solution (constructed by PS5.M and hemin). The mixture is kept for 1.5 h at room temperature and different colors are observed with the naked eye owing to different concentrations of K+. The detection of K+ is based on the integral of different colors observed with the naked eye in 1.5 h after the addition of 180 μL working solution.Results and discussion
K+-sensitive G-quadruplex DNA
It is well known that most of G-quadruplex DNAs can be effectively stabilized by K+. However, only some K+-stabilized G-quadruplexes have the ability to bind with hemin to form the peroxidase-like DNAzymes. To achieve the sensitive and selective DNAzyme-based visual detection of K+, here we investigate six previously reported G-quadruplex-based DNAzymes in the TMB-H2O2 reaction system, to find the best candidate as the sensing element.11,14,17 The fundamental principle is to choose the DNA strand that brings the faintest blank response and the most obvious color discrimination in the presence of K+. Fig. 2 displays the selection of different oligonucleotides for K+. As shown, besides strand T30695, all the DNAzymes based on AG4, PS5.M, PS2.M, AGRO100, and HT-DNA can lead to obvious color differences between blank solutions and K+ containing solutions.11,14,17 But for DNAzymes with strands of AG4 and HT-DNA, both the blank solutions present resolvable blue, so such high blank response is not suitable for the detection of K+ with the naked eye. Among the DNAzymes based on strands of PS5.M, PS2.M, and AGRO100, the one with PS5.M displays the clearest and almost colorless blank solution. The solution including 50 μM K+ shows the deepest blue. Therefore, in terms of the color changes before and after addition of K+ in different cases, PS5.M is proven to be the most sensitive for K+ in the TMB-H2O2 reaction system and chosen as the sensing element for the visual detection of K+ in the following experiments. Note that here we consider that compared to the yellow products, blue products can be observed more sensitively by naked eyes. So we will not use strong acid to end the reaction. | ||
| Fig. 2 Characterizations of the DNAzyme functions of AG4, PS5.M, PS2.M, AGRO100, T30695 and HT-DNA in the presence of 0 μM and 50 μM K+ in the TMB-H2O2 system. Conditions: TMB, 266 μM in Tris-MES buffer (25 mM MES, pH = 5.10); H2O2, 794 μM; DNA, 500 nM; hemin, 500 nM. | ||
Optimization of the colorimetric assay conditions
Some important experimental parameters influencing the colorimetric assay, including the pH of the working solution and the concentrations of hemin and H2O2, are optimized. The influence of the working solution pH on the catalytic activity of hemin-PS5.M DNAzyme in the TMB-H2O2 reaction system is examined. As shown in Fig. S1,† in the investigational concentration range, the color change from blank to K+ containing solutions is most sensitive in the working solution at pH 5.10 among the solutions of different pH values. Therefore, Tris-MES buffer (25 mM MES, pH = 5.10) is chosen as the optimum pH in working solution for the colorimetric assay.The color change also depends on the concentration of hemin. As shown in Fig. S2,† under the condition of the optimum pH, the color change of the TMB-H2O2 system catalyzed by hemin-PS5.M DNAzyme in the presence of 500 nM hemin is distinct while other groups show inconspicuous changes. In the view of high sensitivity and good selectivity, the concentration of TMB is determined to be 500 nM for the following experiments.
Further experiments reveals that the concentration of H2O2 also has an influence on the catalytic activity of hemin-PS5.M DNAzyme in the TMB-H2O2 reaction system. As shown in Fig. S3,† under the condition of the optimum pH and concentration of hemin, the color change of the TMB-H2O2 catalyzed by hemin-PS5.M DNAzyme in the presence of 1.59 mM H2O2 is most obvious while other groups show non-significant color changes. Therefore, the optimized concentration of H2O2 is determined to be 1.59 mM for the colorimetric assay.
Sensitivity and selectivity of K+ detection
The analytic protocol based on the TMB-H2O2 reaction system catalyzed by the hemin-PS5.M DNAzyme is assessed by observing the color change upon the concentration of K+. Under the optimized conditions, the color change upon the concentration of K+ is shown in Fig. 3. The concentration of K+ is in the range from 2 to 1000 μM except for the blank solution (Fig. 3). As shown in Fig. 3, the blue color of the solution grows darker accompanied with the concentration increase of K+. Under the optimized conditions, the lowest detection limit of K+ is 2 μM. This sensitivity is higher than that of most of detection methods reported previously.2–10,29,30 In our previous work,11 we have utilized the G-quadruplex-based DNAzyme to sense K+ at the submicromolar level, with the aid of a UV/Vis spectrophotometer. In contrast, the visual method developed here does not need any special instrument, which makes it relatively simple, rapid and economical. Fig. 3 shows the UV-Vis absorption spectra of different concentrations of K+. As shown in Fig. 3, the values of the maximal absorption at 372 nm and 651 nm grow larger due to the increase of the concentrations of K+.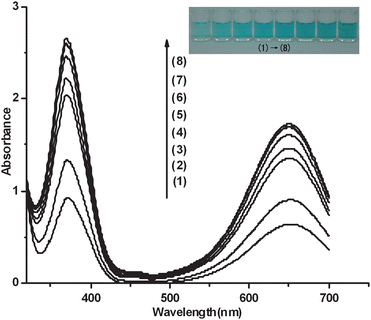 | ||
| Fig. 3 Investigation of the effects of different concentrations of K+ on the formation of hemin-PS5.M DNAzyme by using UV-Vis absorption spectroscopy. Conditions: (1) blank, (2) 2 μM K+, (3) 12.5 μM K+, (4) 25 μM K+, (5) 50 μM K+, (6) 100 μM K+, (7) 200 μM K+, (8) 1 mM K+; TMB, 266 μM in Tris-MES buffer (25 mM MES, pH = 5.10); H2O2, 1.59 mM; hemin, 500 nM; DNA, 500 nM. The inset is the photograph of the detection of K+ of utilizing the color change caused by the catalytic activity of hemin-PS5.M DNAzyme in the TMB-H2O2 reaction system. | ||
To test the specificity of sensing K+ with the utilization of hemin-PS5.M DNAzyme in the TMB-H2O2 system, other metal ions are used in place of K+ and furthermore they are mixed with K+. As shown in Fig. 4A, except for K+, all of the tested metal ions do not cause an observable change in the solution color, suggesting that only the K+-stabilized PS5.M has the superior DNAzyme function. We further investigated the potential influence of coexisting metal ions on K+ detection utilizing the hemin-PS5.M DNAzyme. It is found that most of other monovalent cations do not influence the DNAzyme-based detection of K+ (Fig. 4B). In contrast, there is a little interference from divalent cations such as Mg2+ and Ca2+, but this interference can be overcome by introduction of EDTA (Fig. 4C). These observations indicate the high selectivity of this visual method for K+ detection. Fig. 4D also shows the differences of UV-Vis absorption spectra between K+ and other cations. As shown in Fig. 4D, the values of the maximal absorption at 372 nm and 651 nm of K+ are the largest in these cations. Fig. 4E shows the absorption spectra of K+ and the mixture of K+ and other cations. As shown in Fig. 4E, the values of the maximal absorption at 372 nm and 651 nm decrease a lot after the addition of Mg2+ and Ca2+.
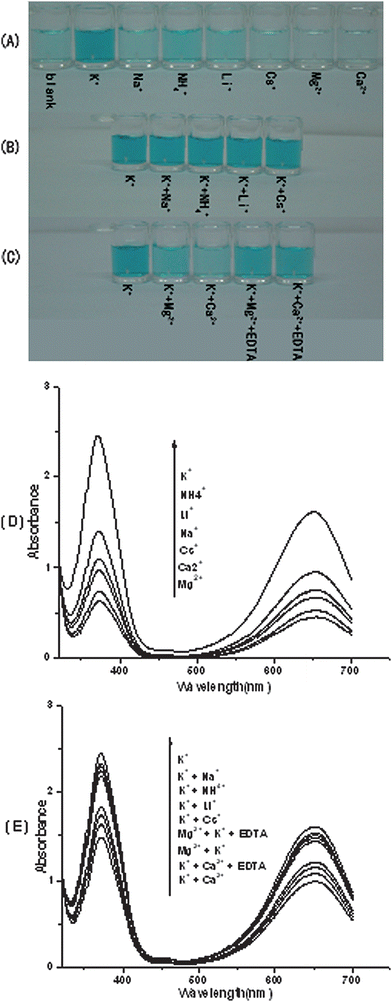 | ||
| Fig. 4 The selectivity of utilizing the hemin-PS5.M DNAzyme to sense aqueous K+. (A) Color responses of K+ and other metal ions, respectively. (B) Color responses of mixtures of K+ and other metal ions. (C) Color responses before and after EDTA was used to eliminate the interference from Mg2+ and Ca2+. (D) (E) Investigation of the effects of the mixture of K+ and other different cations on the formation of hemin-PS5.M DNAzyme by using UV-Vis absorption spectroscopy. In all of Fig. 4A, 4B, 4C, 4D, 4E, the concentration of K+ was 50 μM, and all the other tested metal ions were used at the concentration of 250 μM. TMB, 266 μM in Tris-MES buffer (25 mM MES, pH = 5.10); H2O2, 1.59 mM; hemin, 500 nM; DNA, 500 nM. | ||
In order to perform K+ assays in real samples, we employed hemin-PS5.M DNAzyme in the TMB-H2O2 system to detect human blood serum directly. We obtained a prominent visual photograph and a good UV-Vis absorption spectra, which are shown in Fig. 5.
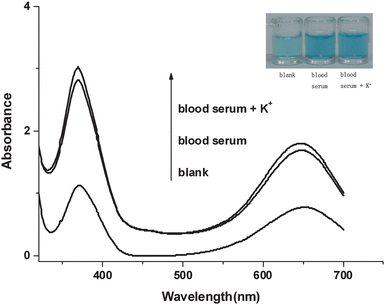 | ||
| Fig. 5 Absorption spectra of deionized water, human blood serum and human blood serum with the addition of 200 μM K+. Conditions: TMB, 266 μM in Tris-MES buffer (25 mM MES, pH = 5.10); H2O2, 1.59 mM; hemin, 500 nM; DNA, 500 nM. The inset is the photograph of deionized water and human blood serum. | ||
Conclusions
In conclusion, a simple, selective, and sensitive visual assay protocol for the detection of K+ with the naked eye has been established based on the catalytic activity of the hemin-PS5.M DNAzyme in the TMB-H2O2 reaction system. As to K+, a sensitive detection limit of 2 μM is obtained. Furthermore, this method has a specific selectivity for K+. Compared with the reported assays for the detection of K+, the present method has the following advantages: 1) it is a simple label-free visual method based on hemin-PS5.M DNAzyme and the concentration of K+ is determined with the naked eye, eliminating the need for any expensive instruments; 2) it extends the applications of DNAzyme based TMB oxidation; 3) it can be used in the detection of K+ in real samples.Acknowledgements
The work was supported by the National Natural Science Foundation of China with grant numbers of 20735003 and the 973 Project 2009CB930100 and 2010CB933601, Chinese Academy of Sciences KJCX2 YW HO9.References
- M. Teresa, S. R. Gomes, K. S. Tavares and J. A. Oliveira, Analyst, 2000, 125, 1983 RSC.
- H. Ueyama, M. Takagi and S. Takenaka, J. Am. Chem. Soc., 2002, 124, 14286 CrossRef CAS.
- F. He, Y. Tang, S. Wang, Y. Li and D. Zhu, J. Am. Chem. Soc., 2005, 127, 12343 CrossRef CAS.
- S. Nagatoishi, T. Nojima, B. Juskowiak and S. Takenaka, Angew. Chem., Int. Ed., 2005, 44, 5067 CrossRef CAS.
- S. Nagatoishi, T. Nojima, E. Galezowska, B. Juskowiak and S. Takenaka, ChemBioChem, 2006, 7, 1730 CrossRef CAS.
- A. E. Radi and C. K. O'Sullivan, Chem. Commun., 2006, 3432 RSC.
- L. H. Wang, X. F. Liu, X. F. Hu, S. P. Song and C. H. Fan, Chem. Commun., 2006, 3780 RSC.
- C. C. Huang and H. T. Chang, Chem. Commun., 2008, 1461 RSC.
- J. Lee, H. J. Kim and J. Kim, J. Am. Chem. Soc., 2008, 130, 5010 CrossRef CAS.
- Z. S. Wu, C. R. Chen, G. L. Shen and R. Q. Yu, Biomaterials, 2008, 29, 2689 CrossRef CAS.
- T. Li, E. Wang and S. Dong, Chem. Commun., 2009, 580 RSC.
- J. T. Davis, Angew. Chem., Int. Ed., 2004, 43, 668–698 CrossRef CAS.
- J. A. Walmsley and J. F. Burnett, Biochemistry, 1999, 38, 14063–14068 CrossRef CAS.
- T. Li, L. Shi, E. Wang and S. Dong, Chem.–Eur. J., 2009, 15, 1036 CrossRef CAS.
- R. R. Breaker, Nat. Biotechnol., 1997, 15, 427 CrossRef CAS.
- G. M. Emilsson and R. R. Breaker, Cell. Mol. Life Sci., 2002, 59, 596 CrossRef CAS.
- P. Travascio, Y. Li and D. Sen, Chem. Biol., 1998, 5, 505 CrossRef.
- P. Travascio, A. J. Bennet, D. Y. Wang and D. Sen, Chem. Biol., 1999, 6, 779 CrossRef.
- T. Li, E. Wang and S. Dong, Chem. Commun., 2008, 3654 RSC.
- T. Li, S. Dong and E. Wang, Chem. Commun., 2007, 4209 RSC.
- T. Li, Y. Du and E. Wang, Chem.–Asian J., 2008, 3, 1942 CrossRef CAS.
- T. Li, E. Wang and S. Dong, Chem. Commun., 2008, 5520 RSC.
- V. Pavlov, Y. Xiao, R. Gill, A. Dishon, M. Kotler and I. Willner, Anal. Chem., 2004, 76, 2152 CrossRef CAS.
- D. Li, B. Shlyahovsky, J. Elbaz and I. Willner, J. Am. Chem. Soc., 2007, 129, 5804 CrossRef CAS.
- B. Shlyahovsky, D. Li, E. Katz and I. Willner, Biosens. Bioelectron., 2007, 22, 2570 CrossRef CAS.
- J. Elbaz, B. Shlyahovsky and I. Willner, Chem. Commun., 2008, 1569 RSC.
- P. D. Josephy, T. Eling and R. P. Mason, J. Biol. Chem., 2008, 3, 1942.
- T. Li, B. Li, E. Wang and S. Dong, Chem. Commun., 2009, 3551 RSC.
- A. Thibon and V. Pierre, J. Am. Chem. Soc., 2009, 131, 434 CrossRef CAS.
- D. Kong, Y. Ma, J. Guo, Wei. Yang and H. Shen, Anal. Chem., 2678 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3. See DOI: 10.1039/b913036e |
| This journal is © The Royal Society of Chemistry 2010 |
