Development and validation of a multiplex bead assay for measuring growth mediators in wound fluid†
Thanasak
Rakmanee
ab,
Irwin
Olsen
*a,
Gareth S.
Griffiths
c and
Nikolaos
Donos
a
aEastman Dental Institute for Oral Health Care Sciences, Division of Biomaterials and Tissue Engineering, UCL-Eastman Dental Institute, 256 Gray's Inn Road, London, UK WC1X 8LD. E-mail: i.olsen@eastman.ucl.ac.uk; Fax: +44(0)207975 1254; Tel: +44(0)207915 1254
bFaculty of Dentistry, Thammasat University, Patumthani, Thailand
cSchool of Clinical Dentistry, University of Sheffield, Sheffield, UK
First published on 17th November 2009
Abstract
Large amounts of biological samples are usually required to measure multiple components by the enzyme-linked immunosorbant assay. However, the amounts of many tissue extracts and fluids, including gingival crevicular fluid (GCF), are generally extremely small. The aim of this study was, therefore, to develop and validate a novel multiplex bead assay (MBA) to simultaneously measure a profile of healing-related mediators in the GCF of treated periodontal wounds. An MBA was developed and validated by assessment of assay selectivity, recovery, precision and sensitivity, using eight recombinant human growth mediators as assay standards. GCF samples were collected on paper strips from healing wound (test) and healthy unaffected (control) sites of 15 patients with periodontitis, seven days post-periodontal surgery. Each GCF sample was eluted and the levels of the mediators measured using the MBA and antibody pairs specific for angiopoietin-1, vascular endothelial growth-factor, bone morphogenetic protein-2, osteoprotegerin, tissue inhibitor of metalloprotease-1 (TIMP-1), basic fibroblast growth-factor, keratinocyte growth-factor, and platelet derived growth-factor. Less than 1.8% of cross-reactivity was observed between antibodies and the eight different analytes, for which the recovery was more than 85%. Mean intra- and inter-assay precision were within the acceptance criteria of 20% and 25%, respectively. Detection of all mediators was highly sensitive (≤70 ng/L) except for TIMP-1 (215 ng/L). Angiogenic factors were the most highly secreted in the GCF seven days post-surgery. This new MBA can simultaneously measure small amounts of eight different growth mediators in the GCF of healing periodontal wounds. It might also be a valuable tool for evaluating the components of wound fluids as a prognostic indicator of the success of therapeutic intervention.
Introduction
Wound healing involves several interrelated stages of coagulation, inflammation, angiogenesis, re-epithelialisation (in some tissues) and remodelling. Many cytokines and growth factors (GF) have been identified and their functions in tissue destruction and in wound healing studied in vitro and in vivo.1–3 These polypeptide mediators are secreted from cells and exert their biological activity by binding to their corresponding transmembrane receptors and initiating an intracellular cascade of molecular signalling events which ultimately activate specific target genes.4 Moreover, the presence of such mediators in body fluids is generally considered to reflect the activity of the underlying tissue, a number of studies reporting the close association between GF in skin drainage fluid,5 nasal fluid6 and periodontal fluid (gingival crevicular fluid; GCF)7 and the wound healing process.3GF are pivotal in mediating periodontal wound healing and regeneration and their effects on periodontal cells in vitro and in vivo have been extensively investigated. For example, platelet derived growth-factor (PDGF) was found to promote regeneration of both hard and soft periodontal tissues,8 while keratinocyte growth-factor (KGF) has a major role in re-epithelialisation,9 including the formation of the long-junctional gingival epithelium.10 Basic fibroblast growth-factor (bFGF), vascular endothelial growth-factor (VEGF) and angiopoietin-1 (Ang-1) play important parts in vascularisation/angiogenesis during the early phase of wound healing,11–13 while bone morphogenetic protein-2 (BMP-2) has been shown to recruit undifferentiated mesenchymal cells and induce bone formation14 and cementogenesis in vivo.15 Osteoprotegerin (OPG) has a major role in bone deposition and bone remodelling,16 and tissue inhibitor of metalloprotease-1 (TIMP-1) is associated with the turnover of extracellular matrix components as well as with cell migration and angiogenesis.17–19
Studies of the molecular events which accompany the healing of damaged tissue in vivo have, however, been limited by the very small amounts of wound fluid which can generally be obtained. Thus, the majority of such studies have examined only one mediator in each situation, usually by enzyme-linked immunosorbant assay (ELISA). Although this technique is relatively specific and sensitive, it precludes the systematic examination of the profile of multiple factors in a small sample of biological material. While a ‘sequential’ ELISA could possibly overcome some of these constraints,20 it is time-consuming, impractical for routine analysis and not cost-effective, therefore there is a need for a procedure which is able to rapidly and accurately determine the profile of multiple components in small amounts of biological fluid.
Multiplex bead technology utilising flow cytometry (FCM) enables several analytes to be measured simultaneously in a small volume of biological material.21 This technique has been used for the assay of cytokines, chemokines and other inflammatory markers, and a number of multiplex panels are now commercially available. However, a multiplex bead assay (MBA) has hitherto not been applied to the measurement of multiple GF in wound fluids. Additionally, the MBA has usually utilised dual-laser FCM, which is not readily available. The present study reports, for the first time, an MBA for simultaneously measuring growth-associated mediators in wound fluids using single-laser FCM (BD FACScan®, BD Biosciences). A Tyramide Signal Amplification (TSA) procedure22 has also been utilised which, in conjunction with the MBA, significantly increases the assay sensitivity for measuring the following eight mediators in the GCF of healing periodontal wounds: Ang-1, VEGF, BMP-2, OPG, TIMP-1, bFGF, KGF, and PDGF.
Materials and methods
Reagents
The recombinant human (rh) proteins and antibodies (Ab) used in the present study are shown in Supplemental Data Table 1.† Carboxylated fluorescent beads (7.6 µm) (excitation 488 nm; emission 635 nm), which comprise a set of eight fluorescent beads each with a different intensity of the same colour fluorescence, and non-fluorescent beads pre-coated with bovine serum albumin (BSA) (0.8 µm, ‘carrier beads’) were obtained from Spherotech Inc. Streptavidin-AlexaFluor488® was used as ‘reporter’ fluorescence (excitation 495 nm; emission 519 nm) (Molecular Probes™). The TSA™ Biotin System kit was obtained from PerkinElmer™, Streptavidin-HRP from R&D Systems, 1-Ethyl-3-(3 dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) from Pierce Biotechnology, 2-(N-morpholino) ethanesulfonic acid (MES), phosphate-buffered saline (PBS), BSA, Tween20 and sodium azide (NaN3) from Sigma-Aldrich.Collection and elution of GCF samples
GCF samples were collected from 15 subjects (age 32 ± 7; 9 males) seven days after periodontal surgery. The subjects were diagnosed with advance periodontitis23 and periodontal surgery was scheduled as part of their corrective treatment. Informed consent was obtained from all participants and the collection of the GCF was reviewed and approved by the Eastman/UCLH Joint Research Ethics Committee, London, UK (study reference: 04/Q0512/93).For each subject, the test GCF was collected from the sites undergoing periodontal surgery (four samples) while the control GCF was collected from healthy unaffected sites (four samples), using an intracrevicular method with pre-cut filter strips as previously described.24 The volume of GCF collected was measured, the strips pooled according to the group category, transferred to plastic micro-centrifuge tubes (0.4 mL) (Alpha Laboratories) and stored at −70°C until elution using a previously described technique.25
Antibody-bead conjugation
‘Capture’ Ab corresponding to each of the eight mediators were conjugated to the beads using a modified two-step protocol,26 with all incubation processes carried out at room temperature (RT) in the dark. 2 × 106 Beads were washed by centrifugation (MicroCentaur centrifuge) at 13000 rpm for 4 min, the supernatant removed and the beads resuspended in 0.2 mL of distilled water, centrifuged again and resuspended in 40 µl of 50 mmol/L MES buffer (pH 5.5). Freshly prepared sulfo-NHS in sterile water (30 µl; 100 g/L) was added, then fresh EDC in sterile water (30 µl; 100 g/L). The suspension was mixed by vortex (IKA Works), gently shaken for 20 min, washed 3 times with 0.1 mL of the MES buffer and centrifuged again. To determine the optimal conjugation efficacy, 10, 20, 50 and 100 µg of the ‘capture’ Ab in 0.2 mL of MES buffer were incubated with the beads for either 2 h or overnight, with end-to-end rotation (Jencons Scientific). After incubation the beads were washed 3 times with wash buffer (PBS containing 0.5 mL/L Tween 20) and resuspended in 0.2 mL of PBS buffer containing 10 g/L BSA, 0.5 mL/L Tween 20 and 0.5 g/L NaN3, then stored in the dark at 4 °C. The relative level of ‘capture’ Ab binding to the beads was assessed by incubating for 1 h with goat anti-mouse Ab conjugated with AlexaFluor488 (1:500 dilution in PBS containing 10 g/L BSA). The beads were then washed and analysed by FCM.MBA protocol for GCF analysis
A schematic illustration of the MBA of the eight mediators is shown in the Supplemental Data.† The MBA was carried out in 0.2 mL polypropylene microcentrifuge tubes (Alpha Laboratories) in the dark at RT, with end-to-end rotation. Following all incubation processes, the beads were washed three times by the addition of 0.1 mL of wash buffer and centrifuged at 13000 rpm for 4 min. Vortex shaking and 1 min of sonication (Elma GmbH & Co.) were used to re-suspend the pellets following centrifugation.Mixtures of the Ab-coated beads were incubated overnight with 0.1 mL of the eluted GCF samples and also with 0.1 mL of a mixture of known concentrations of the eight mediators used as standard samples for calibration (2-fold dilution series of six concentrations of the rh antigens, excluding zero, starting with 10 µg/L of TIMP-1, 4 µg/L of bFGF and 2 µg/L of each of the other mediators). After incubation, carrier beads were added to each sample and washed as above. The beads were re-suspended and incubated for 30 min in 0.5% TSA blocking buffer, centrifuged, washed and incubated for 1.5 h with a mixture of the eight (biotinylated) detection Ab (at the pre-determined optimal concentrations ranging from 45–250 µg/L). To evaluate the use of TSA in the MBA, dilutions (0, 125, 250, 500, 1000 ng/L) of a mixture of the 8 rh antigens were prepared in diluent buffer, in duplicate, and the procedure carried out with and without the addition of TSA. In the MBA without TSA, the beads were incubated for 45 min with 0.1 mL of Streptavidin-AlexaFluor488 (1:1000 dilution in diluent buffer), washed and analysed by FCM. In the MBA with the addition of TSA, the beads were resuspended in 0.1 mL of Streptavidin-HRP (1:50 dilution in the TSA blocking buffer) and incubated for 45 min, washed again as above and incubated for 10 min in 0.05 mL of TSA reagent (1:50 dilution in the amplification buffer). After washing, the beads were incubated for 45 min in 0.1 mL of Streptavidin-AlexaFluor488 (1:1000 dilution in diluent buffer). Finally, the beads were washed and resuspended in 0.1 mL of distilled water and analysed by FCM. CellQuest™ (v 3.3) and BD™ Cytometric Bead Array (CBA) software (BD Biosciences) were used for data acquisition and data analysis, respectively. The absolute amount of the analytes (ng) was determined using the standard curve obtained for each antigen, carried out at the same time. A 4-parameter logistic model of the concentration-response relationship was used for fitting the curve, as previously recommended.27 Because each GCF was eluted in a total volume of 0.1 mL, the absolute amount was calculated by dividing the outcome from the standard curve (ng/L) by 10−4 and expressed as ng/site.
Validation of the bead assay
The MBA was validated according to the guidelines of the US Food and Drug Administration (FDA),28 which included assessment of assay selectivity, precision, recovery and sensitivity, as follows.Selectivity is the ability of the assay to distinguish a specific analyte in the presence of other components in the samples.28 To assess selectivity, a mixture of eight Ab-coated beads was added to 0.1 mL of a high concentration of each of the eight mediators individually (10 µg/L of TIMP-1, 8 µg/L of bFGF and 5 µg/L of each of the other mediators, in diluent buffer) and in duplicate, and the MBA carried out as above. The control was the diluent buffer alone. Selectivity of individual mediators was also evaluated by diluting each of the eight mediators at the above concentration in 10% normal human serum (NHS) (pooled from six healthy donors), which is equivalent to the final 10% concentration of the eluted GCF samples.29
Precision is the closeness of measurements when the procedure is repeated.28 To evaluate precision, the eight mediators were mixed at three concentrations (five replicates each), including the concentrations at the lower limit of quantification (LLOQ), the approximate midrange of the calibration curve and the upper limit of quantification (ULOQ). To be accepted as the lowest and highest concentrations on the standard curve, the LLOQ and ULOQ need to meet the following criteria: (1) the response (median fluorescence intensity, MFI) at the anticipated LLOQ is ≥5 times higher than the MFI of the blank (diluent buffer alone); and (2) the response should be reproducible with a mean coefficient of variation (CV) of <20%, evaluated independently using ten mixtures of the eight mediators at the anticipated LLOQ.28 The CV is defined as the ratio of the standard deviation (SD) to the mean ((SD/mean) × 100). The ULOQ is selected as the highest concentration of the calibration curve and evaluated using the same protocol as for LLOQ. The analysis of intra- and inter-assay precision was carried out three times on three separate occasions, with the acceptance criteria being a mean CV of <20% and <25% for intra-assay and inter-assay precision, respectively.27
Recovery was analysed using three solutions, each containing low, medium or high concentrations of the eight mediators (1, 3 and 10 µg/L) diluted in the 10% pooled NHS.28 Triplicate, 5 µL samples of each of the three mixtures were placed onto the paper strips separately, and the elution process carried out as above.25 The control sample was each of the three mixtures without paper strips and elution.
Assay sensitivity is defined by the limit of detection (LOD) (lowest concentration of analyte for which the response (MFI) can be reliably distinguished from the background noise),28 in which ten blank samples (diluent buffer alone) were incubated with a mixture of the eight Ab-coated beads and the MBA carried out as above. Standard curves of the eight individual mediators were generated using increasing concentrations of each mediator, as noted above. The mean and SD of the MFI of the blank for each bead type were calculated and 3 × SD was added to the mean. The resulting MFI with 3 × SD was then interpolated with the standard curve and expressed as ng/L, as previously described.27
Statistical analysis
SPSS software (v14.0; SPSS Inc.) was used for statistical comparison, the LOD values of each of the mediators being used instead of a zero value when the mediator could not be detected by the MBA. In order to limit the amount of data included in this study, analysis was not carried out for the concentration of the mediators but only for GCF volumes and for the absolute amounts using the paired-t test and the Wilcoxon Signed Rank Test, respectively.Results
Assay development
The effect of concentration of the ‘capture’ mAb on the level of Ab-bead conjugation was found to vary, as measured using goat anti-mouse Alexa488, with 100 µg of ‘capture’ Ab yielding the highest levels of Ab-bead binding for VEGF, BMP-2, TIMP-1 and KGF and 50 µg being optimal for Ang-1, OPG, bFGF and PDGF. 24 h of incubation of the ‘capture’ mAb with the beads generated 5–8 fold greater conjugation than 2 h (data not shown). In addition, the TSA was found to substantially increase the detection of all of the individual mediators with little change in background (blank sample) (Figure 1). At the highest concentration (1000 ng/L), the presence of the TSA caused an 11-fold increase in the MFI of BMP-2 and PDGF, compared with the control. At the same concentrations, the MFI signals for TIMP-1, OPG, KGF and bFGF in the test group were found to increase 5–7 fold, and there was a 3–4 fold signal increase for Ang-1 and VEGF.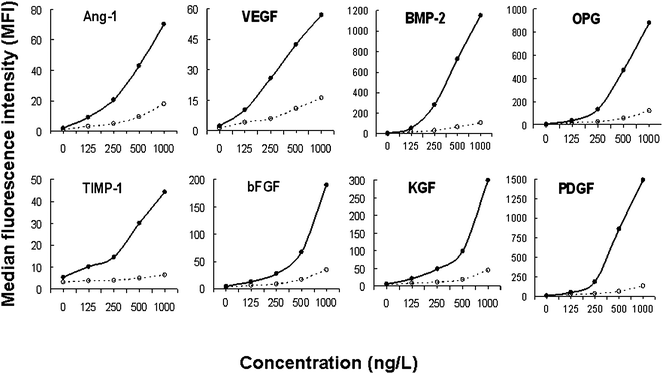 | ||
Fig. 1 Effect of Tyramide Signal Amplification (TSA) on assay sensitivity The curves show the MFI of the MBA carried out with (•![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) •) and without (∘ •) and without (∘![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ∘) the TSA, using increasing concentrations of the eight mediators. ∘) the TSA, using increasing concentrations of the eight mediators. | ||
Validation of the MBA
Analysis of the assay selectivity showed that there was only a very low level of interaction between each specific mediator and the Ab to the other seven mediators (≤1.8%), when using a high concentration of each mediator (Table 1). Evaluation of selectivity in the presence of 10% NHS showed that there was little effect on any of the mediators, despite relatively high levels of endogenous Ang-1 and TIMP-1 in the NHS (data not shown).| Selectivity (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target antigens | Concentration (ng/L) | Corresponding Ab | |||||||
| Ang-1 | VEGF | BMP-2 | OPG | TIMP-1 | bFGF | KGF | PDGF | ||
| a Selectivity of 100% is defined as the median fluorescence intensity (MFI) of each target antigen with its own specific corresponding Ab. | |||||||||
| Ang-1 | 5000 | 100.0 | 0.3 | 0.1 | 0.0 | 0.2 | 0.2 | 0.3 | 0.0 |
| VEGF | 5000 | 1.3 | 100.0 | 0.4 | 0.2 | 1.0 | 1.0 | 0.6 | 0.2 |
| BMP-2 | 5000 | 1.2 | 1.8 | 100.0 | 0.3 | 1.0 | 0.8 | 0.8 | 0.2 |
| OPG | 5000 | 1.7 | 1.8 | 0.5 | 100.0 | 1.4 | 1.0 | 0.8 | 0.2 |
| TIMP-1 | 10000 | 0.6 | 0.3 | 0.2 | 0.1 | 100.0 | 0.4 | 0.9 | 0.1 |
| bFGF | 8000 | 0.8 | 0.8 | 0.1 | 0.1 | 0.6 | 100.0 | 0.6 | 0.1 |
| KGF | 5000 | 1.7 | 0.9 | 0.3 | 0.3 | 1.0 | 0.2 | 100.0 | 0.2 |
| PDGF-AB | 5000 | 0.8 | 0.5 | 0.1 | 0.1 | 0.7 | 0.5 | 0.7 | 100.0 |
Analysis of the intra-assay precision showed that the mean CV of each of the mediators was <20%, thus fulfilling the precision acceptance criteria (Table 2). However, while the mean CV inter-assay of TIMP-1, bFGF and PDGF also fulfilled this criteria, Ang-1, VEGF, BMP-2, OPG and KGF were slightly elevated, but never greater than 25% (Table 2, highlighted in bold).
| Sample | Ang-1 | VEGF | BMP-2 | OPG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | CV | Concentration | CV | Concentration | CV | Concentration | CV | |||||
| ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | |
| a The results show the coefficient of variation (CV) of the intra- and inter-assay for each validation sample and the mean CV of the three concentrations. CV is defined as the ratio of the standard deviation (SD) to the mean (SD/mean) × 100). | ||||||||||||
| LLOQ | 125 | 9.3 | 16.8 | 200 | 9.8 | 20.1 | 62 | 19.3 | 25.5 | 62 | 15.2 | 21.7 |
| Midrange | 500 | 12.2 | 19.5 | 500 | 14.7 | 22.0 | 500 | 13.5 | 26.3 | 500 | 12.5 | 25.2 |
| ULOQ | 2000 | 15.4 | 27.3 | 2000 | 12.9 | 27.8 | 2000 | 14.0 | 22.2 | 2000 | 10.6 | 24.6 |
| MEAN | 12.3 | 21.2 | 12.4 | 23.3 | 15.6 | 24.7 | 12.8 | 23.8 | ||||
| Sample | TIMP-1 | bFGF | KGF | PDGF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | CV | Concentration | CV | Concentration | CV | Concentration | CV | |||||
| ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | ng/L | Intra- | Inter- | |
| LLOQ | 1250 | 7.1 | 10.0 | 400 | 8.1 | 10.5 | 200 | 11.4 | 21.3 | 100 | 9.7 | 24.0 |
| Midrange | 2500 | 14.2 | 17.2 | 1000 | 13.5 | 21.8 | 625 | 18.6 | 24.2 | 500 | 12.4 | 13.4 |
| ULOQ | 10000 | 14.4 | 28.1 | 4000 | 14.8 | 27.5 | 2000 | 14.0 | 20.3 | 2000 | 15.3 | 19.3 |
| MEAN | 11.9 | 18.4 | 12.1 | 19.9 | 14.7 | 21.9 | 13.6 | 18.9 | ||||
Following the elution process, at least 83.4% was recovered when using the lowest concentration (1 µg/L) and >93.5% recovered when using the highest concentration (10 µg/L). Overall, the average analyte recovery was approximately 97.2% (ranging from 78.3–116.5%), with no indication that any particular mediator was differentially affected (Table 3).
| Concentration (µg/L) | Recovery (%)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Ang-1 | VEGF | BMP-2 | OPG | TIMP-1 | bFGF | KGF | PDGF | |
| a % recovery is the MFI of the sample after elution compared with that of the mediator analysed directly without elution, defined as 100%. | ||||||||
| 1 | 83.4 | 101.3 | 98.9 | 84.0 | 99.8 | 111.0 | 88.8 | 91.0 |
| 3 | 91.6 | 107.8 | 93.8 | 78.3 | 102.1 | 111.5 | 79.8 | 85.0 |
| 10 | 116.5 | 99.9 | 97.7 | 94.0 | 103.5 | 113.9 | 93.5 | 102.9 |
| MEAN | 97.2 | 103.0 | 96.8 | 85.5 | 101.8 | 112.1 | 87.9 | 93.0 |
Measurement of the assay sensitivity showed that most of the mediators could be detected at relatively low concentrations: Ang-1, 12.8 ng/L; VEGF, 31.5 ng/L; BMP-2, 20.1 ng/L; OPG, 17.9 ng/L; bFGF, 69.1 ng/L; KGF, 6.8 ng/L, PDGF, 34.2 ng/L, although at least 214.9 ng/L of TIMP-1 was the minimum required for detection by the MBA.
Analysis of GCF samples
The GCF test group was found to have significantly higher volumes than the control (6.7 ± 3.9 µL and 1.7 ± 0.8 µL, respectively) (p < 0.05). The total of 30 GCF (15 test and 15 control) were eluted and analysed by the validated MBA. VEGF was detected in all GCF samples whereas Ang-1, OPG and bFGF were detected in 28 of the 30 samples. While BMP-2 and TIMP-1 were detected in all of the test GCF, these two mediators were only detected in 6 and 7 of the 15 control GCF, respectively. KGF and PDGF were only detected in 6 and 5 of the 15 test GCF, respectively. Since only very small volumes of these GCF were obtained, it was not possible to repeat the assay of any samples or to compare these GCF results with an ELISA assay. However, in experiments not shown here, replicate samples of the standard recombinant growth mediators tested by the MBA and ELISA showed comparable assay accuracy and sensitivity. The results in Table 4 show that the amounts of all the mediators in the test GCF were higher than those in the control GCF; however, significant differences were observed only for Ang-1, VEGF, OPG and TIMP-1 (p < 0.05). Within the GCF groups, the amounts of Ang-1, VEGF, bFGF and TIMP-1 were found to be greater than BMP-2, OPG, KGF and PDGF (Table 4).| Samples | Total amount of mediators in GCF (ng/site) (× 10−3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ang-1 | VEGF | BMP-2 | OPG | TIMP-1 | bFGF | KGF | PDGF | |
| a The results show the median amounts (ng) of the analytes obtained from 15 subjects at the test and control sites with the 25th and 75th percentiles [in brackets]. The differences in amounts of the mediators between the test and control groups in each subject were averaged. b Significant differences between the test and control sites (p < 0.05). | ||||||||
| Test | 338.9 [198.5, 479.0] | 344.4 [266.7, 422.0] | 25.8 [13.9, 37.8] | 33.9 [13.5, 54.4] | 2243.1 [830.4, 3655.8] | 226.9 [126.4, 327.5] | 84.2 [54.3, 114.5] | 127.2 [59.8, 194.6] |
| Control | 130.8 [63.0, 198.6] | 140.5 [98.4, 182.6.0] | 20.8 [17.0, 24.6] | 12.2 [7.8, 16.6] | 162.8 [10.7, 314.8] | 164.5 [113.5, 215.1] | 12.8 [8.6, 17.0] | 57.3 [25.0, 89.7] |
| Average differences | 208.0 [91.1, 324.7] | 203.8 [130.6, 277.0] | 5.0 [-5.4, 15.8] | 21.7 [2.7, 40.7] | 2080.3 [848.0, 3312.7] | 62.4 [-17.1, 141.9] | 71.4 [40.9, 101.9] | 69.9 [-10.5, 150.3] |
Discussion
The present study has developed and validated the MBA for simultaneously measuring the profile of selected growth mediators in very small volumes of wound fluids. For each analyte it was found, in experiments not shown here, that each new Ab batch required an individual validation. In addition, the choice of buffer was found to have a major effect on the assay, with the MES buffer providing the best coupling efficiency.30The TSA procedure, which was found to be rapid, simple and reproducible, markedly improved the sensitivity of the MBA. One concern of using the TSA is that it may generate non-specific signals due to diffusion of the radicalised biotin-tyramine that can react non-specifically and thus increase the assay background.22 However, in experiments not shown here, the use of an optimal concentration of the TSA was able to eliminate this difficulty. In addition, the TSA procedure also allowed low concentrations of the detection Ab to be used, compared with the ELISA.
Selectivity of the MBA for ‘specific’ target Ag is essential because biological samples are usually obtained without extraction, precipitation or further purification and are thus likely to contain multiple components which might interfere. Such endogenous analytes could complicate assay development and validation because calibration curves are generally obtained using standard solutions which do not contain other ‘contaminating’ and possibly cross-reacting factors.27 The present study showed, however, that even in the presence of relatively large amounts of endogenous Ang-1 and TIMP-1 in NHS samples, the MBA was highly selective, with only a maximum of 1.8% cross-reaction between reagents.
The FDA draft guidance acceptance criteria for assay precision is set at a CV of 20%.28 However, while this may be appropriate for validation of chromatographic assays27 which depend on the chemical and/or physicochemical properties of the molecules, it may not be fully suitable for validation of immunoassays27 which rely on the binding between the analyte (Ag) and Ab and endpoint detection system.31 It has therefore been recommended that a CV of ≤ 25% for assay precision of immunological methods is acceptable,27 and in the present study this has been shown to be met for both intra- and inter-assays.
Measurement of LOD showed that most of the mediators, except for TIMP-1, could be detected by the MBA at relatively low concentrations. Analysis of the GCF samples showed that the MBA sensitivity was sufficient to measure most of the mediators in the GCF except for KGF and PDGF, which could be detected in only half of the test samples. It is not yet known why these two mediators were not detected by the MBA in all samples, since other studies have shown them to be present in the GCF of gingival overgrowth patients32,33 and following periodontal surgery,34,35 respectively, as measured by conventional ELISA assay. The reason for this discrepancy may be due to the actual differences between the different GCF samples (e.g., timing of sample collection in relation to different stages of the wound healing process and hence different levels of growth mediators in the wound fluid). It is notable that the amounts of the angiogenic factors Ang-1, VEGF, bFGF and especially TIMP-1 were found here to be present at high levels in the test GCF, consistent with previous reports of high levels of VEGF in skin wound fluid at 7 days and of bFGF and PDGF at earlier time periods.36–38 However, such analyses require relatively large amounts of the wound fluid, in contrast to the very small volumes of GCF used here for measuring a profile of the eight growth mediators simultaneously.
In conclusion, wound healing is a complex process involving several types of cell and a number of growth mediators. The present study has developed and validated an MBA for simultaneously measuring, for the first time, the profile of mediators in a very small volume of wound fluid using single laser FCM. The TSA procedure was found to substantially increase the assay sensitivity and demonstrated that the amounts of all of the mediators were elevated in periodontal wound fluids.
Acknowledgements
This work was in part supported by a scholarship of the Royal Thai Government and the Periodontology Unit, UCL-Eastman, Research Discretionary Account.References
- L. Hakkinen, V. J. Uitto and H. Larjava, Cell biology of gingival wound healing, Periodontology 2000, 2000, 24, 127–152 CrossRef CAS.
- P. Martin, Wound healing–aiming for perfect skin regeneration, Science, 1997, 276, 75–81 CrossRef CAS.
- W. V. Giannobile, Periodontal tissue engineering by growth factors, Bone, 1996, 19, 23S–37S CrossRef CAS.
- K. T. Tran, L. Griffith and A. Wells, Extracellular matrix signaling through growth factor receptors during wound healing, Wound Repair Regener., 2004, 12, 262–268 Search PubMed.
- E. A. Baker, G. S. El, D. G. Aitken and D. J. Leaper, Growth factor profiles in intraperitoneal drainage fluid following colorectal surgery: relationship to wound healing and surgery, Wound Repair Regener., 2003, 11, 261–267 Search PubMed.
- J. B. Watelet, P. Gevaert, C. Bachert, G. Holtappels and C. P. Van, Secretion of TGF-betal, TGF-beta2, EGF and PDGF into nasal fluid after sinus surgery, European Archives of Oto-Rhino-Laryngology, 2002, 259, 234–238 CrossRef.
- J. W. Cooke, D. P. Sarment, L. A. Whitesman, S. E. Miller, Q. Jin, S. E. Lynch and W. V. Giannobile, Effect of rhPDGF-BB delivery on mediators of periodontal wound repair, Tissue Eng., 2006, 12, 1441–1450 CrossRef CAS.
- M. Nevins, M. Camelo, M. L. Nevins, R. K. Schenk and S. E. Lynch, Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone, J. Periodontol., 2003, 74, 1282–1292 CrossRef CAS.
- S. Werner, Keratinocyte growth factor: a unique player in epithelial repair processes, Cytokine Growth Factor Rev., 1998, 9, 153–165 CrossRef CAS.
- M. Li, J. D. Firth and E. E. Putnins, Keratinocyte growth factor-1 expression in healthy and diseased human periodontal tissues, J. Periodontal Res., 2005, 40, 118–128 CrossRef CAS.
- U. Fiedler and H. G. Augustin, Angiopoietins: a link between angiogenesis and inflammation, Trends Immunol., 2006, 27, 552–558 CrossRef CAS.
- N. Ferrara, H. P. Gerber and J. LeCouter, The biology of VEGF and its receptors, Nat. Med., 2003, 9, 669–676 CrossRef CAS.
- S. Javerzat, P. Auguste and A. Bikfalvi, The role of fibroblast growth factors in vascular development, Trends Mol. Med., 2002, 8, 483–489 CrossRef CAS.
- E. A. Wang, V. Rosen, J. S. D'Alessandro, M. Bauduy, P. Cordes, T. Haradaet al., Recombinant human bone morphogenetic protein induces bone formation, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 2220–2224 Search PubMed.
- G. N. King, N. King, A. T. Cruchley, J. M. Wozney and F. J. Hughes, Recombinant human bone morphogenetic protein-2 promotes wound healing in rat periodontal fenestration defects, J. Dent. Res., 1997, 76, 1460–1470 CrossRef CAS.
- L. C. Hofbauer and A. E. Heufelder, Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology, J. Mol. Med., 2001, 79, 243–253 CrossRef CAS.
- E. Dasse, L. Bridoux, T. Baranek, E. Lambert, S. Salesse, M. L. Sowaet al., Tissue inhibitor of metalloproteinase-1 promotes hematopoietic differentiation via caspase-3 upstream the MEKK1/MEK6/p38alpha pathway, Leukemia, 2007, 21, 595–603 Search PubMed.
- R. Chirco, X. W. Liu, K. K. Jung and H. R. Kim, Novel functions of TIMPs in cell signaling, Cancer Metastasis Rev., 2006, 25, 99–113 CrossRef CAS.
- T. Hayakawa, K. Yamashita, K. Tanzawa, E. Uchijima and K. Iwata, Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum, FEBS Lett., 1992, 298, 29–32 CrossRef CAS.
- M. J. Steffen and J. L. Ebersole, Sequential ELISA for cytokine levels in limited volumes of biological fluids, Biotechniques, 1996, 21, 504–509 CAS.
- E. B. Cook, J. L. Stahl, L. Lowe, R. Chen, E. Morgan, J. Wilsonet al., Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics, J. Immunol. Methods, 2001, 254, 109–118 Search PubMed.
- M. N. Bobrow, T. D. Harris, K. J. Shaughnessy and G. J. Litt, Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays, J. Immunol. Methods, 1989, 125, 279–285 CrossRef CAS.
- G. C. Armitage, Development of a classification system for periodontal diseases and conditions, Annals of Periodontology, 1999, 4, 1–6 Search PubMed.
- G. S. Griffiths, J. M. Wilton and M. A. Curtis, Contamination of human gingival crevicular fluid by plaque and saliva, Arch. Oral Biol., 1992, 37, 559–564 CrossRef CAS.
- G. S. Griffiths, M. A. Curtis and J. M. Wilton, Selection of a filter paper with optimum properties for the collection of gingival crevicular fluid, J. Periodontal Res., 1988, 23, 33–38 CrossRef CAS.
- D. Sehgal and I. K. Vijay, A method for the high efficiency of water-soluble carbodiimide-mediated amidation, Anal. Biochem., 1994, 218, 87–91 CrossRef CAS.
- J. W. Findlay, W. C. Smith, J. W. Lee, G. D. Nordblom, I. Das, B. S. DeSilvaet al., Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective, J. Pharm. Biomed. Anal., 2000, 21, 1249–1273 Search PubMed.
- US Food and Drug Administration Guidance for Industry Bioanalytical Method Validation http://www.fda.gov/cder/guidance. 26-7-2008. Ref Type: Electronic Citation.
- I. L. Chapple, G. Landini, G. S. Griffiths, N. C. Patel and R. S. Ward, Calibration of the Periotron 8000 and 6000 by polynomial regression, J. Periodontal Res., 1999, 34, 79–86 CrossRef CAS.
- J. R. Moffett, M. A. Namboodiri and J. H. Neale, Enhanced carbodiimide fixation for immunohistochemistry: application to the comparative distributions of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat brain, J. Histochem. Cytochem., 1993, 41, 559–570 CAS.
- S. Braggio, R. J. Barnaby, P. Grossi and M. Cugola, A strategy for validation of bioanalytical methods, J. Pharm. Biomed. Anal., 1996, 14, 375–388 CrossRef CAS.
- S. J. Das, M. H. Parkar and I. Olsen, Upregulation of keratinocyte growth factor in cyclosporin A-induced gingival overgrowth, J. Periodontol., 2001, 72, 745–752 CrossRef CAS.
- S. J. Das, H. N. Newman and I. Olsen, Keratinocyte growth factor receptor is up-regulated in cyclosporin A-induced gingival hyperplasia, J. Dent. Res., 2002, 81, 683–687 CrossRef CAS.
- J. W. Cooke, D. P. Sarment, L. A. Whitesman, S. E. Miller, Q. Jin, S. E. Lynch and W. V. Giannobile, Effect of rhPDGF-BB Delivery on Mediators of Periodontal Wound Repair, Tissue Eng., 2006, 12, 1441–1450 CrossRef CAS.
- L. Kuru, G. S. Griffiths, A. Petrie and I. Olsen, Changes in transforming growth factor-beta1 in gingival crevicular fluid following periodontal surgery, J. Clin. Periodontol., 2004, 31, 527–533 CrossRef CAS.
- E. Aiba-Kojima, N. H. Tsuno, K. Inoue, D. Matsumoto, T. Shigeura, T. Satoet al., Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet-rich plasma and potential use in cell culture, Wound Repair Regener., 2007, 15, 511–520 Search PubMed.
- V. G. Di, R. Patti, P. D'Agostino, G. Caruso, M. Arcara, S. Buscemiet al., Cytokines and growth factors in wound drainage fluid from patients undergoing incisional hernia repair, Wound Repair Regener., 2006, 14, 259–264 Search PubMed.
- N. N. Nissen, P. J. Polverini, A. E. Koch, M. V. Volin, R. L. Gamelli and L. A. DiPietro, Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing, Am. J. Pathol., 1998, 152, 1445–1452 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Growth mediators and antibodies, and schematic illustration of the multiplex bead assay. See DOI: 10.1039/b911863b |
| This journal is © The Royal Society of Chemistry 2010 |
