On the mechanical stability of polymeric microcontainers functionalized with nanoparticles
Matthieu F.
Bédard
*ad,
Almudena
Munoz-Javier
b,
Renate
Mueller
a,
Pablo
del Pino
b,
Andreas
Fery
c,
Wolfgang J.
Parak
b,
Andre G.
Skirtach
a and
Gleb B.
Sukhorukov
d
aMax Planck Institute of Colloids and Interfaces, Am Mühlenburg, 14424 Potsdam, Germany
bFachbereich Physik, Philipps Universität Marburg, Renthof 7, 35037 Marburg, Germany
cUniversity of Bayreuth, Physical Chemistry II, Universitätsstr. 30, 95447 Bayreuth, Germany
dDepartment of MaterialsQueen Mary University of London, Mile End Road, London, UK E1 4NS. E-mail: m.bedard@qmul.ac.uk
First published on 29th October 2008
Abstract
We present key factors that influence the mechanical stability of polyelectrolyte/nanoparticle composite microcontainers and their encapsulation behavior by thermal shrinkage. Poly(diallyldimethylammonium chloride) (PDADMAC), poly(styrenesulfonate) (PSS) microshells and citrate-stabilized gold nanoparticles are used. The presence of nanoparticles in the microshell renders the encapsulation process by heat-shrinking more difficult. The encapsulation efficiency is found to decrease as the concentration of material to be encapsulated increases. Increasing nanoparticle content in the microshell or the concentration of dextran increases the likelihood of getting fused and damaged capsules during encapsulation. On the other hand, mechanical studies show that doping microshells with gold nanoparticles significantly increases their stiffness and resistance to deformation. Internalization of capsules by cells supports that the incorporation of metal nanoparticles makes the shells more resistant to deformation. This work provides information of significant interest for the potential biomedical applications of polymeric microshells such as intracellular storage and delivery.
Introduction
The use of external stimuli to remotely induce permeability changes in microcontainers has attracted much interest for their potential applications towards remote encapsulation and release of molecules within living tissues.1–4 A delivery system known as polymeric microshells (i.e. microcapsules) was introduced by Möhwald et al. in 1998.5 Microshells are constructed onto sacrificial colloidal particles via a technique known as layer-by-layer (LbL) assembly6 in which oppositely charged polyelectrolytes are sequentially precipitated on a surface.7,8 Some encapsulation methods using microcapsules comprise changing pH,9,10 temperature (via thermal shrinking),11 as well as coprecipitation12 and optical sensitization.13 Recent work mainly focuses on understanding and controlling the release of encapsulated agents towards important potential applications of polymeric microshells such as drug delivery14,15 and anticorrosion coatings.16 An effective way of releasing material from microshells lays in incorporating nanoparticles of metals, such as gold or silver between the capsules' polyelectrolyte layers. Gold and silver nanoparticles are known to produce heat upon light absorption due to an electronic property termed surface plasmon resonance (SPR).14,17,18 The SPR adsorption band of gold nanoparticles is typically situated in the visible range but may be moved to longer wavelengths by changing the particles' size, shape or distribution.19 So-called gold nanorods20 for instance, can absorb light in the near-infrared region at wavelengths that can penetrate deep into biological tissues (biologically friendly).21 Light can be used to remotely open polyelectrolyte/nanoparticle composite shells because the heat generated disrupts the polyelectrolyte network, allowing encapsulated material to flow out.22–24Not much information is yet available on the influence of inserting nanoparticles in the wall of microcapsules with regards to their mechanical stability or encapsulation capabilities. It is imperative to possess all information on such aspects because remotely addressable microcontainers are found to behave very differently in a biological environment whose integrity is possible only under strict conditions that can destabilize the polyelectrolyte shell (e.g. temperature of 37.5 °C, pH = 5.0).25–27 Gao and coworkers recently reported a simple method to evaluate the mechanical properties of microcapsules, in which the osmotic pressure of a solution of capsules is changed using high molecular weight polyelectrolytes.28 At high enough concentration, the pressure caused capsules to deform and thus, statistical analyses permitted to attribute microshells approximate elasticity parameters as a function of shell diameter and number of layer. More recently, the combined work of the groups of Sukhorukov,29,30 Fery31,32 and Vinogradova33 has shed some light on the mechanical processes that govern polyelectrolyte microshells' behavior. The use of the small deformation approach of Fery et al. offers a way to quantitatively assess elastic constants.32 It was shown that thermally shrunken capsules gain significant stability as the temperature of the polyelectrolyte complex exceeds glass transition, allowing entropy-driven rearrangements and thickening of the capsule wall.31 This densification of the wall in turn means that it becomes correspondingly less permeable, allowing encapsulation of materials that prior to heating would have permeated the multilayers.30 Combining heat-shrinking encapsulation with gold nanoparticle doping of the microcapsule wall supplies an easy-to-use, laser-addressable microdelivery system.
One important characteristic that defines a good remotely addressable microdelivery system is its resistance against degradation, deformation or breakage once implemented in the desired environment in order to prevent unwanted early release of the encapsulated material.7Degradation of the shell depends very much on the environment intended for the microcontainers (pH, ionic strength, presence of appropriate enzymes, etc.) but the shell composition can also be chosen according to one’s needs.26,27 Polymeric microshells are essentially semi-permeable membranes, impermeable to relatively large molecules such as polymers but highly permeable to water (solvent) so that they are unaffected by osmotic effects. Capsule breakage is usually the result of mechanical stress and is highly undesirable. An important source of stress that is particularly relevant to this paper is cellular endocytosis, a general term that describes processes by which living cells internalize foreign bodies. There is no advantage in being able to remotely open a delivery system if it gets damaged and loses its cargo before, or in the process of endocytosis.34 The incorporation of gold nanoparticles into polyelectrolyte shells comes with two important advantages with regards to the use of capsules for intracellular delivery: first, the nanoparticles themselves have been established as the most efficient light-addressable agent for the release of material from the capsule cavity, second, the presence of the nanoparticles appear to have intrinsic stabilizing effects on the capsules wall.35
Scheme 1 illustrates an observation repetitively made in our recent work: intact microcontainers are found much more frequently after cellular uptake wherever gold nanoparticles are inserted between the polyelectrolyte layers. Our motivation is therefore to determine the effects of incorporating gold in nanoparticles/polyelectrolyte composite microshells on the mechanical stability and encapsulation efficiency of these shells. This information is necessary in order to select the best encapsulation and release conditions for any polymeric microcontainers intended for biological applications.
 | ||
| Scheme 1 An idealized representation of the internalization of polymeric microcapsules by a living cell. A shell containing nanoparticles of gold (right) keeps its shape while a shell containing no nanoparticles (left) is deformed during internalization. Once internalized, an intact container retains its encapsulated material. Shells functionalized with gold particles can later be remotely activated and release their cargo with the help of an infrared laser. | ||
Experimental
Materials
Poly(diallyldimethylammonium chloride) (PDADMAC, 20% w/v, 200–350 kDa), poly(styrenesulfonate, sodium salt) (PSS, 70 kDa), 5 nm and 20 nm colloidal gold, and hydrofluoric acid were purchased from Sigma-Aldrich, Germany. Monodisperse silica particles with a diameter of 4.78 µm (5% w/v) were obtained from Microparticles GmbH (Berlin, Germany). Alexa Fluor 488 dextran (AF488, 10 kDa) was obtained from Invitrogen (Karlsruhe, Germany). The water used in all experiment was obtained from a Millipore Milli-Q purification system and had a resistivity of 18.2 mΩ cm−1.Preparation and characterization of polyelectrolyte microshells
The fabrication of (PDADMAC/PSS)4 hollow shells doped with gold nanoparticles was done on SiO2 particles using the Layer-by-Layer (LbL) method. Polyelectrolyte hollow shells were constructed by alternatively treating the template with solutions of PDADMAC (2mg/ml, 0.5M NaCl) and PSS (2mg/ml, 0.5M NaCl) starting with PDADMAC. Gold doping was done by directly resuspending the previously washed substrate with a solution of the desired colloidal gold. Gold was added at once, on the second and third PDADMAC layers. After each polyelectrolyte layer addition, the particles were left to vortex for 12 minutes. When adding gold nanoparticles, less than 5 minutes were necessary for all the gold to absorb as indicated by measuring the UV/Visible spectra of the supernatants afterwards. After each layer addition, the suspension was sedimented at 5000 g for 1 minute, the supernatant discarded and the particles washed twice with water, sedimenting each time. The removal of the SiO2 template was done by resuspending 1.5ml of sample solution in 8.5ml of HF (0.3 M) for 20min. The samples were then thoroughly washed with water until the solution's pH > 5. The centrifugation conditions used during the dissolution steps were to 2000 g and 1 hour. Transmission electron microscopy (TEM) was done using a Zeiss Omega EM 912 at an operating voltage of 120 kV. For scanning electron microscopy (SEM), a drop of sample was dried under nitrogen and sputtered with gold. SEM measurements were conducted on a Gemini Leo 1550 instrument (3 eV). Confocal laser scanning microscopy (CLSM) was used to visualize the capsules in solution. LSCM images were recorded by means of a 100x/1.4–0.7 oil immersion objective. A CARY 50 conc. (Varian, Germany) UV/Vis spectrophotometer was used to obtain absorption spectra. For atomic force microscopy (AFM) measurements a drop of sample was dried on mica. AFM measurements were performed using a Nanoscope III Multimode AFM (Digital Instruments, USA) in tapping mode. The wall thickness of capsules was calculated by averaging the thickness of flat regions of at least 30 unshrunk capsules. Measurements were performed at room temperature at constant humidity. Dye encapsulation was done by heating a mixture of capsules and AF488 at temperatures in the range 52 °C–64 °C for 20 minutes depending on the size of capsule needed. After cooling down for 5 minutes, the suspension was washed twice to remove the labeled dextran that was not encapsulated.Force deformation experiments
The colloidal probe technique was used for the force deformation measurements.36,37 A MFP1d (Asylum Research, USA) AFM instrument was used for measurements of shells in water, at room temperature. The AFM was placed atop of an inverted optical microscope (Olympus IX71, Japan) so that fluorescence microscopy could be used to locate and monitor the capsules during the experiment. Colloidal particles (glass beads, diameter ≈ 30–50µm, Polyscience Inc., USA) were attached to tipless cantilevers (MicroMash, Spain) with a two component epoxy glue (UHU Plus endfest 300, UHU GmbH & Co.KG, Germany) using a micromanipulator (Suttner Instrument Co., USA). The spring constants of the cantilevers were determined using the thermal noise method38 and the Sader method.39 Both methods agreed within 10 percent and values of the spring constants were in the range reported by the manufacturer. A droplet of the solution containing the capsules was placed onto a coverslip which was previously coated with poly(ethyleneimine) (PEI) such that the capsule adhered to it. During the experiment, individual capsules were compressed and both the force and the deformation of the capsule were measured.31,40 We limited ourselves to deformations on the order of the wall thickness of the capsules, in order to avoid plasticity and effects due to the permeation of the solvent through the capsule membrane.41 For each data point, 5–45 capsules were measured.General cell culture techniques
MDA-MB-435s breast cancer cells (ATTC, USA) were cultured in L-15 Leibovitz medium (Biochrom AG) supplemented with 1% L-Glutamin (Biochrom AG), 1% Penicillin/Streptomycin (GIBCO), 10% Fetal Bovine Serum (Biochrom AG), 0.1% Insulin (Sigma), and were cultivated at 37 °C in a humidified atmosphere. NRK fibroblast cells (ATTC, USA) were cultured in D-MEM medium (GIBCO) supplemented with 1% Penicillin/Streptomycin (GIBCO), 10% Fetal Bovine Serum (Biochrom AG) and cultivated at 37 °C in a humidified atmosphere containing 5% CO2. To determinate the cellular uptake of the microcapsules, 1 × 104cells/cm2 were seeded in sterile microscopy chambers. When cells were adherent, they were incubated with a concentration of 32 × 104capsules/cm2 (32 capsules/cell) for 15 hours prior to evaluation of capsule deformation. The capsule concentration was determined using a counting chamber (Improved Neubauer, MarienFeld). To visualize the cells and capsules, a fluorescence-transmission microscope (Axiovert 200M with HAL-100 and FluoArc lamps, Zeiss, Germany), equiped with phase contrast and green fluorescence filters (filter set 10 for green fluorescence, Zeiss) was used. Microshells with different stiffness on the capsule wall were used to study deformation of polyelectrolyte capsules upon cell uptake. For each type of capsule, capsules were incubated with the cells for 15 hours. Phase contrast and green fluorescence images were then analyzed to compute the numbers of capsules which were deformed or non-deformed after cellular uptake. More information on the setup can be found in our previous work.34Results and discussion
The heat-induced shrinking behavior of PDADMAC/PSS microshells changes significantly when metallic nanoparticles are embedded within the polyelectrolyte walls. The specific behavior of (PDADMAC/PSS)n in solution upon heating has been explicitly explained in detail with the combined efforts of Koehler and coworkers and Mueller and coworkers.11,29–31 It was shown that once the solution's temperature exceeds that of the glass transition for the polyelectrolyte complex, material constants like the Young's Modulus drop by two orders of magnitude. Ionic bonds between the polyelectrolytes are weakened, resulting in a viscoelastic, fluid-like material, which allows the shell to reorganize depending on the net charge of the wall. In this respect, a capsule with an even number of layers has a near zero net charge and surface tension effect resulting from the hydrophobic polymer-solvent interactions drives the capsules to shrink at elevated temperature. In contrast, a capsule built with an odd number of layers will have a net excess charge which generates an electrostatic repulsion that greatly exceeds the hydrophobic force and drives the shell to expand instead.30 The thermally induced shrinking of capsules is particularly useful, since it comes with a densification of the polymer complex and constitutes one of the most effective methods of encapsulation using polyelectrolyte shells. Fig. 1 illustrates our initial observations concerning the heat-shrinking behavior of standard (PDADMAC/PSS)4 capsules versus shrinking curves of the same shells containing 5 nm and 20 nm citrate-stabilized gold nanoparticles (negatively charged) at identical concentrations (2000 particles/capsule). First, we observed that the average diameter of shells doped with gold nanoparticles is higher at most temperatures in comparison to shells containing no metal particles. Secondly, it appears that the minimum diameter to which the shells can be shrunk increases in samples containing nanoparticles. These observations are reminiscent of the changes in thermal shrinking behavior of polymeric capsules upon increasing the layer number.30,40 | ||
| Fig. 1 General influence of inserting gold nanoparticles on the heat-shrinking behavior of (PDADMAC/PSS)4 microshells. 30 capsules per data point were measured. The capsules doped with nanoparticles were estimated to contain approximately 2000 particles per capsule. | ||
Contructing microcapsules with more polyelectrolyte layers is known to improve their mechanical stability,29 but this comes at the expense of a reduction in shell permeability, which is necessary in order to load the capsules in the first place. However, we found no significant difference in the diffusion rate of fluorescently labeled dextran across the wall of (PDADMAC/PSS)4 capsules and similar microcapsules doped with metal nanoparticles.
In order to better understand these observations, the question addressed was to determine how incorporating different concentration of gold nanoparticles in the polyelectrolyte shell complex changes its thermal and mechanical behavior. We prepared capsules with three different concentrations of 20 nm gold particles. Citrate-stabilized 20 nm gold nanoparticles possess a more efficient heat generation response when irradiated with light than smaller gold particles, and are additionally not so large relative to the thickness of the shells as to reduce the efficiency of thermal encapsulation. Gold nanoparticles 20 nm in size are therefore a good compromise to functionalize polymeric microcapsules. Microshells were constructed from PDADMAC and PSS solutions onto a sacrificial silica template using the layer-by-layer method (LbL). Gold nanoparticles were inserted in the capsule wall by adding the desired volume of colloidal gold solution directly to the coated silica after each positively charged PDADMAC layer. Gold nanoparticles precipitate onto the oppositely charged polyelectrolyte layer via electrostatic interactions. An appropriate volume of hydrofluoric acid (0.3 M) was added to remove the sacrificial silica template, leaving behind a solution of hollow capsules with the following composition: PDADMAC/PSS/PDADMAC/(Au/PSS/PDADMAC)2PSS.†
All colloidal gold was absorbed within five minutes of resuspending the PDADMAC-coated silica particles with gold solution, and the silica particles gradually turned red during this time. The complete absorption of the gold nanoparticles onto PDADMAC layers was confirmed spectroscopically by measuring the absorbance of the supernatant after each sequence of gold addition/sedimentation. The statistical gold nanoparticles concentration in the composite microshells used for this investigation were calculated mathematically in terms of surface filling factors (FS) and found to be 0.07, 0.15, and 0.28 for the low to high gold concentration samples, respectively. FS is defined as the ratio of the total surface coverage of nanoparticles to the available surface area of a microcapsule, based on TEM imaging. (A hypothetical FS ratio equal to 1 would correspond to a complete coverage of metal particles over the capsule surface.) The distribution of gold nanoparticles within the capsule wall of the three samples was visualized by TEM, and representative images are presented in Fig. 2.
 | ||
| Fig. 2 Thermal shrinking behavior of (PDADMAC/PSS)4 capsules containing different concentrations of gold nanoparticles (filling factor 7%, 15%, 28%). The percentages shown represent the calculated surface area coverage of nanoparticles throughout the polymeric shell. The inset shows the linear relationship between the percent gold content in the capsule wall versus the minimum diameter the capsules can be thermally shrunk (before fusion). Each data point is calculated from the diameter of at least 30 capsules; error bars correspond to standard deviation. Bottom: representative TEM images showing the distribution of the gold nanoparticles of a section of polymeric shell before shrinking. Scale bars represent 200 µm. | ||
A homogeneous coverage of the PDADMAC layer by the gold nanoparticles was insured by first resuspending the silica precipitate in a small volume of water followed by rapid addition of the colloid gold solution. However, it was found that using larger concentrations of gold nanoparticles affects their distribution and aggregates formed, which are absent in capsules with a lower concentration of gold nanoparticles. The first aggregates of gold nanoparticles appear in the capsules of the sample with FS ∼ 0.1–0.15. Since the gold content was equally distributed over the two middle layers of PDADMAC, these aggregates of gold are thought to form atop PDADMAC layers when FS ≥ 0.05 for a single gold layer. We had already reported that admixing ligand stabilized gold particles with same charge polymers led to homogeneously distributed nanoparticles, while adding the gold particles alone favors the formation of aggregates on the oppositely charged polyelectrolyte capsule layer.42 These differences in the aggregation mechanisms on gold nanoparticles at the polyelectrolyte-water surface are not fully understood and are currently being investigated.
Aliquots of solutions of capsule doped with the three concentrations of gold nanoparticles were heated at various temperatures from ambient to 80 °C. After thermal treatment, the capsule solutions were left to cool down and a small volume of rhodamine 6G solution (10−7 M) was added to make the shells fluorescent. The shell diameters were averaged from at least 30 capsules using LSCM. The graph in Fig. 2 shows the results obtained. It was found increasingly difficult to thermally shrink polymeric shells as the gold content within the wall is increased. The inset illustrates the linear relationship between gold content and the minimum size a capsule could be shrunk. In addition, it was found that higher gold content limits the minimum diameter the capsules may be shrunk to. This minimal shell diameter as a function of gold concentration is particularly important for encapsulation purposes, because this relationship comes with a contrariety: constructing shells with a high gold content means more efficient light-induced release, but lower encapsulation efficiency. Another factor that may influence the mechanical properties of polymeric microshells with encapsulated material is the concentration of substance to be encapsulated (encapsulate) prior encapsulation. (PDADMAC/PSS)4 shells doped with 7% (FS = 0.07) gold nanoparticles were resuspended in 0.5, 1.0, or 2.0 mg/ml of fluorescently labeled dextran (3 kDa) for 30 minutes and then, heated at various temperatures from room temperature to 80 °C at 5 °C intervals. Fig. 3 displays selected SEM images of capsules containing at relatively low (7%) and high (28%) nanoparticle content after being thermally shrunk at various temperatures. One can clearly see from these images that the high gold content capsules are larger at every temperature compared to shells containing low amounts of gold. Shells that are larger than half their original diameter have low density walls and collapse upon drying as seen for both samples (7% and 28%) that were treated at lower incubation temperatures. At 60 °C, the wall of capsules containing 7% gold is dense enough that some shells are able to support themselves in the dried state, whereas shells carrying a high gold load need further heating before this could be partially achieved. This wall density difference is another experimental evidence that shells with different gold content might have different mechanical properties. Interestingly, it was also found that shells containing high concentration of gold nanoparticles in their wall were fusing more frequently at lower temperature than observed in capsules containing no gold. We observed various changes in capsules after thermal treatment in presence of relatively high concentration of other substances, such as fusion,43‡ or rupture of capsules.
 | ||
| Fig. 3 Representative SEM images of thermally shrunk capsules with a gold nanoparticles surface coverage of 7 and 28% (FS = 0.07 and 0.28), top and bottom row, respectively. Each column shows an image of each type of capsule shrunk under the same conditions with their average diameter (dA). The two dA values that are shaded had a broad size distribution with a significant number of fused or damaged capsules. Capsule average diameters were averaged from at least 30 capsules. | ||
Similar observations were obtained when attempting to thermally encapsulate relatively high concentration of 3 kDa fluorescently labeled dextran. These observations are summarized in Fig. 4. It can be clearly seen that as concentration of the dextran is increased the microshells are more difficult to shrink. In addition, the minimum diameter that capsules with encapsulated material can be shrunk also increased significantly with increased dextran concentration.
 | ||
| Fig. 4 Effect of dextran (3 kDa) concentration on the heat-shrinking curves and encapsulation efficiency of PDADMAC/PSS/PDADMAC/ (Au/PSS/PDADMAC)2PSS microshells with a gold surface coverage of 7%. The capsule diameters were averaged by LSCM from 30 capsules. At higher concentrations, dextran promoted fusion and damaged capsule formation. | ||
This can be attributed to the diffusive force caused by the concentration difference in dextran across the wall of the shrunken capsules. The shell permeability may vary significantly depending on its thickness and constituents, but also on the temperature of incubation and encapsulate used. This is because the permeability of any semi-permeable membrane is a function of the rate of diffusion specific to a particular compound, and depends on the interactions of a molecule with the membrane and its density of the membrane. The shrunk capsule shell with a denser wall is less permeable to any given material than in its unshrunk state. The final concentration of an encapsulated substance by thermal treatment of microcapsules can be approximated by the expression CF = Ci(ri3 − rF3) where Ci is the initial encapsulate concentration and ri, rF are the initial and final capsule diameters, respectively. For example, if we neglect the changes in wall thickness, shrinking capsules of 4.6 µm to 2.3 µm corresponds to a ten-fold decrease of the inner capsule volume. Meanwhile, the capsule wall becomes thicker and rapidly impermeable to molecules as shell shrinking progresses. As a result of the heat treatment, molecules that could previously diffuse across the capsules wall become sequestered inside a shrinking shell, leading to the creation of a diffusive force which opposes the hydrophobic force that drives shell shrinking.
Another interesting feature of the capsules’ shrinking behavior was the appearance of a region where capsules were found to fuse or rupture that was dependant on the dextran concentration. Capsules that were considered “ruptured” were typically deformed and contained no fluorescent material in the cavity after shrinkage. Ruptured and fused capsules appeared at around 60 °C, 65 °C and 75 °C for samples that were thermally treated in presence of dextran concentrations of 2.0 mg/ml, 1.0 mg/ml and 0.5 mg/ml, respectively (Fig. 4). Since the onset for damaged microcontainers decreases as dextran concentration is increased, rupture thought to be the result of accumulating an excessive diffusive pressure across the wall during shrinkage. For a given dextran concentration, to reach the diameter at which ruptured shells appear also means that samples of lesser quality with fewer capsules holding encapsulated material were obtained. We then proceeded to encapsulate 10 kDa fluorescently labeled dextran under the same conditions to determine whether increasing the molecular weight of the encapsulated substance affected the behavior previously observed. The resulting thermal shrinking curves were not significantly different from those obtained with a 3 kDa dextran. In view of these results, we can postulate that the useful range of encapsulate concentration for moderate size polymers using the thermal encapsulation method, should be below 2 mg/ml. Thermally shrinking capsules in the presence of concentrations of material too high to encapsulate brings about undesired side effects such as fusion and rupture of the microshells. The useful range of temperatures at which optimal number of capsules with encapsulated material is obtained at encapsulate concentration below 2 mg/ml is situated between 50 and 60 °C.
The influence of gold content and geometry of the shell on their mechanics was also investigated. An accurate method to determine mechanical properties of polyelectrolyte capsules in solution is AFM force spectroscopy with the colloidal probe technique. In this method, a glass bead is glued to a tipless cantilever, providing a well-defined contact area between the capsule and the bead. This situation can be described within the theoretical model of Reissner.44 It relates the samples' spring constant c to the Young's Modulus of the material E, the wall thickness of the shell h and the radius of the shell R:
 | (1) |
The spring constant is derived from the slope of the force-deformation curve and is a measure for the stability of the shell. For this study, we selected capsules doped with gold nanoparticles with percent filling factors of 1%, 7% and 28%. Exemplified force-deformation data curves of individual capsules are shown in Fig. 5. A clear trend can be seen: increasing gold concentration in the wall of the (PDADMAC/PSS)4 capsules of the same size increases the resistance of microshells to deformation. The increase in the capsules spring constant due to the gold content seems to be non-linear. For FS values of 0.01 and 0.07 there is a relatively small difference in the spring constant of shells of the same size; the mechanical properties are similar to non-doped capsules. When compared to the high gold concentration, there is an increase in the stability of the capsules by a factor of approximately five. This result is in line with the work of Dubreuil et al. who found a considerable increase in the spring constant due to the incorporation of nanoparticles into the shells.45 This is also reflected by the average values of the spring constants of up to 45 individually tested shells which are presented in Table 1. A possible explanation for the non-linear process might be the percolation threshold, which needs a critical concentration of nanoparticles to significantly affect the capsules' stability. Below the critical concentration the mechanical properties are governed by the polyelectrolyte matrix. Above the critical concentration, percolation takes place and the nanoparticles begin to interact with each other, dominating the shell mechanics. The error bars of the average values might stem from small derivations in the capsules' geometry. Usually the capsules are considered to be ideal spheres with a defined radius and wall thickness and a homogeneous wall material. Assuming a variation of 0.2 µm (error of the optical measurement) in the shells' radii results in an error of 75–250 pN/nm in their stiffness (estimated from ref. 31). Also variations in wall thickness may affect the mechanical constants of the capsules. It was previously discussed that increasing the number of layers of microshells of comparable diameter also increases their mechanical stability.31,46 Similarly, thermally shrunk capsules have thicker and denser walls, as a result of enthalpy- driven processes and are much more stable than larger siblings, thus agreeing with our observation: large effects in stability without changing the gold content can be archived by annealing the capsules. Comparing the values in Table 1, it is found that the stability of capsules with a diameter of about 4.5 µm and a high gold content (FS = 0.28) is comparable to capsules with a diameter of 2.5 µm with a medium gold content (FS = 0.07). For biomedical application a higher gold content in wall of the capsules is not desirable because of a less defined capsule wall, so shrinking capsules with a lower gold concentration in the shell might be a suitable way to produce microcontainers with a tailored gold content, as well as a tailored stability (Table 1).
| Capsule | Diameter [µm] | Surface filling factor [%] | Stiffness [pN/nm] |
|---|---|---|---|
| No gold | 4.57 ± 0.2 | 0 | 183.1 ± 74 |
| Low gold | 4.52 ± 0.3 | 1 | 259.2 ± 140 |
| Medium gold | 4.58 ± 0.2 | 7 | 293.3 ± 90 |
| High gold | 4.48 ± 0.3 | 28 | 1447.6 ± 352 |
| Medium gold | ∼2.5 | 7 | 1460.6 ± 588 |
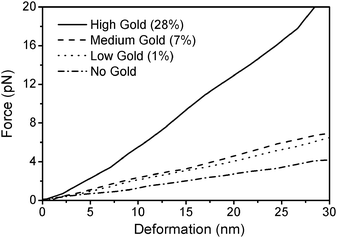 | ||
| Fig. 5 The force-deformation curves of exemplified individual capsules with different gold content and different sizes. A small stiffness increase is found in shells with surface filling ratio of 0.01 or 0.07, whereas a large increase is found in shells with the highest load of gold nanoparticles. | ||
It was observed that incorporating charged colloids into the polyelectrolyte mesh affects the adhesion of subsequent polymeric layers. This may have little impact at the capsule level (macroscopic) but is expected to thoroughly alter the polymer organization within the shells. Due to their large diameter relative to the shell thickness, gold nanoparticles inevitably makes the roughness of the latter increase. Fig. 6 shows the roughness profiles of 1 µm2 area of (PDADMAC/PSS)4 microcapsule (left), and that of an identical shell doped with 7% of gold nanoparticles (right) (not thermally shrunk), as measured by AFM. The average roughness values were measured to be 1.5 nm and 2.1 nm for the reference and the gold-containing samples, corresponding to an increase of about 30% in shell roughness. Roughness measurements represent the average values obtained from measuring 400 nm2 flat areas (containing no folds) of 10 capsules for each type of shell. Alongside roughness, the surface area available for the next layer to adhere is also increased. Therefore thicker shells were expected for when doped with nanoparticles since more material is expected to precipitate onto the colloid particles during subsequent layer additions. The average wall thickness of 30 capsules of the aforementioned samples (Fig. 6) was measured using AFM and revealed to be 18.4 nm (±1.5 nm) for the control sample and 20.4 nm (±1.8 nm) for capsules containing 7% gold nanoparticles. The large thickness variations due to the presence of gold particles were accounted for by specifically taking thickness values at the lowest point of each measured capsule. The measured wall thicknesses suggest that a slight increase in wall thickness of approximately 10% exists in capsules containing gold nanoparticles with respect to capsules containing no gold. Increased wall thickness may further contribute to the shell stability of microcapsules containing nanoparticles as a direct consequence of an increased shell roughness.
 | ||
| Fig. 6 Comparative AFM topographic representations of the surface roughness of a 1 µm2 section of an unshrunk (PDADMAC/PSS)4 capsule (left) and a similar capsule doped with 7% of gold nanoparticles (right). The x and z axes measure 200 nm. The range of height (y axis) of the surfaces is ca. 50 nm, as depicted by the scale in the center. Measurements were made at room temperature. | ||
Based on all this information, we then conducted a series of experiments in which microcontainers were incubated in presence of living cells in an environment of potential application. The capsules that were internalized by the cells could be monitored by a camera equipped with fluorescence and phase contrast imaging.47 The proportion of microshell that deformed in the uptake process was determined as a function of the gold nanoparticle content found in the microcapsules, as well as the size of the shrunk capsules (Fig. 7). In addition, two different types of cell lines were used in order to determine whether the degree of shell deformation found with a given cell line compares to that a different type of cells. Capsules (FS = 0.07) were loaded with dextran and shrunk to an average diameter of 4.0 µm, 2.8 µm and 2.3 µm. Two different cell lines were used: fibroblast and breast cancer cells, which are refered to as NRK and MDA-MB-435s, respectively. Fig. 8 summarizes our findings. First, after only a very slight shrinkage (ca. 10% from original size), an average of 97% of all microshells were found to deform no matter which cell line was used. When shrunk to an average of 2.8 µm, only the shells doped with 20 nm gold particles were found to have a significant improvement in shell stability, with about 11% of shells that remained intact after being incorporated by the NRK cells. However, still no significant improvement was observed in any of the shells ingested by the cells MDA-MB-435s cells. It is only at a diameter of 2.3 µm, which corresponds to 50% of their initial diameter that the shells became truly more resistant to deformation. The best results were obtained with NRK cells and capsules containing 20 nm gold particles for which 81% of the capsules remained intact after being internalized. Using the same type of cells, 38% of the shells containing no gold and 60% of shells containing 5 nm gold particles were found intact. These results illustrate that the presence of gold nanoparticles in the shells can greatly improve the shell stability over mechanical deformation, but this stability becomes significant only for microshells shrunk to smaller diameters. Similarly, the mechanical stability of shells shrunk to 2.3 µm containing no gold, as well as 5 nm and 20 nm gold nanoparticles was found to greatly improve when internalized by MDA-MB-435s cells. However, in this case, while both control capsules and shells doped with 20 nm particles displayed a 79% of intact shell after cellular uptake, shells doped with 5 nm particles were found to be much less with a 42% success rate. This discrepancy is not currently understood but could be related to the uptake process of MDA-MB-435s cells.
 | ||
| Fig. 7 Microscopy images of MDA-MDA-435s cells with internalized capsules. On the left, capsules without gold particles thermally shrunk to 4 µm (a) and to 2.3 µm (b). On the right, capsules containing gold nanoparticles shrunk to 4 µm (c) and to 2.3 µm (d). All capsules that were internalized in a, b and c were found to be deformed after internalization by the cells. Most capsules in d were found to be unaffected by the cellular uptake. White arrows indicate non-deformed microcontainers outside the cell. A dashed line indicates the position of the nucleus of the cell that internalized capsules. The scale bars measure 10 µm. | ||
 | ||
| Fig. 8 Statistics of the deformation of (PDADMAC/PSS)4 microshells containing gold nanoparticles or not, as a function of nanoparticle size (5 nm, 20 nm), capsule diameter and cell type (NRK, MDA-MB-435s). Shells doped with gold nanoparticles both contained a similar number of citrate-stabilized gold nanoparticles per capsule, as monitored by TEM. Average capsule diameters were calculated from 30 capsules. At least 400 capsules were monitored for each experiment. | ||
Conclusion
In this work, using PDADMAC- and PSS-based microcapsules as a model intracellular delivery vehicle, doped with citrate-stabilized gold nanoparticles as a remotely accessible release mediator, we have shown the large differences in sample qualities that must be taken into consideration when using polymeric microshells doped with metal nanoparticles. Increasing the surface filling factor of gold nanoparticles decreases the ability of microshells to thermally shrink and limit the lower size they may be shrunk to. Increasing the concentration of material to be encapsulated has similar effects. In general, increasing either or both gold content and encapsulate concentration increases the probability of finding ruptured or fused microcontainers at low incubation temperature. Rupture and fusion has consequences for reduction of sample quality and appears to be related to the shells’ higher surface roughness for samples of increasing gold content, and weakened electrostatic interaction in the wall when using higher concentration of encapsulation material. Force-deformation measurements show that in their unshrunk state, there are some improvement in shell stiffness with low and moderate gold content inside the wall, but a five-fold increase in stability was achieved by doping a high amount of gold into the wall of unshrunk capsules or shrinking the shells to about half of their diameter. The improvement in shell stiffness due to gold content is non-linear. At low gold content, shell mechanics are thought to be dominated by the polymer matrix, but are dictated by the nanoparticles above a critical concentration. Various shells with and without gold nanoparticles were incubated with cells and monitored for deformation after cellular uptake. While the all microcapsules displayed a poor resistance to deformation when thermally shrunk to larger volumes, shells shrunk to about 50% of their original size showed significant improvements, with around 80% of intact capsules found in some cases. Shells doped with larger particles and shrunk to smaller diameter were found to be generally more stable, and less inclined to deformation.Acknowledgements
We would like to acknowledge Prof. Helmuth Möhwald for valuable discussions and financial support and Rona Pitschke for technical assistance. WJP, PdP and AMJ are grateful to the EU (project Nanointeract) for funding.References
- K. Glinel, C. Dejugnat, M. Prevot, B. Scholer, M. Schonhoff and R. V. Klitzing, Colloids Surf., A, 2007, 303, 3–13 CrossRef CAS.
- L. Hartmann, M. Bedard, H. G. Borner, H. Mohwald, G. B. Sukhorukov and M. Antonietti, Soft Matter, 2008, 4, 534–539 RSC.
- D. G. Shchukin, D. A. Gorin and H. Moehwald, Langmuir, 2006, 22, 7400–7404 CrossRef CAS.
- T. Shutava, Z. G. Zheng, V. John and Y. Lvov, Biomacromolecules, 2004, 5, 914–921 CrossRef CAS.
- E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Mohwald, Angew. Chem., Int. Ed., 1998, 37, 2202–2205 CrossRef.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- G. B. Sukhorukov, A. L. Rogach, M. Garstka, S. Springer, W. J. Parak, A. Munoz-Javier, O. Kreft, A. G. Skirtach, A. S. Susha, Y. Ramaye and R. Parankar, Small, 2007, 3, 944–955 CrossRef CAS.
- Z. H. Lu, T. Shutava, N. Sahiner, V. John and Y. Lvov, Chem. Lett., 2005, 34, 1536–1537 CrossRef CAS.
- K. Itano, J. Y. Choi and M. F. Rubner, Macromolecules, 2005, 38, 3450–3460 CrossRef CAS.
- C. Dejugnat and G. B. Sukhorukov, Langmuir, 2004, 20, 7265–7269 CrossRef CAS.
- K. Kohler and G. B. Sukhorukov, Adv. Funct. Mater., 2007, 17, 2053–2061 CrossRef.
- O. Kreft, A. G. Skirtach, G. B. Sukhorukov and H. Mohwald, Adv. Mater., 2007, 19, 3142–3145 CrossRef CAS.
- M. Bedard, A. G. Skirtach and G. B. Sukhorukov, Macromol. Rapid Commun., 2007, 28, 1517–1521 CrossRef CAS.
- A. G. Skirtach, A. M. Javier, O. Kreft, K. Kohler, A. P. Alberola, H. Mohwald, W. J. Parak and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2006, 45, 4612–4617 CrossRef CAS.
- Y. J. Wang, V. Bansal, A. N. Zelikin and F. Caruso, Nano Lett., 2008, 8, 1741–1745 CrossRef CAS.
- D. G. Shchukin and H. Mohwald, Adv. Funct. Mater., 2007, 17, 1451–1458 CrossRef CAS.
- H. H. Richardson, Z. N. Hickman, A. O. Govorov, A. C. Thomas, W. Zhang and M. E. Kordesch, Nano Lett., 2006, 6, 783–788 CrossRef CAS.
- A. O. Govorov and H. H. Richardson, Nano Today, 2007, 2, 30–38 CrossRef.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS.
- A. V. Alekseeva, V. A. Bogatyrev, B. N. Khlebtsov, A. G. Mel'nikov, L. A. Dykman and N. G. Khlebtsov, Colloid J., 2006, 68, 661–678 CrossRef CAS.
- X. Huang, W. Qian, I. H. El-Sayed and M. A. El-Sayed, Lasers in Surgery and Medicine, 2007, 39, 747–753 Search PubMed.
- A. G. Skirtach, A. A. Antipov, D. G. Shchukin and G. B. Sukhorukov, Langmuir, 2004, 20, 6988–6992 CrossRef CAS.
- M. F. Bédard, D. Braun, G. B. Sukhorukov and A. G. Skirtach, ACS Nano, 2008, 2, 1807–1816 CrossRef CAS.
- B. Radt, T. A. Smith and F. Caruso, Adv. Mater., 2004, 16, 2184–2189 CrossRef CAS.
- O. Kreft, A. M. Javier, G. B. Sukhorukov and W. J. Parak, J. Mater. Chem., 2007, 17, 4471–4476 RSC.
- B. G. De Geest, R. E. Vandenbroucke, A. M. Guenther, G. B. Sukhorukov, W. E. Hennink, N. N. Sanders, J. Demeester and S. C. De Smedt, Adv. Mater., 2006, 18, 1005–1009 CrossRef.
- S. De Koker, B. G. De Geest, C. Cuvelier, L. Ferdinande, W. Deckers, W. E. Hennink, S. De Smedt and N. Mertens, Adv. Funct. Mater., 2007, 17, 3754–3763 CrossRef CAS.
- C. Y. Gao, S. Leporatti, S. Moya, E. Donath and H. Mohwald, Langmuir, 2001, 17, 3491–3495 CrossRef CAS.
- K. Kohler, H. Mohwald and G. B. Sukhorukov, J. Phys. Chem. B, 2006, 110, 24002–24010 CrossRef.
- K. Kohler, D. G. Shchukin, H. Mohwald and G. B. Sukhorukov, J. Phys. Chem. B, 2005, 109, 18250–18259 CrossRef.
- R. Mueller, K. Kohler, R. Weinkamer, G. Sukhorukov and A. Fery, Macromolecules, 2005, 38, 9766–9771 CrossRef CAS.
- A. Fery and R. Weinkamer, Polymer, 2007, 48, 7221–7235 CrossRef CAS.
- B. S. Kim, O. V. Lebedeva, K. Koynov, H. F. Gong, G. Glasser, I. Lieberwith and O. I. Vinogradova, Macromolecules, 2005, 38, 5214–5222 CrossRef CAS.
- A. M. Javier, O. Kreft, M. Semmling, S. Kempter, A. G. Skirtach, O. Bruns, P. del Pino, M. F. Bedard, J. Radler, J. Kas, C. Plank, G. B. Sukhorukov and W. J. Parak, Adv. Mater., 2008 Search PubMed DOI: 10.1002/adma.200703190.
- C. Y. Jiang, S. Markutsya, Y. Pikus and V. V. Tsukruk, Nat. Mater., 2004, 3, 721–728 CrossRef CAS.
- W. A. Ducker, T. J. Senden and R. M. Pashley, Nature, 1991, 353, 239 CrossRef CAS.
- H. J. Butt, Biophysical Journal, 1991, 60, 1438–1444 Search PubMed.
- J. L. Hutter and J. Bechhoefer, Rev. Sci. Instrum., 1993, 64, 1868 CrossRef CAS.
- J. E. Sader, Journal of Applied Physics, 1998, 84, 64–76 Search PubMed.
- F. Dubreuil, N. Elsner and A. Fery, Europhys. J. E, 2003, 12, 215–221 Search PubMed.
- A. Fery, F. Dubreuil and H. Möhwald, New Journal of Physics, 2004, 6, 18 Search PubMed.
- A. G. Skirtach, C. Dejugnat, D. Braun, A. S. Susha, A. L. Rogach and G. B. Sukhorukov, J. Phys. Chem. C, 2007, 111, 555–564 CrossRef CAS.
- K. Köhler, PhD Thesis, Universität Potsdam, 2006.
- E. Reissner, Journal Of Mathematics And Physics, 1946, 25, 279–300 Search PubMed.
- F. Dubreuil, D. G. Shchukin, G. B. Sukhorukov and A. Fery, Macromol. Rapid Commun., 2004, 25, 1078–1081 CrossRef CAS.
- N. Elsner, F. Dubreuil, R. Weinkamer, M. Wasicek, F. D. Fischer and A. Fery, Progress in Colloid and Polymer Science, 2006, 132, 117–123 Search PubMed.
- A. M. Javier, O. Kreft, A. P. Alberola, C. Kirchner, B. Zebli, A. S. Susha, E. Horn, S. Kempter, A. G. Skirtach, A. L. Rogach, J. Radler, G. B. Sukhorukov, M. Benoit and W. J. Parak, Small, 2006, 2, 394–400 CrossRef.
Footnotes |
| † Note that “Au/” does not indicate the presence of a distinct layer of gold nanoparticles but instead is written in this manner to indicate the point of insertion of gold nanoparticles. |
| ‡ Fusion of polymeric microshells is a complex phenomenon that was discovered very recently and is still under investigation. Although this topic is outside the scope of this work, it should be noted that it is necessary to have a certain degree of aggregation to obtain fused microcapsules. |
| This journal is © The Royal Society of Chemistry 2009 |
