Structure-property relationships in flavour-barrier membranes with reduced high-temperature diffusivity
Kevin A.
Heitfeld
and
Dale W.
Schaefer
*
Department of Chemical and Materials Engineering, University of Cincinnati, Cincinnati, OH 45221-0012, USA. E-mail: dale.schaefer@uc.edu; heitfeka@yahoo.com; Fax: +1 206 600 3191 (Schaefer); Tel: +1 513-948-2187 (Heitfeld) Tel: +1 513 556 5431 (Schaefer)
First published on 28th October 2008
Abstract
Encapsulation is used to decrease the premature release of volatile flavour ingredients while offering protection against environmental damage such as oxidation, light-induced reactions, etc. Hydroxypropyl cellulose (HPC) is investigated here as a “smart,” temperature responsive membrane for flavour encapsulation and delivery. Gel films were synthesized and characterized by diffusion and small-angle neutron and X-ray scattering techniques. Increasing temperature typically increases the diffusion rate across a membrane; HPC, however, can be tailored to give substantially improved elevated temperature properties. Scattering results indicate processing conditions have a significant impact on membrane morphology (micro phase separation). Under certain synthetic conditions, micro phase separation is mitigated and the membranes show temperature-independent diffusivity between 25 °C and 60 °C.
1. Introduction
Temperature responsive gels (TRGs) are an interesting class of hydrogels that change their volume in response to some environmental stimuli such as temperature1–3 or pH.4–7 A TRG can alternate between a swollen state (absorbing water) and a shrunken state (expelling water) at a critical temperature. The response is commonly realized as a change in volume as well as a change in other characteristics such as opacity and mechanical properties.8–10 This paper explores a modified hydroxypropyl cellulose (HPC) TRG that, above a critical temperature, shrinks with the concomitant expulsion of water. On the contrary, when the temperature decreases below the critical temperature, the gel reversibly swells and absorbs water. This type of transition occurs in polymers that exhibit a lower critical solution temperature (LCST).Current encapsulation technology is passive. The shell material does not respond to the environment.11 For instance, if temperature is increased, the volatile flavour core will be released more rapidly. A shell material that reacts to the environment by changing its diffusive properties is needed to control flavour loss at increased temperature. A TRG could be used as the shell material where at temperatures below the LCST the shell exists in its swollen state, and above the LCST the shell collapses resulting in a diffusion barrier membrane.
This paper investigates the potential of a TRG as a diffusion barrier membrane. The diffusion properties of small flavour molecules across HPC films have been determined at room temperature and elevated temperatures. We demonstrate the ability to tailor the HPC diffusion properties by controlling processing conditions such that the permeability is temperature independent. The encapsulation of flavours in HPC has been previously described by Heitfeld et al.12–14
We avoid temperature-responsive synthetic polymers in favor of HPC, which is approved as a food additive by the US Federal Drug Administration (FDA). However, the HPC was modified to form a gel. The modification procedure is not FDA approved.
2. Experiment
2.1 Materials
HPC (Mw ∼ 80,000 Mn ∼ 10,000), Methacryloyl chloride (MAC), poly(ethylene glycol) dimethacrylate (PEG-DMA), 30% hydrogen peroxide solution, and n,n-dimethylacetamide (DMAc) were obtained from Sigma-Aldrich. Benzaldehyde (model flavour), sodium alginate, calcium chloride, and food grade oil Miglyol 812 were provided by Givaudan Flavours, Cincinnati, OH.2.2 Crosslinking
Sodium alginate is a popular material used in flavour encapsulation. Sodium alginate films were therefore prepared for comparison with HPC. A 2.5 wt% sodium alginate solution in water was poured into custom molds measuring approximately 75 × 50 × 1 mm. The molds were made by adhering microscope slides on a glass pane and covering the glass with Bytac® surface protector. The solution was allowed to dry, and the dry films were placed into a 2 wt% CaCl2 solution for two hours.HPC films were prepared by ultra-violet (UV) light. This method limits the use of toxic crosslinkers that would ultimately inhibit FDA approval. The method employed here requires the HPC to be modified by an acid chloride15,16 to add sites of unsaturation for free radical crosslinking.
 | ||
| Fig. 1 Representative chemical structure of (a) hydroxypropyl cellulose (b) methacryloyl chloride (c) modified hydroxypropyl cellulose with one substituted hydroxypropyl group. | ||
Vinylization was accomplished by dissolving 10 wt% HPC in DMAc at 60 °C with nitrogen purge. The amount of MAC added for these experiments ranges from 0.10 – 0.18 ml of MAC per gram of HPC in solution. Under nitrogen purge, the MAC was added to 100 ml of DMAc in a separate flask. The MAC/DMAc solution was then added dropwise to the HPC solution at 60 °C. The HPC solution was left to react for 24 h and then removed from heat. The vinylized HPC was precipitated in ether and purified by dissolving the modified polymer in methanol and then precipitating with ether for three cycles. After final precipitation the modified HPC was placed in a vacuum oven to remove residual solvent.
Bulk crosslinked films were prepared by casting the HPC solution in the molds and allowing the water to evaporate from solution over night in the hood. These dry films were then crosslinked under the UV lamp. The lamp was warmed up as previously described, and the dry films were removed from the mold and placed 2.5 cm under the UV lamp. The lamp was turned on for 1 h to crosslink the films.
Unmodified HPC phase separates above 40 °C. Several films were crosslinked at different temperatures to determine the effect of crosslinking temperature on microstructure. These films were crosslinked in solution. A circulation bath was used along with copper tubing and an aluminum heat sink to control the crosslinking temperature. A thermocouple was placed between the glass mold and the aluminum heat sink to monitor the temperature.
Several films were crosslinked with the addition of the crosslinking agent PEG-DMA and hydrogen peroxide. PEG serves as an oligomeric crosslinker. The same 5 wt% HPC solutions were made with the addition of 1.1 wt% PEG-DMA by weight of HPC in solution. This solution was crosslinked in bulk and in solution following the previous procedures.
3. Results
3.1 NMR
The HPC modification was confirmed by 1H NMR with a 300 MHz Varian Gemini 2000 spectrometer. All samples were referenced to deuterated methanol-d3 at 3.31 ppm. NMR spectra of pure HPC, pure MAC, and modified HPC were taken. The modified HPC spectrum was compared with the MAC spectrum to establish the presence of new peaks.The NMR spectra of unmodified HPC as received from Aldrich (Fig. 2a), MAC (Fig. 2b), and the 0.15 ml/g modified HPC (Fig. 2c) are compared. The HPC spectrum shows peaks near 1.2 ppm from the methyl groups in the hydroxypropyls, peaks from 3.4 to 3.8 ppm from the cyclic glucose units, and a peak at 4.8 from the methanol. The methacryloyl chloride spectrum shows three strong peaks with two peaks near 5.6 and 6.1 ppm from the hydrogen at the carbon-carbon double bond and a peak near 1.9 ppm from the methyl group. After modification, these peaks appear in the modified HPC NMR spectrum.
 | ||
| Fig. 2 NMR spectra of (a) unmodified HPC (b) MAC (c) modified HPC. The three strong peaks for the MAC near 6.1, 5.6, and 1.9 show up in the modified HPC spectrum indicating modification has occurred. | ||
The NMR spectra show that the HPC was successfully modified with double bonds. Modification is also apparent during the crosslinking step since unmodified HPC does not crosslink. The modification also causes the LCST to lower with increasing modifier concentration.
Fatty acid methyl ester analysis was used to determine the extent of modification. The ester linkage formed in the modification reaction is hydrolyzed to remove the methacrylic acid. Gas chromatography was used to determine the percentage of methacrylic acid to be 1.8% by weight of dry polymer. It is likely there are unreacted hydroxyl groups in the HPC and these unreacted groups are not expected to participate in the crosslinking reaction.
3.2 Rheometry
Rheometry was performed using a Malvern Instruments Gemini Rheometer in viscosity mode at a shear rate of 100 s−1. HPC solutions (10-wt% HPC in water) were tested with a 2° cone and plate while sweeping the temperature from 25 °C to 60 °C. Viscosity measurements were reported as viscosity (centipoise) versus temperature ( °C). The LCST was determined as the temperature when viscosity begins to decrease from the initial plateau.Viscosity was plotted versus temperature for modified HPC at several modification conditions indicated in milliliters of modifier per gram of HPC (Fig. 3). The data show an abrupt drop in viscosity at the LCST and a decreasing LCST with increasing modifier concentration (Fig. 4).
 | ||
| Fig. 3 Viscosity of modified HPC solutions with a temperature sweep from 25 °C to 60 °C. Data was collected at a spindle rate of 100 s−1. Modification increases the viscosity and adds hydrophobicity causing the LCST to decrease (shifting the curves to the left). | ||
 | ||
| Fig. 4 The LCST was determined at the point of intersection between the slope of the initial plateau and the slope of the decreasing viscosity region in figure 3. The LCST lowers with increasing modifier concentration. | ||
3.3 Swelling
The swelling response of the films was investigated. Films were placed in distilled water at 25 °C and 60 °C. The swollen gels were periodically removed from the water and weighed using the blot-and-weigh method where excess water was removed from the polymer with Kimwipes. The swelling degree, Q, was calculated according to equation 1, where w is the weight of the swollen gel and w0 is the weight of the dry gel.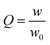 | (1) |
Swelling ratios, Q, were calculated and plotted versus time. At room temperature, solution crosslinked gels swell more than bulk crosslinked gels (Fig. 5). The gels crosslinked at 5 °C swell significantly more than the other gels and take over 72 h to fully equilibrate. At 60 °C all gels collapse (Fig. 6). Both gels crosslinked at 25 °C reach equilibrium before the 5 °C gel.
 | ||
| Fig. 5 Swelling ratio of gels in 25 °C water. Solution crosslinked gels swell more than bulk crosslinked gels. The gels crosslinked at 5 °C swell significantly more than the other gels indicating a homogeneous gel. Decreasing the crosslink temperature creates a more homogeneous gel. | ||
 | ||
| Fig. 6 Collapse transition of gels in 60 °C water. When placed in water above the LCST the gels begin to collapse. | ||
3.4 Diffusion
Diffusion measurements were performed with a vertical diffusion cell. The films were tested with a Hanson Research MicroettePlus autosampling system. The diffusion cells are water jacketed allowing for diffusion testing above room temperature. The model flavour benzaldehyde was used for these experiments. Benzaldehyde is a small, volatile flavour molecule and is stable under these testing conditions. Benzaldehyde was mixed with food grade oil Miglyol 812 at 1000 ppm benzaldehyde in oil.The test was setup by filling the acceptor cell with distilled water. Gel films were swollen in distilled water for 72 h before testing. The films were placed on top of the acceptor cell making sure no air bubbles form beneath the film. The donor cell was clamped on top of the film and was filled with 5 ml of benzaldehyde solution. The MicroettePlus system automatically dispenses aliquots from the acceptor cell at specified time intervals. These aliquots were taken to a UV-visible spectrophotometer to determine the diffusing concentration based on an absorbance calibration curve for known benzaldehyde concentrations.
Data were collected as absorbance (a.u.) versus time (s) and then converted to benzaldehyde concentrations. Diffusion coefficients, D, were calculated using equations 2–4. Here, C0 is the initial concentration of the benzaldehyde in migloyl, C*0 is the initial concentration of benzaldehyde at the oil-water interface, Pow is the oil-water partition coefficient of benzaldehyde, Ct is the benzaldehyde concentration in the acceptor cell at time t, A is the area of the gel membrane, V is the volume of the acceptor cell, P is the membrane permeability, l is the membrane thickness, and Kd is the partition coefficient of the membrane. The oil-water partition coefficient was found to be 38 by the analytical group at Givaudan Flavours. The membrane partition coefficient did not change significantly between samples and was taken as 1 since the membrane is a hydrogel, allowing the use of the following equations.17
 | (2) |
 | (3) |
 | (4) |
Diffusion coefficients are calculated for HPC gel films at room temperature and 60 °C (Fig. 7). No change in diffusion properties is observed for crosslinking times greater than 1 h. Diffusion data for bulk crosslinked films at room temperature show decreasing diffusion coefficient with increasing modifier concentration. Increasing the modifier concentration increases the number of crosslinking sites leading to higher crosslink density. Higher crosslink density causes the films to act more like a solid material thus slowing flavour diffusion. Solution crosslinked films do not follow this trend; the factors governing the diffusion properties of these gels are more complicated and are yet to be determined.
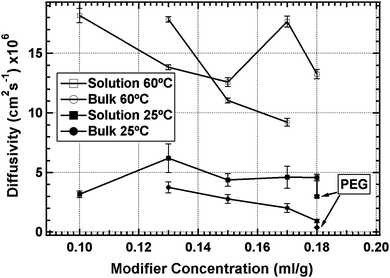 | ||
| Fig. 7 Diffusion coefficients of benzaldehyde through HPC crosslinked at 25 °C. The temperature in the legend indicates the temperature of the diffusion test. Bulk crosslinked films show trends of decreasing diffusivity with increasing modifier concentration. Solution crosslinked films do not show this trend. Increasing the temperature to 60 °C causes the diffusivity to increase. The addition of PEG-DMA (indicated by arrows) decreases the diffusivity. | ||
The lowest diffusion coefficients are obtained from the films crosslinked with PEG-DMA. The PEG-DMA acts as a crosslinking agent. The short chain polymer acts as a bridge to link crosslink points, which, by virtue of spatial separation, may have gone unreacted without the oligomeric crosslinking agent. The result is higher crosslinking density typically leading to reduced diffusivity. It is also possible that the PEG modified the domain structure leading to decreased diffusion coefficient. Small angle scattering would have to be performed on these samples to determine any structural changes.
Diffusion data at 60 °C show an increase in diffusion coefficient compared to room temperature. The ratio between the diffusion coefficient at 60 °C and the coefficient at room temperature is used as the performance measure. A lower ratio indicates better performance. Both bulk and solution crosslinked films have an average ratio near 4 (Fig. 7). Based on Fig. 7, HPC modified with 0.18 ml/g was chosen for crosslinking at 5 °C. HPC films crosslinked at 5 °C have the lowest ratio near 1 (Fig. 9). That is, diffusivity for the 5 °C gel is independent of temperature between room temperature and 60 °C.
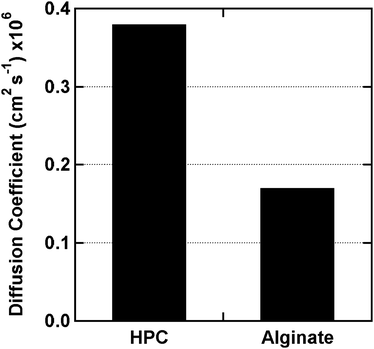 | ||
| Fig. 8 Diffusion coefficients of HPC and alginate films crosslinked and tested at 25 °C. The HPC films were modified with 0.18 ml of MAC per g of HPC and crosslinked with the PEG-DMA oligomer. The HPC films are comparable to highly crosslinked alginate films. | ||
The HPC performance is compared with alginate films (Figs. 8 & 9). Alginate gels were chosen since they are a common shell material in the flavor industry and considered to be good performers for hydrogels. For the technology in this paper to be viable, it would have to perform as well as current alginate films in the industry. The alginate film has a lower diffusion coefficient at room temperature than HPC (Fig. 8). At 60 °C however the alginate diffusivity increases by a factor of 7 (Fig. 9), whereas the HPC diffusivity is nearly independent of temperature. Reducing the high temperature diffusivity is the key objective of this study. It is nearly impossible to prevent diffusion; therefore, even a slight change in diffusion coefficient would bring an advantage to barrier properties. The food industry is interested in slowing diffusion. A rate change by a factor of even 2 would prove significant, i.e. the capsule would retain the flavour twice as long.
 | ||
| Fig. 9 HPC diffusion ratio (diffusion coefficient data at 60 °C divided by diffusion coefficient data at 25 °C) compared with alginate. A lower ratio indicates better high temperature performance. The HPC film was crosslinked at 5 °C while the alginate was crosslinked at 25 °C. Alginate increases in diffusivity by a factor of 7 at increased temperature while HPC can be processed to show no change. | ||
3.5 Scattering
Scattering measurements were performed on the gels to correlate gel morphology to bulk diffusive properties. Ultra-small angle neutron scattering (USANS) was performed using the Perfect Crystal Diffractomer on beam port BT-5 at the NIST Center for Neutron Research at the National Institute for Standards and Technology (NIST) in Gaithersburg, Maryland. Ultra-small angle X-ray scattering (USAXS) was performed at the 33ID beamline operated by X-ray Science Division of the Advanced Photon Source, Argonne National Laboratory in Argonne, Illinois, USA.Small-angle data were analyzed as intensity, I, versus the modulus of the momentum transfer wave vector, q, where q is related to the scattering angle θ by q = (4π/λ)sin(θ/2), and λ is the wavelength of the X-rays or neutrons. The USANS q range is ∼ 0.00003 ≤ q ≤ 0.01 Å−1 and the USAXS q range is ∼ 0.0001 ≤ q ≤ 1.0 Å−1. The collected data were analyzed using a unified fitting method to extract Guinier length scales and power-law slopes.18–20
Films characterized by USANS were hydrated in D2O and placed in a sample cell between two quartz windows with a path length of 1 mm. The sample cell was filled with D2O while ensuring no air bubbles were present.
Films prepared for USAXS at Argonne were hydrated in H2O and placed in a sample cell between two Kapton windows with a path length of 1 mm. The cell was filled with H2O while ensuring no air bubbles were present.
Fig. 10 shows the influence of modifier concentration on the morphology of gels bulk-crosslinked at ambient temperature and observed at 25 °C. The 0.17 and 0.13-ml/g modified samples have a shoulder region indicative of a morphological feature (presumably a phase-separated domain) with a radius-of-gyration, Rg, of 0.8 ± 0.3 µm and a power-law slope of −3.6 ± 0.5. By contrast, the 0.10-ml/g sample shows no shoulder in the USANS q range. Power-law slopes of −3.6 are indicative of a rough domain interface. The 0.17 ml/g sample shows the highest intensity in the shoulder region while the 0.13 ml/g sample is less intense and the 0.10 ml/g sample has no shoulder. The featureless scattering in the last case is typical of parasitic scattering from a homogeneous film. The intensity trend shows that the degree of phase separation increases with increasing modifier concentration. This behavior is typical of polymerization induced phase separation. Since the gels are crosslinked, phase separation is limited to micrometer length scales.
 | ||
| Fig. 10 USANS data of bulk crosslinked gels collected at 25 °C. All gels were crosslinked at 25 °C and swollen in D2O. A D2O background was used. As modifier concentration increases a shoulder appears near 0.8 µm. The shoulder indicates phase separation is occurring. | ||
USAXS was used to study the influence of crosslinking temperature on the morphology of the solution crosslinked gels. Films modified at 0.17-ml/g were examined at 25 °C (Fig. 11) and 60 °C (Fig. 12) in the wet state. These data show features similar to the bulk-crosslinked gels (Fig. 10) although the relevant length scales are smaller and the features are less distinct at 25 °C. Tested at 25 °C, the gel crosslinked at 5 °C shows no shoulder while all other samples have shoulder regions. The shoulder is present in the 25 °C-crosslinked gel with an Rg of 189 ± 30 Å and the Rg shifts to larger sizes (lower q) as the crosslink temperature increases (Fig. 11). For the 60 °C crosslinked gel, Rg approaches that observed by USANS for the gel crosslinked in bulk.
 | ||
| Fig. 11 USAXS data on solution-crosslinked gels collected at 25 °C. All gels were swollen in H2O with an H2O background. The legend indicates the crosslinking temperature varying from 5 °C to 60 °C. At a crosslinking temperature of 25 °C a shoulder appears. The shoulder shifts to lower q as crosslinking temperature increases. | ||
 | ||
| Fig. 12 USAXS data of solution crosslinked gels collected at 60 °C. The gels were swollen in H2O with an H2O background. The legend indicates the crosslinking temperature varying from 5 °C to 60 °C. Each data set has a shoulder that shifts to lower q with increasing crosslinking temperature. | ||
The USAXS data measured at 60 °C show distinct evidence of microphase separation. Regardless of crosslinking temperature, all samples show a shoulder region followed by a power law slope of −4.0 ± 0.2, the value characteristic of a sharp interface. The gel crosslinked at 5 °C has the smallest Rg. The Rg of each sample increases with increasing crosslink temperature (Fig. 12).
4. Discussion
Since the UV light crosslinks the HPC, it is clear the HPC has been successfully vinylized. Interestingly, the modification reaction causes the LCST to decrease; as the modifier concentration increases the HPC phase separates at lower temperature. The LCST is governed by a balance between hydrophobicity and hydrophilicity in the polymer solution. The MAC adds hydrophobicity to the HPC causing the LCST to decrease. Enhanced hydrophobicity and subsequent increase in the degree of phase separation is evidenced by the emergence of shoulders attributed to domain formation in the USANS and USAXS data.The USANS shoulder is attributed to micro-phase separation. The degree of phase separation increases with increasing modifier concentration and increasing crosslinking temperature. By degree of phase separation we mean the discontinuity in the polymer concentration between the polymer-rich and polymer-lean domains.
We envision the relationship between micro phase separation and processing protocol to proceed as follows. The hydrophobicity of MAC leads to cooperative concentration fluctuations of the modifier during the modification step. That is, hydrophobicity causes non-random modification whereby modification sites are clustered. This clustering then leads to topological fluctuations during crosslinking. Since crosslinking acts similarly to an attractive interaction between polymer chains, the LCST is lower in the more densely crosslinked regions of the gel, leading to microphase separation of these regions on increasing the temperature.
Since the UV lamp heats the sample, it plays a significant role in controlling gel properties. The temperature rise caused by the UV lamp induces phase separation of the more densely crosslinked regions, cooperatively enhancing further crosslinking in these regions (cooperative hydrophobic effect). The crosslinking reaction then “locks in” the phase-separated domains even if the polymer is subsequently cooled below the LCST. The final gel consists of localized regions of high and low crosslink density.12
The morphological evolution described above is exploited to prepare microporous gels using thermally induced phase separation (TIPS).21 The TIPS technique requires crosslinking to occur near or above the LCST. The result is a matrix of interconnected micropores. For our application, however, the goal is to avoid microporosity, which would lead to enhanced permeability at high temperature.
The reverse of the TIPS strategy can be exploited to prevent microphase separation. If the polymer solution is cooled sufficiently below the LCST, phase separation is suppressed, resulting in a more homogeneous gel. The validity of this hypothesis is apparent in the swelling and USAXS data. The gel crosslinked at 5 °C swells significantly more than the other gels indicating a homogeneous gel (Figs. 5 and 6). A gel with phase-separated morphology is unlikely to swell as much as a homogeneous gel, since the phase separated regions act as physical crosslinks. Also the phase-separated domains will have a higher crosslink density thereby limiting the gel swelling.
The room temperature USAXS data (Fig. 11) show that the gel crosslinked at 5 °C has no morphological features in the observed q range. The absence of any shoulder in the USAXS data indicates crosslinking at 5 °C yields a homogeneous gel.
However, a shoulder does appear for gels crosslinked at 25 °C and higher (Fig. 11). This shoulder shifts to lower q with increasing crosslinking temperature indicative of increasing domain size. The USAXS data observed at 60 °C show that all gels micro-phase separate at high temperature. Phase separation is expected since above the LCST the gels collapse. When gels crosslinked at 5 °C collapse the phase separated domains are smaller. Gels crosslinked at higher temperatures already exist in a partially collapsed state, so they don't collapse as efficiently at 60 °C as the lower temperature crosslinked gels. The collapsed state of these gels consists of interconnected micropores leading to enhanced high-temperature permeability. The formation of interconnected micropores and inefficient collapse explains the unpredicted diffusion behavior of the solution crosslinked films in Fig. 7.
5. Conclusion
The diffusion properties of HPC barrier membranes can be manipulated by controlling crosslink density, crosslink temperature, and hydrophobicity. The room temperature diffusion properties are similar to baseline alginate membranes. With proper choice of synthetic protocol, however, HPC membranes show substantially reduced high-temperature diffusivity compared to alginate. Although factors other than morphology could impact transport properties, we were able to reduce high-temperature diffusivity by manipulation of the morphology. Using processing conditions to control molecular structure makes it possible to reach desired transport properties. HPC, therefore, is a credible encapsulant candidate with improved high temperature retention of flavours.6. Acknowledgement
We thank George Yang for his insights regarding modification chemistry. This work was supported by the Membrane Applied Science and Technology (MAST) Center at the University of Cincinnati through funding by the National Science Foundation and Givaudan Flavours. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. We acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce in providing the neutron research facilities used in this work. These facilities22 are supported in part by the National Science Foundation under Agreement No. DMR-0454672.7. Notes and references
- Y. H. Bae, T. Okano and S. W. Kim, J. Polymer. Sci. B Polymer Phys., 1990, 28, 923–936 Search PubMed.
- H. G. Schild, Progr. Polymer Sci., 1992, 17, 163–249 Search PubMed.
- T. Tanaka, Scientific American, 1981, 244, 124 CrossRef CAS.
- L. Brannon-Peppas and N. A. Peppas, Chem. Eng. Sci., 1991, 46, 715–722 CrossRef CAS.
- L. C. Dong and A. S. Hoffman, J. Controlled Release, 1991, 15, 141–152 CrossRef.
- S. H. Gehrke and E. L. Cussler, Chem. Eng. Sci., 1989, 44, 559–566 CrossRef CAS.
- T. G. Park and A. S. Hoffman, J. Appl. Polym. Sci., 1992, 46, 659–671 CrossRef CAS.
- D. Gan and L. A. Lyon, J. Am. Chem. Soc., 2001, 123, 7511–7517 CrossRef CAS.
- J. A. Hinkley, L. D. Morgret and S. H. Gehrke, Polymer, 2004, 45, 8837–8843 CrossRef CAS.
- C. D. Jones and L. A. Lyon, Macromolecules, 2000, 33, 8301–8306 CrossRef CAS.
- S. J. Risch, Encapsulation - Overview Of Uses And Techniques. In Encapsulation And Controlled Release Of Food Ingredients, 1995; Vol. 590, pp 2–7 Search PubMed.
- K. A. Heitfeld. Hydroxypropyl Cellulose for Flavor Encapsulation. University of Cincinnati, Cincinnati, 2006 Search PubMed.
- K. A. Heitfeld. Smart Membranes: Hydroxypropyl Cellulose for Flavor Delivery. University of Cincinnati, Cincinnati, 2007 Search PubMed.
- K. A. Heitfeld, T. Guo, G. Yang, D. W. Schaefer. Materials Science & Engineering, C: Biomimetic and Supramolecular Systems 2008 Search PubMed.
- E. Marsano, L. De Paz, E. Tambuscio and E. Bianchi, Polymer, 1998, 39, 4289–4294 CrossRef CAS.
- E. Marsano, S. Gagliardi, F. Ghioni and E. Bianchi, Polymer, 2000, 41, 7691–7698 CrossRef CAS.
- J. Zhang and N. A. Peppas, Macromolecules, 2000, 33, 102–107 CrossRef CAS.
- G. Beaucage, J. Appl. Crystallogr., 1996, 29, 134–146 CrossRef CAS.
- R. S. Justice, D. W. Schaefer, R. A. Vaia, D. W. Tomlin and T. J. Bunning, Polymer, 2005, 46, 4465–4473 CrossRef CAS.
- D. W. Schaefer, T. Rieker, M. Agamalian, J. S. Lin, D. Fischer, S. Sukumaran, C. Y. Chen, G. Beaucage, C. Herd and J. Ivie, J. Appl. Crystallogr., 2000, 33, 587–591 CrossRef CAS.
- B. G. Kabra, S. H. Gehrke and R. J. Spontak, Macromolecules, 1998, 31, 2166–2173 CrossRef CAS.
- J. G. Barker, C. J. Glinka, J. J. Moyer, M. H. Kim, A. R. Drews and M. Agamalian, J. Appl. Crystallogr., 2005, 38, 1004–1011 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
