Host–guest interaction of 1,4-dihydroxy-9,10-anthraquinone (quinizarin) with cyclodextrins
Noufal
Kandoth
,
Sharmistha Dutta
Choudhury
*,
Tulsi
Mukherjee
and
Haridas
Pal
*
Radiation & Photochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai, 400 085, India. E-mail: sharmidc@barc.gov.in; hpal@barc.gov.in; Fax: 91-22-25505151/25519613
First published on 29th October 2008
Abstract
The interaction of 1,4-dihydroxy-9,10-anthraquinone, (quinizarin; QZ), with α-, β- and γ-cyclodextrin (CD) hosts, has been investigated using absorption, and steady-state and time-resolved fluorescence measurements, in order to understand the effects of cavity size of CDs on the binding of QZ molecule and the changes in the photophysical properties of QZ in the microenvironment of the hosts. The results demonstrate that QZ forms inclusion complexes with all the CDs. The low binding constants as well as the thermodynamic parameters indicate that the mode of interaction between QZ and CDs is mainly hydrophobic in nature. The relative magnitudes of the binding constants and the differential enhancements in the fluorescence intensity of QZ upon complexation with the CDs can be explained by considering the relative dimensions of the host cavity and the guest molecule, as well as the orientation of the guest molecule inside the CD cavity. It is proposed that the unsubstituted benzene ring of QZ is encapsulated within α- and β-CD cavities whereas the dihydroxy-substituted aromatic ring is encapsulated within the γ-CD cavity. This is further supported by the complexation studies of the QZ·CD systems with Al(III) ions. It is observed that the complexation of QZ with the metal ion is enhanced in the QZ·α-CD and QZ·β-CD systems whereas it is significantly reduced in the QZ·γ-CD system, due to shielding of the chelating groups of the dye inside the CD cavity in the latter case.
1. Introduction
In recent years supramolecular host–guest interaction has been the subject of very active research due to its wide spread applications in various areas like pharmaceuticals, food technology, chemical industry, sensors, etc.1–6 Among the different types of available host molecules, cyclodextrins (CDs) have been the most extensively investigated due to their higher solubility in aqueous medium. CDs are cyclic oligosaccharides composed of D-glucopyranose units joined by ether linkages.2–4 Depending upon the number of monomer units, CD homologues of different sizes are obtained, the most common members of this family being α-, β-, and γ-CDs, which are made up of six, seven and eight D-glucopyranose units, respectively. The structure of the CDs is that of a truncated cone, having an inner hydrophobic cavity and outer hydrophilic edges consisting of hydroxyl groups. Solute molecules of suitable dimensions can interact and bind to the CD cavity by inclusion complex formation.7–9 The reduced polarity and restricted microenvironment provided by the CD cavity can markedly and advantageously influence a number of properties of the included solute.7–11 In this respect, the complexation of fluorescent dyes by macrocyclic hosts is of considerable interest because the host molecules can tune the photophysical characteristics of the dyes, often by enhancing the fluorescence quantum yields, fluorescence lifetimes and photostability of the molecules.11–14In this article we present a systematic study on the inclusion complex formation of 1,4-dihydroxy-9,10-anthraquinone (quinizarin; QZ) with α-, β- and γ-CD hosts, using absorption, and steady-state and time-resolved fluorescence measurements. The photophysics of QZ has generated much interest because this molecule undergoes both intra- and inter-molecular hydrogen bonding, which has a profound effect on its geometric, electronic, vibrational and photodynamic radiationless transition properties.15–21QZ and related compounds are also widely used as vat dyes, as fungicides, photo-initiators and additives in lubricants.22,23 In addition, QZ serves as a model for anthracycline antitumour antibiotics.24–26 The fluorescent complexes of QZ with many metal ions are used as spectrophotometric analytical reagents for the estimation of the ions.27–32 In this work, we have studied the effect of cavity size of the CDs on the binding of QZ and the consequent changes in the photophysical properties of the dye in the microenvironment of the CD receptors. The reported complexation of QZ with Al(III) ions,28 has also been exploited to reveal the orientation of QZ molecule inside the CD cavities. Chemical structures of the dye, QZ, and the CDs used are shown in Scheme 1 along with their relative dimensions.
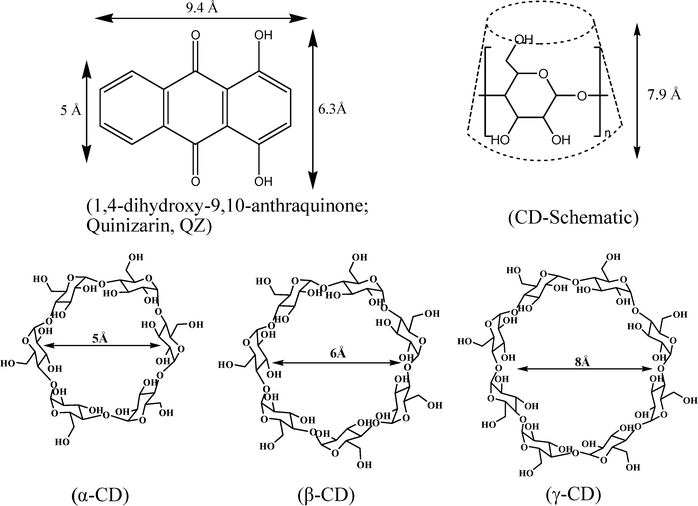 | ||
| Scheme 1 Chemical structures of QZ and α-, β- and γ-cyclodextrins. A schematic presentation of the truncated cone shape of the CDs is also shown. | ||
2. Experimental
Quinizarin (QZ) was obtained from Aldrich and was used after recrystallization from water-methanol mixture. The cyclodextrins (α-, β- and γ-CDs) were purchased from TCI Mark, Tokyo and were used as received without further purification. A concentrated stock solution of QZ was prepared in methanol and small aliquots were added to water for preparing aqueous solutions of QZ with concentration around 10–20 μM. The inclusion behavior of QZ with α-, β- and γ-CDs was studied by adding different weighed amounts of the corresponding CDs to the aqueous solution of QZ. Nanopure water with conductivity less than 0.1 μS cm−1 was obtained from a Millipore Elix3/A10 water purification system and was used to prepare all sample solutions.Absorption spectra were recorded with a Shimadzu UV-vis spectrophotometer (model UV-160A) and steady-state fluorescence spectra were recorded with a Hitachi spectrofluorimeter (F-4010). An IBH instrument, based on the time-correlated single-photon-counting (TCSPC) principle, was used for the time-resolved fluorescence measurements. A 440 nm diode laser (∼100 ps, 1 MHz repetition rate) was used as the excitation source and a MCP-PMT based detection module was used for the measurement of the fluorescence decays. Except for anisotropy measurements, all other fluorescence decays were collected at magic angle (54.7°) with respect to the vertically polarized excitation light, to avoid the effect of rotational depolarization of the dye on the measured fluorescence lifetimes.33,34 The DAS-6 software from IBH was used for the deconvolution analysis of the observed decays, considering either mono-exponential or multi-exponential decay functions. The quality of the fits and consequently the mono- and bi-exponential natures of the decays were judged by the reduced chi-square (χ2) values and the distribution of the weighted residuals among the data channels. For a good fit, the χ2 value was close to unity and the weighted residuals were distributed randomly among the data channels.33,34 For fluorescence anisotropy measurements, the polarized fluorescence decays for the parallel (III(t)) and perpendicular (I⊥(t)) emission polarizations with respect to the vertical excitation polarization were first collected. Using these III(t) and I⊥(t) decays, the anisotropy decay function, r(t), was constructed as,
 | (1) |
Unless otherwise stated, all measurements were carried out at ambient temperature, 25 ± 1 °C. Some of the binding studies for the present systems were, however, carried out with changing temperature. In these studies, a microprocessor based temperature controller (model DS from IBH) was used to adjust the temperatures of the solutions (within ± 1°C) with the help of a cold finger arrangement.
3. Results and discussion
3.1. Absorption spectral characteristics of QZ in the presence of the CDs
In aqueous solution, QZ shows an absorption peak at ∼470 nm with a shoulder at ∼538 nm. On addition of α-CD to the aqueous solution of QZ, the absorption band shows ∼8 nm bathochromic shift along with a gradual decrease in the absorbance, finally attaining a limiting value. These changes in the absorption characteristics of QZ in presence of varying α-CD concentrations are shown in Fig. 1a. In the presence of β-CD, there is a slight initial decrease followed by a gradual increase in the absorbance of QZ with increasing CD concentration, and concomitantly, the hump around 538 nm is blue shifted to ∼520 nm (Fig. 1b). On addition of γ-CD, a gradual increase in OD is observed along with a blue shift of the hump around 538 nm to ∼520 nm (Fig. 1c). These results indicate that QZ interacts with all the three CDs used. However, the interactions in all the three cases appear to be significantly different, suggesting the possible differences in the nature of interaction depending on the cavity sizes of the CDs involved. The significant decrease in absorbance in the presence of α-CD can arise either due to some structural change of the dye on inclusion, or due to the reduced solubility of the QZ·α-CD complex in aqueous solution. To be noted, that at high α-CD concentration, fine particles were observed in the solution by visual inspection, which suggests the reduced solubility of the QZ·α-CD complex as the main cause for the decrease in absorbance with increasing α-CD concentration.![a Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different α-CD concentrations. [α-CD]/mM: (1) 0.0, (2) 1.5, (3) 3.5, (4) 6.3, (5) 23.0 and (6) 28.0. b Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different β-CD concentrations. [β-CD]/mM: (1) 0.0, (2) 2.3, (3) 10.0 and (4) 13.5. c Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different γ-CD concentrations. [γ-CD]/mM: (1) 0.0, (2) 6.5, (3) 14.0 and (4) 29.0.](/image/article/2009/PP/b815294b/b815294b-f1.gif) | ||
| Fig. 1 a Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different α-CD concentrations. [α-CD]/mM: (1) 0.0, (2) 1.5, (3) 3.5, (4) 6.3, (5) 23.0 and (6) 28.0. b Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different β-CD concentrations. [β-CD]/mM: (1) 0.0, (2) 2.3, (3) 10.0 and (4) 13.5. c Representative absorption spectra of QZ (20 × 10−6 M) in aqueous solution at different γ-CD concentrations. [γ-CD]/mM: (1) 0.0, (2) 6.5, (3) 14.0 and (4) 29.0. | ||
The dihydroxy-substituted ring of QZ has a dimension of about 6.3 Å where as the unsubstituted aromatic ring on the other side has a dimension of ∼5 Å. Therefore, considering the size of the α-CD cavity, (inner diameter ∼5 Å), it is expected that the dihydroxy-substituted ring of the dye possibly cannot enter the α-CD cavity (Scheme 1). Hence, it is likely that the inclusion of QZ inside α-CD cavity takes place with the unsubstituted ring of the dye entering the host cavity through its wider rim (Scheme 2). Such an orientation leaves not only the dihydroxy-substituted ring but also the quinonoid oxygens of the dye projected outside the host cavity towards the bulk aqueous phase. Since with such an orientation the quinonoid oxygens are placed close to the OH groups of α-CD at the wider rim, they can participate in strong intermolecular hydrogen bonding interaction in the QZ·α-CD complex. This intermolecular interaction can thus disrupt the cyclic intramolecular hydrogen bonding network present in the dye15–20 and accordingly can reduce the quasi-aromatic character of the dye inside the α-CD cavity. This could cause an additional effect towards the reduced absorbance of the dye in the presence of α-CD along with the solubility limitation as mentioned previously. That for QZ·α-CD complex, the dihydroxy-substituted ring of the dye is exposed to the bulk aqueous phase is also indicated by the fact that the shape of the absorption spectrum, especially the hump at ∼538 nm, remains effectively unchanged even after inclusion of the dye in α-CD.
 | ||
| Scheme 2 Schematic representation of the orientation of QZ inside α-, β- and γ-CD cavities depending on their relative dimensions. | ||
In the case of γ-CD, since the cavity size is large enough, it can allow the inclusion of almost the whole QZ molecule inside the host cavity (Scheme 2). Moreover, the inclusion of QZ into γ-CD cavity can take place with any of the two possible orientations, i.e. the unsubstituted ring placed close to the narrow rim or the wider rim of the host. With either of these orientations, the quinonoid oxygens cannot have an access to the portal OH groups of the host and accordingly no intermolecular hydrogen bonding is possible between them. Thus, the intramolecular hydrogen bonding network and the inherent quasi-aromatic characteristic of QZ molecule remains unaffected inside the γ-CD cavity. This is possibly reflected as the marginal increase in the peak absorbance and as the blue shift in the longer wavelength hump of the dye in presence of γ-CD. With β-CD, since the inner diameter of the cage (∼6 Å) is just similar to the width of the dihydroxy-substituted ring of QZ, it is likely that the dye enters the β-CD cavity with its unsubstituted ring placed close to the narrow rim of the host to provide maximum inclusion of the dye in the host cavity. Though this orientation of the dye in β-CD cavity is similar to that anticipated in the case of α-CD, it is expected that in β-CD, the dye can enter much deeper into the host cage such that the quinonoid ring is completely buried inside the cage (Scheme 2). So a comparison of the relative sizes of the host cavities and the dimension of the dye can reasonably justify the observed changes in the absorption characteristics for the present systems. As the changes in the absorption characteristics of QZ dye in presence of β- and γ-CDs are not that large and since in the case of α-CD these changes are indicated to be due to solubility problem, we did not use these absorbance changes for any quantitative estimation of the binding constants for the inclusion complexes. For the present systems, all quantitative analyses were done following the changes in the fluorescence characteristics of the dye, as discussed in the next section.
3.2. Steady-state fluorescence characteristics of QZ in the presence of CDs
In aqueous solution, the dye QZ shows an emission peak at ∼570 nm with a prominent shoulder at 545 nm. The fluorescence intensity is found to increase on addition of α-, β- or γ-CD. To be mentioned that in the case of α-CD, the enhancement is not that large as such but becomes quite significant after making a correction for the reduced absorbance of the solution in presence of α-CD. For β- and γ-CD, however, the fluorescence enhancements are quite prominent as such. The steady-state fluorescence characteristics of QZ in the presence of the three CDs are shown in Fig. 2a–c.![a Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution (after correcting for absorbance changes) at different α-CD concentrations, [ α-CD]/mM: (1) 0.0, (2) 0.6, (3) 1.5, (4) 3.5, (5) 6.3, (6) 10.3, (7) 15.8 and (8) 23.0. Excitation wavelength was 490 nm. Inset-I: Shows the fluorescence spectra under similar conditions but without correcting for the decrease in the absorbance. Inset-II: Shows the binding isotherm for QZ·α-CD complex using the data corrected for the absorbance changes. b Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution at different β-CD concentrations, [ β-CD]/mM: (1) 0.0, (2) 1.2, (3) 2.0, (4) 3.4, (5) 5.3, (6) 8.8, (7) 12.8, (8) 15.8 and (9) 19.2. Excitation wavelength was 490 nm. Inset shows the binding isotherm for QZ·β-CD complex using the data corrected for the absorbance changes. c Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution at different γ-CD concentrations, [γ-CD]/mM: (1) 0.0, (2) 0.6, (3) 1.8, (4) 3.6, (5) 6.4, (6) 10.3, (7) 14.5, (8) 18.8 and (9) 24.1. Excitation wavelength was 490 nm. Inset shows the binding isotherm for QZ·γ-CD complex using the data corrected for the absorbance changes.](/image/article/2009/PP/b815294b/b815294b-f2.gif) | ||
| Fig. 2 a Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution (after correcting for absorbance changes) at different α-CD concentrations, [ α-CD]/mM: (1) 0.0, (2) 0.6, (3) 1.5, (4) 3.5, (5) 6.3, (6) 10.3, (7) 15.8 and (8) 23.0. Excitation wavelength was 490 nm. Inset-I: Shows the fluorescence spectra under similar conditions but without correcting for the decrease in the absorbance. Inset-II: Shows the binding isotherm for QZ·α-CD complex using the data corrected for the absorbance changes. b Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution at different β-CD concentrations, [ β-CD]/mM: (1) 0.0, (2) 1.2, (3) 2.0, (4) 3.4, (5) 5.3, (6) 8.8, (7) 12.8, (8) 15.8 and (9) 19.2. Excitation wavelength was 490 nm. Inset shows the binding isotherm for QZ·β-CD complex using the data corrected for the absorbance changes. c Steady-state fluorescence spectra of QZ (20 × 10−6 M) in aqueous solution at different γ-CD concentrations, [γ-CD]/mM: (1) 0.0, (2) 0.6, (3) 1.8, (4) 3.6, (5) 6.4, (6) 10.3, (7) 14.5, (8) 18.8 and (9) 24.1. Excitation wavelength was 490 nm. Inset shows the binding isotherm for QZ·γ-CD complex using the data corrected for the absorbance changes. | ||
As evident from Fig. 2a–c, the extent of the changes in the fluorescence characteristics of the dye in the presence of the three CDs are significantly different. In the presence of α-CD, the nature of the fluorescence spectrum remains effectively unchanged but there is about 4 times enhancement in the fluorescence intensity especially after correction for the decrease in the absorbance, though the fluorescence enhancement as such (without absorbance correction) is just marginal (Inset-I, Fig. 2a). In case of β-CD, the enhancement in the fluorescence intensity is as such very significant (∼9 times) and is also accompanied by a small blue shift (∼5 nm) in the emission peak along with a relative increase in the resolution of the vibrational bands in the spectrum. For γ-CD, the enhancement in the fluorescence intensity is about 5 times but the nature of the fluorescence spectrum remains effectively unchanged. Most interestingly, in the case of γ-CD, the changes in the steady-state fluorescence characteristics of QZ appear to get saturated at about 20 mM of γ-CD concentration, though at the similar range of α-CD and β-CD concentrations, the fluorescence characteristics still continue to change. This indicates that the binding constant for QZ·γ-CD system is much higher than QZ·α-CD and QZ·β-CD systems. This is expected since the dye can enter the γ-CD cavity with either of the two possible orientations as discussed earlier, but in the case of α-CD and β-CD only one of the two orientations is sterically allowed for the dye inclusion.
The enhancement in the emission intensities for the present systems is certainly due to the confinement of the QZ molecule inside the CD cavities, as these confinements will restrict the vibrational and rotational motions of the dye that are coupled to its nonradiative deexcitation process.7,9–14 So the steady-state fluorescence results clearly indicate the formation of inclusion complexes of QZ with the three CDs used. The differences in the effect of the three CDs on the fluorescence properties of QZ are thus proposed to be due to the combined effect of the differences in the orientation of the dye molecule inside the host cavities and the differential extent of restrictions imposed by the hosts to the vibrational and rotational motions of the encapsulated dye.9 As proposed earlier, the orientation of QZ inside α-, and β-CD is such that the dihydroxy-substituted ring of the dye is fully or partially exposed, respectively, to the aqueous phase outside (Scheme 2). Thus for QZ·β-CD complex, the dye experiences much more rigidity inside the host cavity than in the case of QZ·α-CD complex. Accordingly, the nonradiative processes for excited QZ are much efficiently reduced in QZ·β-CD complex, leading to a significant enhancement in the radiative fluorescence intensity. The largely reduced mobility of QZ inside β-CD is also reflected from the better resolution of the vibrational bands in the fluorescence spectra. In the case of QZ·α-CD complex, the lower enhancement in the fluorescence intensity is further aggravated due to the reduced solubility of the complex in the solution. Compared to QZ·β-CD system, although in QZ·γ-CD system the bulkier dihydroxy-subsituted ring of QZ is encapsulated within the host cavity, the larger cavity dimension of γ-CD compared to the size of the guest molecule causes the dye to experience relatively less restriction for its mobility and consequently the nonradiative processes are not arrested to the same extent as in β-CD cavity. Thus fluorescence enhancement for QZ·γ-CD complex is comparatively less than that for QZ·β-CD complex.
In order to determine the binding constants (Keq) for different QZ·CD complexes, we adopted the widely used fluorescence titration method,7,9–14 considering the 1:1 complexation model for the dye with the three CDs (eqn (2)).
 | (2) |
Taking [Dye]0 and [CD]0 as the total concentrations of QZ and CD, respectively, eqn (3) can be derived for the concentration of the free (uncomplexed) dye in the solution in equilibrium with the dye·CD complex:
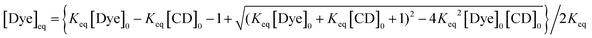 | (3) |
Exchange of the dye, i.e., the conversion of the uncomplexed dye to the complexed one and vice versa, can be excluded within the excited-state lifetime of the dye (<2 ns, see below), because the corresponding guest exchange rate constants are very small for cyclodextrin.11 The fluorescence intensity can therefore be considered as a composite of the fluorescence intensity contributions from the complexed and uncomplexed forms of the dye and should be expressed as eqn (4):
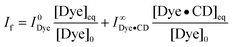 | (4) |
 | (5) |
In the fluorescence titration studies, the changes in the emission intensities in the presence of β-CD and γ-CD were employed as the experimental measures, after correcting for the small absorbance changes. The fluorescence titration curves thus obtained for QZ dye in the presence of varying β- and γ-CD concentrations are shown in the insets of Fig. 2b and c, respectively. The binding constant values estimated from the fitting of the fluorescence titration curves following eqn (5) are 39 M−1 and 96 M−1 respectively, for QZ·β-CD and QZ·γ-CD systems. It is indicated from these values that QZ binds more strongly in γ-CD cavity than β-CD cavity. This is possibly due to better accommodation of the dye in the larger γ-CD cavity (almost the whole QZ molecule can enter the γ-CD cavity) in comparison to that in β-CD cavity (the dihydroxy-substituted ring remains outside the cavity). Moreover, in γ-CD, the dye can adopt either of the two possible orientations as discussed earlier and this in effect causes a better binding affinity for the dye with γ-CD cavity than with β-CD cavity. For α-CD, since the changes in the absorbance values were quite large, and since there was an indication of reduced solubility for the QZ·α-CD complex in solution, we anticipate that a considerably large error is involved in correcting the fluorescence intensity for the absorbance changes (Fig. 2a). Still we followed the similar procedure to construct the fluorescence titration curve (Fig. 2a, Inset II) and an approximate value for Keq is estimated, ∼80 M−1, for the QZ·α-CD system. Considering the significant error involved in the analysis, we feel that this is an upper limit of the Keq value for the QZ·α-CD system. In general, the Keq values obtained for the present QZ·CD systems are not that large, suggesting that the inclusion complexes in the present systems are formed mainly due to hydrophobic interaction between the encapsulated dye and the CD cages.1–6
To have an idea about the thermodynamics of the binding process, we also studied the temperature dependence of the binding constant for QZ·γ-CD complex as a representative case, since the Keq value was the highest for this system. It is found that the binding constant value reduces with an increase in the temperature (Fig. 3). The enthalpy and entropy changes for the binding of QZ to γ-CD cavity were determined following the van't Hoff equation (eqn (6));35 and the corresponding plot is shown in the inset of Fig. 3.
 | (6) |
 | ||
| Fig. 3 Binding isotherms for γ-CD·QZ system at 30 °C (▲), 40 °C (■) and 60 °C (●). Inset shows the van't Hoff plot for the same system. | ||
The results show that the complexation process is driven by the combined effect of a favorable enthalpy change (−ΔH° = 1.2 kcal mol−1) and a gain in the entropy (TΔS° = 1.5 kcal mol−1 at 298 K) of the system. The observed enthalpy change, which is not very large, is quite expected because the weak hydrophobic interaction mainly stabilizes the inclusion complexes for the present systems. The entropy gain possibly results from the release of the high energy water molecules from the host cavity during the encapsulation of the dye into the host cages.3,5
3.3. Effect of inclusion complex formation on the fluorescence lifetime of QZ
Fluorescence decays of QZ in aqueous solution were investigated both in the presence and absence of different CD concentrations (Fig. 4). The fluorescence decay of QZ is single exponential in the absence of the host molecules and the fluorescence lifetime (τ) is measured to be 1.4 ns (Table 1). In the presence of α-CD, the fluorescence lifetime remains almost unchanged. This is quite understandable because in QZ·α-CD complex all the substituent groups of the encapsulated QZ molecule remain essentially exposed to the bulk water outside and hence the dye does not experience any significant reduction in its nonradiative deexcitation rate. In the presence of β-,and γ-CDs, fluorescence decays of QZ are seen to fit well with a bi-exponential function, with a shorter lifetime component (∼1.4 ns) corresponding to the uncomplexed QZ and a longer lifetime component (∼1.8 ns) for the QZ·CD complex. With increasing CD concentration, the contribution of the shorter lifetime component decreases and the contribution of the longer lifetime component increases. Representative fluorescence lifetime results are shown in Table 1.| Medium | A 1 (%) | τ 1/ns | A 2 (%) | τ 2/ns | χ2 |
|---|---|---|---|---|---|
| water | 100 | 1.4 | — | — | 1.21 |
| aq. α-CD (6 mM) | 100 | 1.4 | — | — | 1.23 |
| aq. α-CD (25 mM) | 100 | 1.4 | — | — | 1.28 |
| aq. β-CD (5 mM) | 50 | 1.4 | 50 | 1.96 | 1.20 |
| aq. β-CD (19 mM) | 30 | 1.4 | 70 | 2.0 | 1.09 |
| aq. γ-CD (6 mM) | 40 | 1.4 | 60 | 1.85 | 1.26 |
| aq. γ-CD (25 mM) | 20 | 1.4 | 80 | 1.86 | 1.17 |
 | ||
| Fig. 4 Fluorescence decay traces for (1) QZ (20 × 10−6 M) and QZ in presence of (2) γ-CD (25 × 10−3 M) and (3) β-CD (19 × 10−3 M). L is the lamp profile. | ||
An increase in the fluorescence lifetime of QZ on complexation with β-,and γ-CDs is due to the inclusion of the dye into the CD cavity. As discussed earlier, the inclusion of the dye into CD cavity would lead to a decrease in the freedom for the vibrational and rotational motions of the dye and hence will cause a reduction in the nonradiative deexcitation channel of the excited molecule.11,13 Thus, inclusion of the dye in β-, and γ-CD cavities causes an increase in the fluorescence lifetime as well as an increase in the fluorescence intensity as observed in the present study. No change in the fluorescence lifetime for QZ·α-CD complex is tentatively in accordance with the very small fluorescence enhancement observed for this complex (cf. Section 3.2). The situation for QZ·α-CD system might also be aggravated due to the reduction in the solubility of this complex in solution as discussed earlier (cf. Section 3.1).
3.4. Time-resolved fluorescence anisotropy studies on QZ·CD systems
Time-resolved fluorescence anisotropy decays were measured for QZ in the absence and in presence of the three CDs. The fluorescence anisotropy decay rate and hence the rotational relaxation time of any fluorescent molecule depends upon its size and shape as well as on the viscosity of its local environment.33 So it is expected that the rotational relaxation time (τr) of QZ should increase upon complexation, as it will increase the effective size of the encapsulated fluorophore (dye + host).7 The expected increase in τr values has been observed very clearly in the cases of QZ·β-CD and QZ·γ-CD systems. The anisotropy decay traces were found to be effectively single exponential in both the cases, with similar rotational correlation times of ∼500 ps. The anisotropy decay trace for the free QZ molecule in aqueous solution, on the other hand, was very fast (<100 ps) and beyond the time resolution of the present TCSPC set-up (∼100 ps). For QZ·α-CD systems, the anisotropy decay was found to be bi-exponential in nature, with a very fast component (<100 ps, relative contribution, 55%) possibly corresponding to the rotation of the free dye and a slower component (∼500 ps, relative contribution, 45%), corresponding to the rotation of the complexed form. The lower contribution of the complexed form, in this case, could be due to the solubility problem of the QZ·α-CD complex as indicated earlier. However, the 500 ps component of the rotational time indicates that an inclusion complex is indeed formed between α-CD and QZ. The anisotropy decay traces for QZ in the absence and presence of the CDs are shown in Fig. 5 and the decay parameters are presented in Table 2.| A 1 (%) | τ r1 /ps | A 2 (%) | τ r2 /ps | r 0 | χ2 | |
|---|---|---|---|---|---|---|
a Anisotropy decays were analyzed as,  for single and bi-exponential decays. The relative contributions were estimated as for single and bi-exponential decays. The relative contributions were estimated as 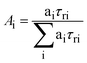 .
b Based on the time resolution of our TCSPC set-up (∼100 ps), the fast decay component is considered to be <100 ps for all practical purposes. .
b Based on the time resolution of our TCSPC set-up (∼100 ps), the fast decay component is considered to be <100 ps for all practical purposes.
|
||||||
| QZ | 100 | 70b | — | — | 0.26 | 1.21 |
| QZ·α-CD | 55 | 70b | 45 | 446 | 0.26 | 1.23 |
| QZ·β-CD | 100 | 513 | — | — | 0.26 | 1.18 |
| QZ·γ-CD | 100 | 476 | — | — | 0.26 | 1.10 |
 | ||
| Fig. 5 Fluorescence anisotropy decays, r(t), for QZ in (1) water and in the presence of (2) α-CD (25 mM), (3) γ-CD (25 mM) and (4) β-CD (19 mM). The smooth lines show the best fit curves. | ||
3.5. Interaction of QZ·CD complexes with Al(III) ions
It is reported that QZ forms strong complexes with many metal ions in which the hydroxyl and quinonoid groups of the dye coordinate to the metal ions.27–32 Recently Quinti et al. have demonstrated the supramolecular and/or polymeric nature of the fluorescent, QZ/Al(III) complexes.28 We have exploited this interaction of QZ with Al(III) to understand the orientation of the QZ molecule inside the CD cavities. It is expected that if the hydroxyl and quinonoid groups of QZ are encapsulated inside the CD cavities, complexation with Al(III) should be significantly reduced.On addition of Al(NO3)3, to each of the QZ·CD systems (corresponding to the highest CD concentrations used in the fluorescence titrations), a fluorescent pink color develops as reported in the literature, due to metal complex formation with QZ.28 Since it is reported that the complexation of Al(III) ions with QZ is a relatively slow process, in the present cases, the spectra were recorded after about 90 min from the mixing of the reagents, assuming that the reaction attains its equilibrium.28 Accordingly, the absorption of QZ at 470 nm decreases with the simultaneous appearance of two new absorption bands having maxima around 515 and 550 nm, respectively (Fig. 6). It is, however, observed that the changes in the absorption bands are significantly lower for the QZ·γ-CD system compared to QZ·α-CD and QZ·β-CD systems. The observation that the changes in the absorption spectra for QZ alone is less than that of QZ·α-CD and QZ·β-CD systems is possibly due to the fact that the complexation of the dye with Al(III) is assisted by the presence of the host, as also reported in the literature.27 Concomitant with the changes in the absorption spectra, the metal complex formation also resulted in the formation of two new emission bands and a shoulder at around 565, 605 and 655 nm, respectively, in the fluorescence spectra. The typical fluorescent spectra are shown in Fig. 7 after correction for the differences in OD at the excitation wavelength (530 nm). In this case also it is observed that the fluorescence intensity for the newly developed bands are quite lower in case of the QZ·γ-CD system compared to that of QZ alone. This is a clear indication that QZ has a better binding affinity for γ-CD host and that the hydroxyl and quinonoid groups of QZ are well shielded within the γ-CD cavity in the QZ·γ-CD complex. Whatever little interaction is observed for the QZ·γ-CD system, could be due to the complex formation of Al(III) with the fraction of free dyes available in the aqueous phase. Since complex formation is a dynamic process, it is expected that the complexation equilibrium of QZ with γ-CD will be shifted towards the free dye in the presence of Al(NO3)3. For QZ·α-CD and QZ·β-CD, on the other hand, the complexation with Al(III) appears to be more efficient compared to QZ alone. This is in accordance with an earlier report on the fluorimetric determination of scandium by means of the QZ ligand partially encapsulated in the cavity of a β-CD molecule.27 It was observed that QZ·β-CD forms a chelate with scandium which exhibits characteristic emission, whereas no emission was observed with scandium and QZ in absence of β-CD. It is likely that in this case, since the dihydroxy-substituted ring of QZ is exposed to the bulk water, they can participate along with the portal hydroxyl groups of the CD molecule to form complexes with the metal ions. Obviously the fraction of free QZ molecules in the solution also participates in the complex formation with Al(III) ion, giving an enhanced effect. Hence, the present observations on the interaction of QZ·CD complexes with Al(III) ions, support the proposition that the dihydroxy-substituted ring is indeed entrapped within the γ-CD cavity but it is quite exposed to the bulk water outside in the cases of QZ·α-CD and QZ·β-CD systems. Observed results in the presence of Al(NO3)3 are also in accordance with the fact that QZ has a much higher affinity to be encapsulated in the γ-CD cavity than in α-CD and β-CD cavities.
 | ||
| Fig. 6 Absorption spectra of (1) QZ (20 × 10−6 M) in the absence of Al(NO3)3. Spectra (2) to (5) are in presence of Al(NO3)3 (1.2 × 10−3 M) for QZ, QZ·α-CD (20 mM), QZ·β-CD (19 mM) and QZ·γ-CD (28 mM), respectively. | ||
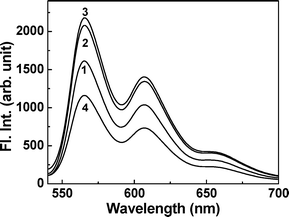 | ||
| Fig. 7 Fluorescence spectra in presence of Al(NO3)3 (1.2 × 10−3 M) for (1) QZ (20 × 10−6 M), (2) QZ·α-CD (20 mM), (3) QZ·β-CD (19 mM) and (4) QZ·γ-CD (28 mM), respectively. The spectra are shown after correction for the differences in OD at the excitation wavelength (530 nm). | ||
Conclusions
The results obtained from ground-state absorption, and steady-state and time-resolved fluorescence studies indicate that QZ forms inclusion complexes with the α-, β- and γ-CD hosts. The low binding constants as well as the estimated thermodynamic parameters indicate that the hydrophobic interaction is mainly responsible for the inclusion complex formation in the present systems. The enhancement in the fluorescence intensity of QZ upon complexation is found to be maximum for β-CD whereas the magnitude of the binding constant is highest for γ-CD. These observations are rationalized by considering the relative dimensions of the host cavities and the guest molecule, as well as the orientation of the guest molecule inside the CD cavities. The results indicate that the unsubstituted ring of QZ enters the α-CD and β-CD cavities through the wider rim of the hosts while the dihydroxy-substituted ring of the dye remains exposed to the bulk water. In case of γ-CD, the encapsulated dye can adopt both the possible orientations with respect to the host and almost the whole QZ molecule enters the host cavity. Comparing QZ·α-CD and QZ·β-CD systems, it is indicated that the dye penetrates deeper into the β-CD cavity and thus experiences more restrictions for its rotational and vibrational motions. Hence, the nonradiative rates are reduced to a greater extent for QZ·β-CD system, leading to the maximum enhancement in the fluorescence intensity. On the other hand, due to better accommodation of the dye inside γ-CD (with dihydroxy-substituted ring also going inside the γ-CD cavity), the binding constant is the highest for QZ·γ-CD system. However, the larger cavity size of γ-CD cannot impart much rigidity to the encapsulated dye and accordingly the enhancement in the fluorescence intensity is lower than in QZ·β-CD complex. The different orientations of QZ within the CD cavities has also been supported by the different extent of interactions of the QZ·CD systems with Al(III) ions. As the hydroxyl and/or the quinonoid groups of QZ are expected to be projected toward the aqueous phase in case of QZ·α-CD and QZ·β-CD complexes, they show better efficiency for the complex formation with Al(III) ions. On the contrary, complexation with Al(III) ions is significantly reduced in case of QZ·γ-CD, due to the higher binding of the dye with γ-CD and also due to the shielding of the hydroxyl and quinonoid groups of QZ inside the γ-CD cavity. Thus, present results demonstrate how hydrophobic and steric control of the inclusion process drastically influence the mode of interaction and reactivity of guests in the cavity, much resembling the way in which enzymes bind substrates and carry out specific catalytic processes.Acknowledgements
We would like to acknowledge Dr J. Mohanty for many fruitful discussions. We are also thankful to all the members of Molecular Photochemistry Section, BARC, RPCD, for their help and cooperation. Thanks are also due to Dr S. K. Sarkar, Head, RPCD, BARC for his constant encouragement and support. NK acknowledges IASc, INSA and NASI for his fellowship under the Summer Research program, 2008.References
- J. Szejtli, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, Marcel Dekker, Inc., 2004, pp. 405–413 Search PubMed.
- S. Li and W. C. Purdy, Cyclodextrins and their Applications in Analytical Chemistry, Chem. Rev., 1992, 92, 1457–1470 CrossRef CAS.
- M. V. Rekharsky and Y. Inoue, Complexation Thermodynamics of Cyclodextrins, Chem. Rev., 1998, 98, 1875–1917 CrossRef CAS.
- K. A. Connors, The Stability of Cyclodextrin Complexes in Solution, Chem. Rev., 1997, 97, 1325–1357 CrossRef CAS.
- Y. Liu, B.-H. Han and Y.-T. Chen, Molecular recognition and complexation thermodynamics of dye guest molecules by modified cyclodextrins and calixarenesulfonates, J. Phys. Chem. B, 2002, 106, 4678–4687 CrossRef CAS.
- R. Villalonga, R. Cao and A. Fragoso, Supramolecular Chemistry of Cyclodextrins in Enzyme Technology, Chem. Rev., 2007, 107, 3088–3116 CrossRef CAS.
- M. K. Singh, H. Pal, A. S. R. Koti and A. V. Sapre, Photophysical Properties and Rotational Relaxation Dynamics of Neutral Red Bound to b-Cyclodextrin, J. Phys. Chem. A, 2004, 108, 1465–1475 CrossRef CAS.
- H.-J. Buschmann and E. Schollmeyer, Cucurbituril and β-cyclodextrin as hosts for the complexation of organic dyes, J. Incl. Phenom. Mol. Recog. Chem., 1997, 29, 167–174 CrossRef CAS.
- M. Shaikh, J. Mohanty, A. C. Bhasikuttan and H. Pal, Tuning dual emission behavior of p-dialkylaminobenzonitriles by supramolecular interactions with cyclodextrin hosts, Photochem. Photobiol. Sci., 2008, 7, 979–985 RSC.
- K. Kalyanasundaram, Photochemistry in Microheterogeneous Systems, Academic Press, Orlando, FL, 1987 Search PubMed.
- J. Mohanty, A. C. Bhasikuttan, W. M. Nau and H. Pal, Host–guest complexation of neutral red with macrocyclic host molecules: Contrasting pKa shifts and binding affinities for cucurbit[7]uril and β-cyclodextrin, J. Phys. Chem. B, 2006, 110, 5132–5138 CrossRef CAS.
- J. Mohanty and W. M. Nau, Ultrastable Rhodamine with Cucurbituril, Angew. Chem. Int. Ed., 2005, 44, 3750–3754 CrossRef CAS.
- W. M. Nau and X. Zhang, An exceedingly long-lived fluorescent state as a distinct structural and dynamic probe for supramolecular association: An exploratory study of host–guest complexation by cyclodextrins, J. Am. Chem. Soc., 1999, 121, 8022–8032 CrossRef CAS.
- A. C. Bhasikuttan, J. Mohanty, W. M. Nau and H. Pal, Efficient Fluorescence Enhancement and Cooperative Binding of an Organic Dye in a Supra-biomolecular Host–Protein Assembly, Angew. Chem. Int. Ed., 2007, 46, 4120–4122 CrossRef CAS.
- D. K. Palit, H. Pal, T. Mukherjee and J. P. Mittal, Photodynamics of the S1 state of some hydroxy- and amino-substituted naphthoquinones and anthraquinones, J. Chem. Soc. faraday Trans., 1991, 86, 3861–3869 Search PubMed.
- M. L. Ferreiro and J. Rodríguez-Otero, Ab initio study of the intramolecular proton transfer in dihydroxyanthraquinones, J. Mol. Struct. (Theochem), 2001, 542, 63–77 CrossRef CAS.
- M. Hovaneissian, P. Archier and C. Vieillescazes, Influence of cetophenolic and diphenolic intramolecular hydrogen bonding on the chromatographic and spectroscopic properties of hydroxyanthraquinones, Dyes and Pigments, 2007, 74, 706–712 CrossRef CAS.
- D. K. Palit, H. Pal, T. Mukherjee and J. P. Mittal, Anisotropic rotational behaviour of quinizarin in solution, Chem. Phys., 1988, 126, 441–452 CrossRef CAS.
- T. P. Carter, D. Gillispie and M. A. Connolly, Intramolecular Hydrogen Bonding Probed by Laser- Induced Fluorescence. 1. 1,4-Dihydroxyanthraquinone (Quinizarin), J. Phys. Chem., 1982, 86, 192–196 CrossRef CAS.
- P. Dahiya, M. Kumbhakar, T. Mukherjee and H. Pal, Effect of amino and hydroxy substituents on the photophysical properties of 1,4-disubstituted-9,10-anthraquinone dyes, J. Mol. Struct., 2006, 798, 40–48 CrossRef CAS.
- H. Inoue, M. Hida, N. Nakashima and K. Yoshihara, Picosecond fluorescence lifetimes of anthraquinone derivatives. Radiationless deactivation via intra- and intermolecular hydrogen bonds, J. Phys. Chem., 1982, 86, 3184–3188 CrossRef CAS.
- M. W. Rembold and H. E. A. Kramer, The role of anthraquinonoid dyes in the catalytic fading of the dye mixtures: substituent dependent triplet yield of diaminoanthraquinone, J. Soc. Dyers Colour., 1980, 96, 122–126 CAS.
- M. W. Rembold and H. E. A. Kramer, Singlet oxygen as an intermediate in the catalytic fading of dye mixtures, J. Soc. Dyers Colour., 1978, 94, 12–17 CAS.
- B. Kalyanraman, E. Perez-Reyes and R. P. Mason, Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs, Biochim. Biophys. Acta, 1980, 630, 119–130 CrossRef.
- W. A. Remers, The Chemistry of Antitumour Antibiotics, Wiley Interscience, New York, 1979, vol. 1, ch. 2 Search PubMed.
- Topics in antibiotic chemistry, ed. P. G. Sammers, Ellis Horwood, Chichester, UK, 1978, vol. 12, Part-C Search PubMed.
- F. G. Sánchez, M. H. Lopez and J. C. M. Gómez, Fluorimetric Determination of Scandium Using the Cyclodextrin-1,4-Dihydroxyanthraquinone Inclusion Complex, Analyst, 1987, 112, 1037–1040 RSC.
- L. Quinti, N. S. Allen, M. Edge, B. P. Murphy and A. Perotti, A study of the strongly fluorescent species formed by the interaction of the dye 1,4-dihydroxyanthraquinone (quinizarin) with Al(III), J. Photochem. Photobiol., A, 2003, 155, 79–91 CrossRef CAS.
- L. Quinti, N. S. Allen, M. Edge, B. P. Murphy and A. Perotti, A study of the luminescent complexes formed by the dye 1,4-dihydroxyanthraquinone (quinizarin) and Ga(III) and In(III), J. Photochem. Photobiol., A, 2003, 155, 93–106 CrossRef CAS.
- L. G. Gracia, L. C. Rodriguez and M. R. Ceba, Spectrophotometric determination of lithium with quinizarin in drugs and serum, Talanta, 1997, 44, 75–83 CrossRef.
- H. Drechsel, M. M. L. Fiallo, A. Garnier-Suillerot, B. F. Matzanke and V. Schünemann, Spectroscopic Studies on iron complexes of different anthracyclines in aprotic solvent systems, Inorg. Chem., 2001, 40, 5324 CrossRef CAS.
- S. S. Massoud and R. B. Jordan, Kinetic and Equilibrium Studies of the Complexation of Aqueous Iron(III) by Daunomycin, Quinizarin, and Quinizarin-2-sulfonate, Inorg. Chem., 1991, 30, 4851–4856 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- D. V. O'Connor and D. Phillips, Time Correlated Single Photon Counting, Academic Press, New York, 1984 Search PubMed.
- P. W. Atkins, Physical Chemistry, Oxford University Press, Oxford, 1998 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2009 |
