Thermal epimerization of diastereomeric Grignard reagents†
Jens
Beckmann
* and
Alexandra
Schütrumpf
Institut für Chemie und Biochemie, Anorganische und Analytische Chemie, Freie Universität Berlin, Fabeckstr. 34–36, 14195, Berlin, Germany. E-mail: beckmann@chemie.fu-berlin.de
First published on 13th November 2008
Abstract
Thermal epimerization is the key to changing the endo-to-exo ratio of the diastereomeric bornyl and fenchyl Grignard reagents from 67 : 33 to 96 : 4 and from 20 : 80 to 80 : 20, respectively.
The formation of Grignard reagents by direct synthesis from alkyl halides proceeds via single electron transfer (SET) at the Mg surface. It involves alkyl radical intermediates and consequently affords racemic Grignard reagents even if optically active alkyl halides with a stereogenic α-carbon atom as the sole chiral centre are applied.1 Consequently, very few enantiomerically enriched Grignard reagents are known and their use for synthetic applications is somewhat limited.1 The menthyl Grignard reagent consists of a configurationally stabile diastereomeric mixture of menthylmagnesium chloride and neomenthylmagnesium chloride in a ratio of 50 : 50, regardless of whether menthyl chloride or neomenthyl chloride was used as starting material (Scheme 1).3
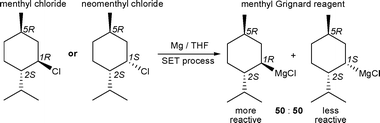 | ||
| Scheme 1 Composition of the menthyl Grignard reagent. | ||
Interestingly, the reactivity of menthylmagnesium chloride is substantially higher than that of neomenthylmagnesium chloride, which can be used to prepare enantiomerically pure menthyl compounds if a twofold excess of the menthyl Grignard reagent is applied.3
The Grignard reagents derived from endo-borneol and exo-isoborneol were investigated previously by a number of researchers, however, with inconsistent and conflicting results, which were critically reviewed in 1966.4 At this point it was concluded that the bornyl Grignard reagent consists of a mixture of bornyl magnesium halides and isobornyl magnesium halides, which allegedly interconvert slowly at room temperature.
We have now reinvestigated the composition of the bornyl Grignard reagent and the closely related fenchyl Grignard reagent by means of diagnostic substitution reactions at the soft model electrophile triphenyltin chloride (Schemes 2 and 3).5 These substitution reactions proceed with low activation barriers and yield air stable mixtures of diastereomeric bornyltriphenyltin (3a) and isobornyltriphenyltin (3b) as well as α-fenchyltriphenyltin (4a) and β-fenchyltriphenyltin (4b), respectively. These stannane mixtures provide indicative information of the diastereomeric composition of the Grignard reagents and were quantitatively and qualitatively assessed by 1D and 2D 119Sn, 13C and 1H-NMR spectroscopy.6 Assignment of the endo- and exo-configuration was supported by comparison of indicative 3J(119Sn-CC-13C) couplings with those of endo- and exo-norbornyltrimethyltin based on the Karplus relation.7
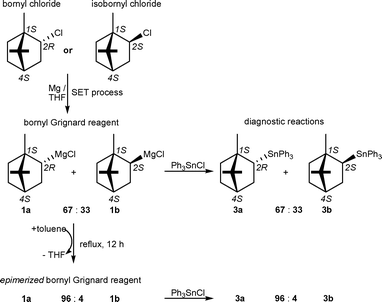 | ||
| Scheme 2 Synthesis and epimerization of the bornyl Grignard reagent. | ||
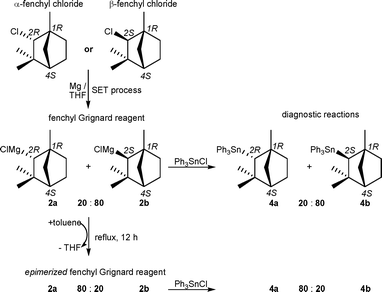 | ||
| Scheme 3 Synthesis and epimerization of the fenchyl Grignard reagent. | ||
The bornyl Grignard reagents prepared from either bornyl chloride or isobornyl chloride and Mg in THF under standard conditions consists of a configurationally stable mixture of bornylmagnesium chloride (1a) and isobornylmagnesium chloride (1b) with an endo-to-exo ratio of 67 : 33 (Scheme 2).
The fenchyl Grignard reagent prepared under identical conditions comprises a configurationally stable mixture of α-fenchylmagnesium chloride (2a) and β-fenchylmagnesium chloride (2b) with an endo-to-exo ratio of 20 : 80, regardless of whether α- or β-fenchyl chloride was used as starting material (Scheme 3). The fact that the formation of both Grignard reagents is independent of the initial configuration of the α-carbon atom is consistent with the SET mechanism.1 This implies that the stereogenic information of the α-carbon atom is lost at the stage of the intermediately formed bornyl and fenchyl radicals, however, their rigid bicyclic structures seem to bias the diastereomeric ratios of the Grignard reagents formed. It is worth emphasizing that in the case of the fenchyl Grignard reagent, the thermodynamically less favoured exo-diastereomer is formed in large excess, which points to a kinetically controlled reaction pathway.
Interestingly, the initial endo-to-exo ratio of both Grignard reagents can be changed dramatically by thermal epimerization at temperatures above 100 °C (Schemes 2 and 3). Therefore, the original solvent (THF) was replaced with toluene by distillation at normal pressure.5 Thermal equilibrium was reached when the Grignard reagents were heated under reflux in toluene for 12 h. Upon cooling to room temperature, the epimerizedGrignard reagents are configurationally stable again and then contain the thermodynamically favoured endo-diastereomers in large excess. In this manner, the diastereomeric purity of the bornyl Grignard reagent can be significantly improved from an endo-to-exo ratio of 67 : 33 to 96 : 4. Similarly, the composition of the fenchyl Grignard reagent can be entirely inverted from an endo-to-exo ratio of 20 : 80 to 80 : 20. While the yield of bornyl and fenchyl Grignard reagents as determined by titration8 is about 80%, the yield of the epimerizedGrignard reagents is only slightly decreased and still ranges between 65–75%. Attempts were made to change the diastereomeric composition of the Grignard reagents to even more favourable endo-to-exo ratios using higher boiling xylene isomers for the thermal epimerization. However, in this case rapid metalation of the solvent occurred and the yield of the bornyl and fenchyl Grignard reagents diminished below 10%.9
To the best of our knowledge, the epimerized bornyl Grignard reagent is the first chiral Grignard reagent of high diastereomeric purity (92% d.e.) that can be prepared easily from readily available starting materials. The fenchyl Grignard reagent can be prepared easily with moderate diastereomeric purity (60% d.e.) but with either of the two diastereomers being in excess. Chiral Grignard reagents hold great potential in mechanistic studies of substitution reactions,2 which, however, has not yet been exploited in organometallic chemistry. We are currently investigating the reaction of the bornyl and fenchyl Grignard reagents with chlorosilanes and chlorophosphanes, respectively, to examine the possibility if SET processes are involved in substitution reactions at these rather hard electrophiles. Preliminary experiments revealed that the substitution reactions of the bornyl and fenchyl Grignard reagents with Ph2PCl are independent of the diastereomeric composition of the Grignard reagents (e.g. the endo-to-exo ratio) and presumably proceed with an SET transfer prior to combination of the radical pair formed (‘electron motion precedes nuclear motion’).2 The Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged for financial support.
Notes and references
- Handbook of Grignard Reagents, ed. G. S. Silverman and P. E. Rakita, Marcel Dekker, New York, 1996 Search PubMed.
- R. W. Hoffmann, Chem. Soc. Rev., 2003, 32, 225 RSC and references cited therein.
- J. Beckmann, D. Dakternieks, M. Dräger and A. Duthie, Angew. Chem., Int. Ed., 2006, 45, 6509 CrossRef CAS.
- A. Hill, J. Org. Chem, 1966, 31, 20 CrossRef and references cited therein.
- Synthesis of the bornyl and fenchyl Grignard reagent. A solution of isobornyl chloride or β-fenchyl chloride (17.2 g, 100 mmol) in THF (180 mL) was slowly added to a suspension of activated Mg turnings (2.7 g, 110 mmol) in THF (20 mL). After the addition was completed, the mixture was heated under reflux for 12 h. Prior to use, the clear solution was separated from the excess of Mg via cannula. The yield determined by titration was about 80%. Synthesis of the epimerized bornyl and fenchyl Grignard reagent. A solution of the bornyl or fenchyl Grignard reagent was slowly distilled while toluene (220 mL) was constantly added to replace the original solvent THF. The temperature was slowly raised to 111 °C and kept there for 12 h. The yield determined by titration was between 65 and 75%.
- Results of the 119Sn NMR analysis. In case of the bornyl Grignard reagent regardless whether it was prepared from bornyl chloride or isobornyl chloride, the 119Sn NMR spectrum revealed signals at δ−49.0 (integral 16%), −85.5 (integral 3%), −101.4 (integral 54%) and −108.1 (integral 27%), which were assigned to unreacted triphenyltin chloride, triphenyltin hydroxide (formed by hydrolysis during the work up), bornyltriphenyltin (3a) and isobornyltriphenyltin (3b), respectively. In the case of the epimerized bornyl Grignard reagent, the 119Sn NMR spectrum revealed signals at δ−49.0 (integral 24%), −85.5 (integral 9%), −101.4 (integral 64.5%) and −108.1 (integral 2.5%), which were assigned to unreacted triphenyltin chloride, triphenyltin hydroxide, bornyltriphenyltin (3a,) and isobornyltriphenyltin (3b), respectively. In the case of the fenchyl Grignard reagent regardless whether it was prepared from α- or β-fenchyl chloride, the 119Sn NMR spectrum revealed signals at δ−49.0 (integral 15%), −85.5 (integral 6%), −123.5 (integral 16%) and −126.4 (integral 63%), which were assigned to unreacted triphenyltin chloride, triphenyltin hydroxide, α-fenchyltriphenyltin (4a) and β-fenchyltriphenyltin (4b), respectively. In the case of the epimerized fenchyl Grignard reagent, the 119Sn NMR spectrum revealed signals at δ−49.0 (integral 13%), −85.5 (integral 5%), −123.5 (integral 57.5%) and −126.4 (integral 14.5%), which were assigned to unreacted triphenyltin chloride, triphenyltin hydroxide, α-fenchyltriphenyltin (4a) and β-fenchyltriphenyltin (4b), respectively.
- D. Doddrell, I. Burfitt, W. Kitching, M. Bullpitt, C.-H. Lee, R. J. Mynott, J. L. Considine, H. G. Kuivila and R. H. Sarma, J. Am. Chem. Soc., 1974, 96, 1640 CrossRef CAS.
- A. Krasovskiy and P. Knochel, Synthesis, 2006, 890 CAS.
- The two main signals in the 119Sn NMR spectrum were detected at δ 118.9 and 122.3, which compare well with the chemical shift reported for PhCH2SnPh3 (δ 117.1). D. Marton, U. Russo, D. Stivanello and G. Tagliavini, Organometallics, 1996, 15, 1645 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/b819178f |
| This journal is © The Royal Society of Chemistry 2009 |
