Synthesis of telluroamino acid derivatives with remarkable GPx like activity†
Antonio L.
Braga
*,
Eduardo E.
Alberto
,
Letiére C.
Soares
,
João B. T.
Rocha
,
Jéssie H.
Sudati
and
Daniel H.
Roos
Departamento de Química, Universidade Federal de Santa Maria, 97.105–900, Santa Maria, RS, Brazil. E-mail: albraga@quimica.ufsm.br
First published on 13th November 2008
Abstract
A series of modular telluroamino acid derivatives with remarkable GPx-like behavior was prepared in an efficient and short two-step synthesis.
Glutathione peroxidase enzymes (GPx) are excellent catalysts for antioxidant reactions. They protect our body against the potentially damaging effects of reactive oxygen species (ROS), such as hydrogen peroxide and some organic hydroperoxides, formed during aerobic metabolism. Several neurodegenerative diseases, including Alzheimer's and Parkinson's disease are linked to ROS activity.1 Since the discovery that selenium plays a pivotal role in GPx enzymes, catalyzing the reduction of hydroperoxides at the expense of glutathione (GSH),2 synthetic developments and design of new chalcogen-based catalytic antioxidants have attracted considerable attention.3 Synthetic organoselenium and organotellurium compounds have emerged as excellent candidates to act as GPx mimics, due their well-known ability to undergo two-electron redox cycle between chalcogen (II) and (IV) species.4
The cyclic selenenamide ebselen 1 (Fig. 1), was the first synthetic compound suggested for hydrogen peroxide inactivating therapy in the presence of glutathione.5
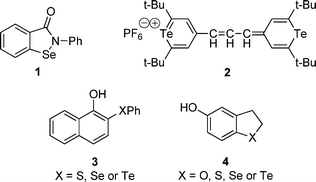 | ||
| Fig. 1 Representative examples of GPx mimics. | ||
Based on the recognized GPx like activity of ebselen, several papers have appeared describing simple synthetic organoselenium compounds with this property (e.g., benzoselenazinones,6 benzoselenazolinones,7camphor-derived selenenamide,8 diaryl diselenides9 and dendrimers with a diselenide core10).
On the other hand, the first organotellurium compound 2, described as a GPx mimic, was reported by Detty.11 After that, a series of diaryl ditellurides12 and diaryl tellurides13 have been reported. Engman and collaborators have developed the synthesis and studied the antioxidant properties of compounds 314 and 4.15 A noteworthy characteristic of these compounds is the much improved antioxidant activity of tellurides, when compared with their selenium and sulfur analogues.
Amino groups that are capable of interacting with selenium, through Se–N nonbonded interactions, are known to play a significant role in modulating the redox proprieties of seleno-based antioxidants.16 However, to the best of our knowledge, there are just a few reports concerning the synthesis and GPx like evaluations of aminoacid derivatives containing selenium17 and none about tellurium. As part of our growing interest in aminoacid derivatives containing chalcogen,3c,18 we report herein the synthesis of novel telluroaminoacid derivatives, easily obtained in a simple, modular and efficient two-step synthesis. We envisioned that this modular characteristic would allow us to investigate the structure–activity relationship of these compounds. Their GPx like activity was evaluated, and we promoted the variation of aminoacid residues, the chain length between the chalcogen atom and the aminoacid moiety in order to find a more efficient catalyst.
GPx mimics 7a–g were prepared in two steps, in 51–78 overall yield, from readily available L-aminoesters and the appropriate bromo-carboxylic acid, as shown in Scheme 1.
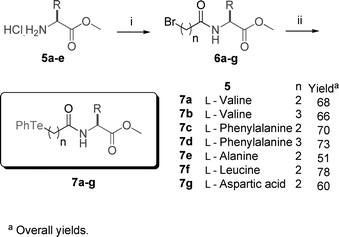 | ||
| Scheme 1 Synthetic strategy to prepare compounds 7a–g. Reagents: i) bromo-carboxylic acid, NMM, ethyl chloroformate, CHCl3; ii) PhTe)2, NaBH4, THF, EtOH. | ||
The catalytic activity of tellurides 7a–g, as a GPx model enzyme, was evaluated according to the Tomoda method15 using benzenethiol as a glutathione alternative. The reduction of H2O2 was monitored through the UV absorption increase at 305 nm, due to diphenyl disulfide formation.
Our prime concern in the evaluation of these compounds as GPx mimics was the influence of the chain length between the tellurium atom and aminoacid moiety. It should be noted that compounds 7a (n = 2) and 7b (n = 3) derived from L-Valine showed the same activity. Linear increases in absorbance (Fig. 2) were observed by mixing MeOH, catalyst 7a–d (2.6 mol%), PhSH and H2O2. Taking advantage of the modular characteristic of our synthetic route, we explored the influence of the aminoacid residue in the reduction of hydrogen peroxide in the presence of an excess of thiol. Compounds 7c (n = 2) and 7d (n = 3) derived from L-Phenylalanine were tested in the same conditions. Compound 7c, with a shorter chain length, showed the same catalytic activity that compounds 7a and 7b. However, 7d was less effective in the reduction of hydrogen peroxide.
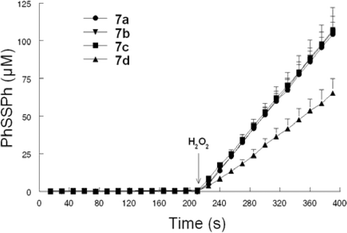 | ||
| Fig. 2 GPx like behavior of compounds 7a-d. | ||
The time required to reduce the thiol concentration to a half (T50) is shown in Table 1. Compounds 7a (n = 2) and 7b (n = 3), derived from L-valine and 7c (n = 2) from L-phenylalanine showed almost the same T50, near to 13 min (Entries 1–3). For compound 7d (n = 3) derived from L-phenylalanine it was 21.61 min (Entry 4).
|
|
|||||
|---|---|---|---|---|---|
| Entrya | Catalyst b | Y | n | R | T 50 c , d |
| a Under this condition addition of H2O2 in the absence of telluride did not produce any significant oxidation of PhSH. b MeOH (1 mL); catalyst [0.05 mmol L−1]; PhSH [1.9 mmol L−1]; H2O2 [8.8 mmol L−1]. c T 50 is the time required, in min, to reduce the thiol concentration with 50% after the addition of H2O2; d Data in parentheses: experimental error. | |||||
| 1 | 7a | Te | 2 | i-Pr | 13.43 (±0.15) |
| 2 | 7b | Te | 3 | i-Pr | 13.14 (±0.27) |
| 3 | 7c | Te | 2 | Bn | 13.43 (±0.31) |
| 4 | 7d | Te | 3 | Bn | 21.61 (±1.10) |
| 5 | 7e | Te | 2 | Me | 10.41 (±0.52) |
| 6 | 7f | Te | 2 | i-Bu | 12.75 (±1.03) |
| 7 | 7g | Te | 2 | CH2COOMe | 8.15 (±0.61) |
| 8 | 7h | Se | 2 | i-Pr | 1543 (±1.82) |
On the basis of these initial observations, we used telluroaminoacids with n = 2 as standard catalysts, and promoted the variation of amino acid moiety to enhance their ability to mimic the glutathione peroxidase enzyme. L-Alanine 7e (Entry 5) and L-leucine 7f (Entry 6) derivatives showed T50 of 10.41 and 12.75 min. respectively, and to our delight we found that telluride 7g derived from L-aspartic acid (Entry 7) was the best catalyst, promoting the reduction of H2O2 concentration to a half in 8.15 min. On the other hand, a seleno derivative 7h (Entry 8) was not able to promote the reduction of H2O2 in an appreciable rate (T50 = 1543 min). Diphenyl ditelluride was also employed as reducing agent, and it was found that it is less effective than compounds 7a–g, (T50 = 16.73 (±0.26) min). We also investigated if the tellurium moiety remains intact after reaction with H2O2. To address this issue we performed a GC analysis of telluride 7g, and another one with the reaction of GPx using 7g as catalyst (see supporting information). To our delight, compound 7g was also present after its application in the standard reaction. Apart from the main product, diphenyldisulfide, some minor side products are formed which may originate in some decomposition of the catalyst or of other components involved.
Encouraged by these results, we next explored the effects of substituents in the aryl group attached to tellurium in 7g derivatives. A new series of telluroaminoacids were prepared with electron donating 7i–k, electron withdrawing 7l and 7m, as well as with steric hindrance substituents 7n and 7o (Fig. 3).
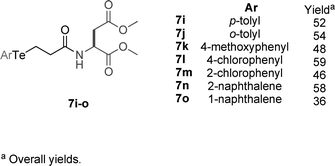 | ||
| Fig. 3 | ||
A new set of experiments to screen the GPx activity of these new catalysts was performed with 7i–o (2 mol%), in a more concentrated solution (Table 2). It was found that the GPx behavior is strongly influenced by steric effects. The T50 of para substituted compounds were lower when compared with the ortho analogues (Entries 2 and 3, 5 and 6). The changing of electronic environment at the tellurium atom did not produce a pronounced change to the thiol peroxidase activity of these compounds. The T50 of 7g (Ar = Ph, Entry 1), 7i (Ar = p-tolyl, Entry 2), 7k (Ar = 4-MeOC6H4, Entry 4), and 7l (Ar = 4-ClC6H4, Entry 5) was between 2.11 and 2.98 min.
| Entrya | Catalyst b | Ar | T50c,d |
|---|---|---|---|
| a Under this condition addition of H2O2 in the absence of telluride did not produce any significant oxidation of PhSH. b MeOH (1 mL); catalyst [0.1 mmol L−1]; PhSH [5 mmol L−1]; H2O2 [5 mmol L−1]. c T 50 is the time required, in min, to reduce the thiol concentration with 50% after the addition of H2O2. d Data in parentheses: experimental error. | |||
| 1 | 7g | phenyl | 2.98 (±0.21) |
| 2 | 7i | p-tolyl | 2.78 (±0.20) |
| 3 | 7j | o-tolyl | 4.78 (±0.12) |
| 4 | 7k | 4-methoxyphenyl | 2.11 (±0.24) |
| 5 | 7l | 4-chlorophenyl | 2.94 (±0.19) |
| 6 | 7m | 2-chlorophenyl | 8.14 (±1.52) |
| 7 | 7n | 2-naphthalene | 3.65 (±0.18) |
| 8 | 7o | 1-naphthalene | 4.27 (±0.32) |
Concerning mechanistic aspects, and in agreement with Detty's study,19 we believe that initially Te(II) compounds 7a-o react with H2O2 to form the Te(IV) oxides 8a–o, and H2O (Scheme 2). Addition of one equivalent of PhSH to these compounds generate tellurenyl sulfides 9a–o which react with another equivalent of PhSH to regenerate 7a–o to the catalytic cycle and produce PhSSPh and H2O.
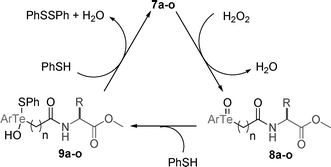 | ||
| Scheme 2 | ||
In conclusion, we have prepared a series of telluroamino acid derivatives, in a short, modular and efficient synthetic route. These compounds were tested as GPx mimics, catalyzing the reduction of H2O2 to water at expense of thiophenol using a very low amount of catalyst. We found that the time required to reduce the concentration of the PhSH to a half, T50, is strongly influenced by the aminoacid residue, as well as by steric effects. New studies to investigate the influence of amino acid residues of telluroamino acid derivatives have been performed in our lab using glutathione as reducing agent.
Acknowledgements
The authors thank CNPq, CAPES, and FAPERGS for financial support.Notes and references
-
Free Radicals in Biology, ed. L. Flohe and W. A. Pryor, Academic Press, New York, 1982 Search PubMed
.
-
(a)
J. T. Rotruck, A. L. Pope, H. E. Ganther, A. B. Swanson, D. G. Hafeman, W. G. Hoekstra, Science 1973, 179, 588 Search PubMed
; (b) L. Flohé, E. A. Günzler and H. H. Schock, FEBS Lett., 1973, 32, 132 CrossRef CAS
; (c) Selenium in Biology and Human Health, ed. R. F. Burk, Springer-Verlag, New York, 1994 Search PubMed
.
-
(a) G. Mugesh and H. Singh, Chem. Soc. Rev., 2000, 29, 347 RSC
; (b) G. Mugesh, W. W. -du Mont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS
; (c) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS
; (d) B. K. Sarma and G. Mugesh, Org. Biomol. Chem., 2008, 6, 965 RSC
.
-
(a) H. J. Reich, Acc. Chem. Res., 1979, 12, 22 CrossRef CAS
; (b) K. B. Sharpless, K. M. Gordon, R. F. Lauer, D. W. Patrick, S. P. Singer and M. W. Young, Chem. Scr., 1975, 8A, 9 CAS
; (c) M. R. Detty, Organometallics, 1991, 10, 702 CrossRef CAS
; (d) M. R. Detty, P. B. Merkel and S. K. Powers, J. Am. Chem. Soc., 1988, 110, 5920 CrossRef CAS
.
-
(a) A. Müller, E. Cadenas, P. Graf and H. Sies, Biochem. Pharmacol., 1984, 33, 3235 CrossRef CAS
; (b) A. Wendel, M. Fausel, H. Safayhi, G. Tiegs and R. Otter, Biochem. Pharmacol., 1984, 33, 3241 CrossRef CAS
; (c) M. J. Parnham and S. Kindt, Biochem. Pharmacol., 1984, 33, 3247 CrossRef CAS
.
- P. V. Jacquemin, L. E. Christiaens and M. J. Renson, Tetrahedron Lett., 1992, 33, 3663 CrossRef CAS
.
- V. Galet, J. L. Bernier, J. P. Hénichart, D. Lesieur, C. Abadie, L. Rochette, A. Lindenbaum, J. Chalas, J. F. R. de la Faverie, B. Pfeiffer and P. Renard, J. Med. Chem., 1994, 37, 2903 CrossRef CAS
.
- T. G. Back and B. P. Dick, J. Am. Chem. Soc., 1997, 119, 2079 CrossRef CAS
.
-
(a) S. R. Wilson, P. A. Zucker, R. R. C. Huang and A. Spector, J. Am. Chem. Soc., 1989, 111, 5936 CrossRef CAS
; (b) T. Wirth, Molecules, 1998, 3, 164 Search PubMed
; (c) G. Mugesh, A. Panda, H. B. Singh, N. S. Punekar and R. Butcher, J. Chem. Commun., 1998, 2227 RSC
; (d) G. Mugesh, A. Panda, H. B. Singh, N. S. Punekar and R. J. Butcher, J. Am. Chem. Soc., 2001, 123, 839 CrossRef CAS
.
- X. Zhang, H. Xu, Z. Dong, Y. Wang, J. Liu and J. Shen, J. Am. Chem. Soc., 2004, 124, 10556 CrossRef
.
- M. R. Detty and S. L. Gibson, Organometallics, 1992, 11, 2147 CrossRef CAS
.
- L. Engman, D. Stern, I. A. Cotgreave and C. M. Andersson, J. Am. Chem. Soc., 1992, 114, 9737 CrossRef CAS
.
-
(a) L. Engman, D. Stern, M. Pelcman and C. M. Andersson, J. Org. Chem., 1994, 59, 1973 CrossRef CAS
; (b) K. Vessman, K. Ekström, M. Berglund, C. M. Andersson and L. Engman, J. Org. Chem., 1995, 60, 4461 CrossRef CAS
.
- L. Engman, D. Stern, H. Frisell, K. Vessman, M. Berglund, B. Ek and C. M. Andersson, Bioorg. Med. Chem., 1995, 3, 1255 CrossRef CAS
.
-
(a) L. Engman, M. Laws, J. Malmström, C. H. Schiesser and L. M. Zugaro, J. Org. Chem., 1999, 64, 6764 CrossRef CAS
; (b) J. Malmström, M. Jonsson, I. A. Cotgreave, L. Hammarström, M. Sjödin and L. Engman, J. Am. Chem. Soc., 2001, 123, 3434 CrossRef CAS
.
- M. Iwaoka and S. Tomoda, J. Am. Chem. Soc., 1994, 116, 2557 CrossRef CAS
.
-
(a) P. P. Phadnis and G. Mugesh, Org. Biomol. Chem., 2005, 3, 2476 RSC
; (b) K. P. Bhabak and G. Mugesh, Chem. Eur. J., 2007, 13, 4594 CrossRef CAS
.
-
(a) A. L. Braga, J. A. Sehnem, F. Vargas and R. C. Braga, J. Org. Chem., 2005, 70, 9021 CrossRef CAS
; (b) A. L. Braga, D. S. Lüdtke, M. W. Paixão, E. E. Alberto, H. A. Stefani and L. Juliano, Eur. J. Org. Chem., 2005, 4260 CrossRef CAS
; (c) A. L. Braga, L. Wessjohann, M. W. Paixão, O. E. D. Rodrigues, A. Schneider and H. R. Appelt, Tetrahedron Lett., 2006, 47, 1019 CrossRef
; (d) A. L. Braga, D. P. Bottega, M. W. Paixão, A. M. Deobald, C. Peppe and P. H. Schneider, J. Org. Chem., 2006, 71, 4305 CrossRef CAS
.
- Y. You, K. Ahsan and M. R. Detty, J. Am. Chem. Soc., 2003, 125, 4918 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures, 1H and 13C NMR spectra of selected compounds. See DOI: 10.1039/b814990a |
| This journal is © The Royal Society of Chemistry 2009 |