Nanoparticle-supported and magnetically recoverable palladium (Pd) catalyst: a selective and sustainable oxidation protocol with high turnover number†
Vivek Polshettiwar and Rajender S. Varma*
Sustainable Technology Division, National Risk Management Research Laboratory, U. S. Environmental Protection Agency, MS 443, Cincinnati, Ohio 45268, USA. E-mail: polshettiwar.vivek@epa.gov; varma.rajender@epa.gov
First published on 13th November 2008
Abstract
A magnetic nanoparticle-supported Pd catalyst was readily prepared from inexpensive starting materials and shown to catalyze various oxidation reactions with high turnover number (TON) and excellent selectivity. The ease of recovery using an external magnetic field, high activity, and the intrinsic stability of the catalyst make this protocol economic and sustainable.
The oxidation of alcohols and olefins to carbonyl compounds is of paramount importance in synthetic organic chemistry because of their use in a wide range of products, such as drugs, agro-chemicals, and fragrances.1 They have conventionally been oxidized in non-catalytic ways with stoichiometric oxidants such as chromium and manganese compounds in the presence of strong mineral acids, which generate enormous amounts of poisonous metal salts as waste.2 Due to the increasing environmental concerns, many efforts have been made to develop the environmentally benign oxidation systems.2 Various heterogeneously catalyzed protocols were developed, including the use of silver nanoparticles supported on hydrotalcites,3 gold nanoparticles supported on metal oxides,4 metallic iron nanoparticles on MCM-41,5 and palladium nanoparticles on hydroxyapatite,6 mesoporous carbon,7 silica-alumina,8 and titiania.9 Among the various protocols, ruthenium hydroxide supported on Fe3O410 and palladium (Pd) nanoparticles supported on SBA-1511 show promising results. However, most systems can be applicable for the oxidations of only activated substrates or large quantities of additives such as bases and electron transfer mediators were used. Recently, Beller and co-workers reported the use of nano-γ-Fe2O3 for oxidation of alcohols with high selectivity.12 Although this work led the way to advance oxidation catalysis, the TON of the reaction is very low, ranging from 6 to 36. As clearly stated by Beller et al.“for general applications in organic synthesis, the activity of catalyst system should be further improved”.12 There is an urgent need to develop less expensive and sustainable oxidation catalyst systems with high TON.
Recently, functionalized magnetic nanoparticles have emerged as viable alternatives to conventional materials, as robust, readily available, high-surface-area heterogeneous catalyst supports.13 They offer an added advantage of being magnetically separable, thereby eliminating the requirement of catalyst filtration after completion of the reaction. Engaged in the development of greener and sustainable pathways for organic transformations,14 nanomaterials,15 and nano-catalysis,16 herein, we report a simple and efficient synthesis of a nano-ferrite-supported, magnetically recyclable, and inexpensive Pd catalyst and its application in oxidation reactions. At the outset of this study, no example of magnetic nanoparticle-supported Pd catalysts had been reported for oxidation reactions, despite the potential inherent stability and activity of such materials.
The first step in the accomplishment of this goal was the synthesis and functionalization of magnetic nanoparticles (Scheme 1). The catalyst was prepared by sonicating nano-ferrites with dopamine (which acts as a robust anchor and avoids Pd-leaching) in water for 2 h, followed by addition of sodium palladium (Pd) chloride at a basic pH. Material with Pd nanoparticles on the amine-functionalized nano-ferrites was obtained in excellent yield.
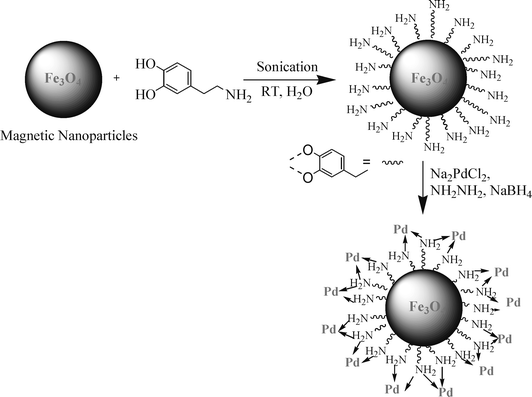 | ||
| Scheme 1 Synthesis of functionalized nano-ferrite-Pd. | ||
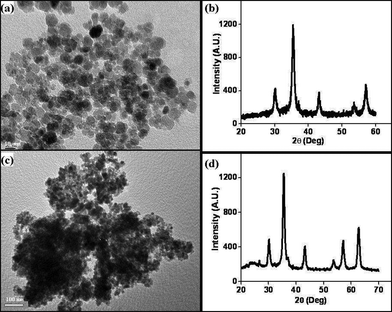
Catalyst characterization by X-ray diffraction (XRD) (Fig. 1b and d) and transmission electron microscopy (TEM) (Fig. 1a and c) confirm formation of single-phase Fe3O4 nanoparticles, with spherical morphology and a size range of 10–16 nm, which is comparable with the crystallite size calculated from the X-ray spectrum, by using the Scherer formula (10.23 nm). FT-IR evidently confirms the anchoring of dopamine on ferrite surfaces (S4, ESI†). The signals of palladium metal were not detected in XRD, showing that the Pd species is highly dispersed on ferrites. The weight percentage of palladium was found to be 10.23% by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis.
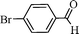
This Pd-coated nanomaterial was then explored as a heterogeneous catalyst for the oxidation of alcohols (Scheme 2).
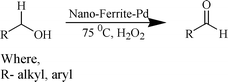 | ||
| Scheme 2 Nano-ferrite-Pd catalyzed alcohol oxidation. | ||
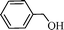
Initially, experiments were performed to optimize reaction conditions for oxidation of benzyl alcohol as a substrate, using one equivalent of hydrogen peroxide, to get a high TON of product (Table 1). First the reaction was conducted using nano-α-Fe2O3 with particle sizes from 2–3 μm (see ESI S7 for TEM images and XRD data†) and conversion with low TON (160) was observed. Then, we tested nano-Fe3O4 with particle sizes from 10–15 nm (Fig. 1a and b) as catalyst, and the TON reached 240 with high selectivity. However, we wanted to develop a high TON protocol and thus prepared Pd-coated nano-Fe3O4 (as discussed in Scheme 1) with particle sizes from 10–16 nm (Fig. 1c) and tested for this oxidation protocol. We observed conversion with very high TON (720), with >99% selectivity.
| Entry | Catalyst | Reaction time/h | TONb | Selectivity (%)c |
|---|---|---|---|---|
| a Reactions were carried out with 10 mmol of benzyl alcohol, 0.025 mole% of catalyst, and 1 equiv of H2O2, at 75–80 °C.b Number of moles of aldehyde formed per mole of catalyst.c Selectivity based on alcohol conversion. | ||||
| 1 | nano-α-Fe2O3 | 6 | 160 | >99 |
| 2 | nano-α-Fe2O3 | 12 | 200 | >99 |
| 3 | nano-Fe3O4 | 6 | 240 | >99 |
| 4 | nano-Fe3O4 | 12 | 280 | >99 |
| 5 | nano-Fe3O4-Pd | 6 | 720 | >99 |
| 6 | nano-Fe3O4-Pd | 12 | 760 | >99 |
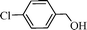
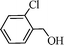
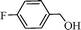
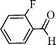
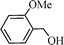
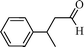
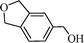
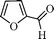
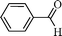
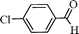
Using the above optimized condition, this catalyst was then explored for oxidation of a variety of alcohols (Table 2). Excellent turnover numbers were observed for various aromatic alcohols (entries 1 to 7) as well as aliphatic alcohols (entries 8, 9) within 6 h. However, in the case of hexanol, conversion with low TON is due to volatility of the ensuing aldehyde (entry 9). Heterocyclic alcohols (entries 10, 11), extensively used building-blocks in drug discovery, also underwent oxidation reaction with high TON, proving the suitability of this protocol for assembly of biomolecules. In all the reactions, we observed more than 95% selectivity because of the very low catalyst amount (0.025 mole%). This will be very useful in total synthesis of drug molecules, where it is required that an alcoholic group be selectively oxidized to its aldehyde without any over-oxidation. The catalytic activity of our system is superior to that of Beller's,8i.e., TON of benzyl alcohol oxidation is 720 in 6 h, as compared to TON of 32 in 12 h, with comparable selectivity.
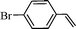
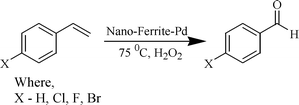
The oxidation of olefinic compounds to the corresponding aldehydes (Scheme 3) is a synthetically important transformation and notably, this catalytic system also oxidized aromatic olefins with high TON and the results are summarized in Table 3.
 | ||
| Scheme 3 Nano-ferrite-Pd catalyzed olefin oxidation. | ||
The scope of the catalyst was established for a range of aromatic olefins and the reactions were completed within 6 h. In all the reactions, TON ranging from 600 to 680 were observed, which is superior as compared to the TON of known protocols, which range from 19 to 36.8
Separation and recovery of the catalyst is also an important aspect for the synthesis of fine chemicals; which is generally performed by filtration with reduced efficiency. In our catalytic system, because of the super-paramagnetic nature of the nano-Fe3O4-Pd catalyst, it can be recovered by simply using external magnets, with high efficiency and up to 99% recovery of catalyst (Fig. 2). After completion of the reaction, within 30–45 sec, the reaction mixture turned clear and catalyst was deposited on the magnetic bar (Fig. 2b), which was easily removed from reaction mixture using an external magnet (Fig. 2c).
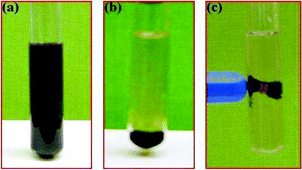 | ||
| Fig. 2 Oxidation of alcohols using nano-ferrite-Pd catalyst, (a) during reaction under stirring, (b) after completion of reaction without stirring, (c) catalyst removal by external magnet. | ||
For practical applications of heterogeneous systems, the lifetime of the catalyst and its level of reusability are very important factors. To clarify this issue, we established a set of experiments for the oxidation of benzyl alcohol using the recycled nano-Fe3O4-Pd catalyst. After the completion of the first reaction to afford the corresponding aldehyde, the catalyst was recovered magnetically, washed with methanol, and finally dried at 60 °C for 10 min. A new reaction was then performed with fresh reactants and H2O2, under the same conditions. The nano-ferrite-supported Pd catalyst could be used at least 5 times without any change in activity.
Metal leaching was studied by ICP-AES analysis of the catalyst before and after the five reaction cycles. The Pd concentration was found to be 7.91% before reaction and 7.86% after the reaction, and also no Pd metal was detected in the final oxidation product (see ESI S5 for details†), which confirmed negligible Pd leaching. The negligible Pd leaching is due to a well defined amine-binding site located on the surface of the nano-ferrite (Scheme 1), which acts as a pseudo-ligand by non-covalent binding with Pd through a metal–ligand interaction. This non-covalent anchoring minimizes deterioration and metal leaching and allows efficient catalyst recycling. Also, the most important criteria in choosing a catalyst is metal recovery. It would be preferable to use a more accessible, economic Pd catalyst, provided that the process works at high TON and that the catalyst leaves no remnants of metal in the end product, since metal contamination is highly regulated by the pharmaceutical industry. All above conditions were well satisfied by our recyclable nano-ferrite-supported Pd catalyst.
In summary, we have developed a convenient synthesis of a nano-ferrite-supported Pd catalyst which was readily prepared from inexpensive starting materials in water. This catalyst then catalyzed the oxidation of alcohols and olefins with high TON and excellent selectivity. Also, being magnetically separable eliminated the requirement of catalyst filtration after completion of the reaction, which is an additional sustainable attribute of this oxidation protocol.
Experimental section
Synthesis of magnetic nano-ferrites
FeSO4·7H2O (13.9 g) and Fe2(SO4)3 (20 g) were dissolved in 500 mL water in a 1000 mL beaker. Ammonium hydroxide (25%) was added slowly to adjust the pH of the solution to 10. The reaction mixture was then continually stirred for 1 h at 60 °C. The precipitated nanoparticles were separated magnetically, washed with water until the pH reached 7, and then dried under a vacuum at 60 °C for 2 h. Ferrite was characterized by X-ray diffraction (XRD) (Fig. 1b) and transmission electron microscopy (TEM) (Fig. 1a). This magnetic nano-ferrite (Fe3O4) was then used for further chemical modification.Surface modification of nano-ferrites
Nano-Fe3O4 (2 gm) was dispersed in 25 mL water by sonication for 30 min. Dopamine hydrochloride (2 gm) dissolved in 5 mL of water was added to this solution and again sonicated for 2 h. The amine-functionalized nanomaterial was then precipitated using acetone, isolated by centrifugation, and dried under vacuum at 60 °C for 2 h.Synthesis of nano-ferrite-Pd catalyst
Amine-functionalized nano-Fe3O4 (1 gm) was dispersed in water and sodium palladium chloride solution in water was added to the mixture to get 10 wt% of Pd. Hydrazine monohydrate solution in water was added dropwise to bring the pH of this mixture to 9, followed by addition of 0.1 gm of NaBH4. The reaction mixture was then stirred for 24 h at room temperature. The product was allowed to settle, washed several times with water and acetone, and dried under vacuum at 60 °C for 2 h. Catalyst characterization by X-ray diffraction (XRD) (Fig. 1d) and transmission electron microscopy (TEM) (Fig. 1c) confirm the anchoring of Pd nanoparticles on ferrite surfaces. The weight percentage of Pd in the catalyst was found to be 7.91% by ICP-AES analysis.Oxidation of alcohols using nano-ferrite-Pd catalyst
10 mmol of alcohol and 0.025 mole% of nano-ferrite-Pd catalyst were placed in a 10 mL glass tube with a magnetic stirrer bar. H2O2 (1 equiv) was then added dropwise to the reaction mixture under stirring at room temperature. The reaction was continuously stirred at 75–80 °C for 6 h. Conversion and selectivity were periodically determined by GC analysis. After completion of the reaction, stirring was stopped. Within 30–45 s, the reaction mixture turned clear and catalyst was deposited on the magnetic bar, which was easily removed from the reaction mixture using an external magnet. The crude product obtained was further purified by column chromatography. All products are known in the literature and were identified by comparison of their 1H and 13CNMR with literature data and comparing their GC-MS spectra with standard Wiley mass spectral library.Oxidation of olefins using nano-ferrite-Pd catalyst
10 mmol of olefins and 0.025 mole% of nano-ferrite-Pd catalyst were placed in a 10 mL glass tube with a magnetic stirrer bar. H2O2 (2 equiv) was then added dropwise to the reaction mixture under stirring at room temperature and the same procedure described above was followed.Acknowledgements
Vivek Polshettiwar was supported by the Postgraduate Research Program at the National Risk Management Research Laboratory administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency.References
- (a) P. T. Anastas, L. B. Bartlett, M. M. Kirchhoff and T. C. Williamson, Catal. Today, 2000, 55, 11 CrossRef CAS; (b) R. Sheldon and J. K. Kochi, in Metal Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- (a) T. Matsumoto, M. Ueno, N. Wang and S. Kobayashi, Chem. Asian J., 2008, 3, 196 CrossRef CAS; (b) T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS; (c) N. Jamwal, M. Gupta and S. Paul, Green Chem., 2008, 10, 999 RSC; (d) S. F. J. Hackett, R. M. Brydson, M. H. Gass, I. Harvey, A. D. Newman, K. Wilson and A. F. Lee, Angew. Chem., Int. Ed., 2007, 46, 8593 CrossRef CAS.
- T. Mitsodume, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2008, 47, 138 CrossRef CAS.
- N. Zheng and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 14278 CrossRef CAS.
- C. Gonzalez-Arellano, J. M. Campelo, D. J. Macquarrie, J. M. Marinas, A. A. Romero and R. Luque, ChemSusChem, 2008, 1, 746 CrossRef CAS.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657 CrossRef CAS.
- A.-H. Lu, W.-C. Li, Z. Hou and F. Schüth, Chem. Commun., 2007, 1038 RSC.
- J. Chen, Q. Zhang, Y. Wang and H. Wan, Adv. Synth. Catal., 2008, 350, 453 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS.
- M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC.
- B. Karimi, S. Abedi, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2006, 45, 4776 CrossRef CAS.
- F. Shi, M. K. Tse, M. M. Pohl, A. Bruckner, S. Zhang and M. Beller, Angew. Chem., Int. Ed., 2007, 46, 8866 CrossRef CAS.
- (a) A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS; (b) N. T. S. Phan, C. S. Gill, J. V. Nguyen, Z. J. Zhang and C. W. Jones, Angew. Chem., Int. Ed., 2006, 45, 2209 CrossRef; (c) R. Abu-Reziq, H. Alper, D. Wang and M. L. Post, J. Am. Chem. Soc., 2006, 128, 5279 CrossRef; (d) C. O. Dalaigh, S. A. Corr, Y. Gunko and S. J. Connon, Angew. Chem., Int. Ed., 2007, 46, 4329 CrossRef CAS; (e) T. Hara, T. Kaneta, K. Mori, T. Mitsudome, T. Mizugaki, K. Ebitanic and K. Kaneda, Green Chem., 2007, 9, 1246 RSC; (f) A.-H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS; (g) A. H. Latham and M. E. Williams, Acc. Chem. Res., 2008, 41, 411 CrossRef CAS.
- (a) V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546 RSC; (b) V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629 CrossRef CAS; (c) V. Polshettiwar and R. S. Varma, Curr. Opin. Drug Discov. Devel., 2007, 10, 723 Search PubMed; (d) V. Polshettiwar and R. S. Varma, J. Org. Chem., 2008, 73, 7417 CrossRef CAS; (e) V. Polshettiwar and R. S. Varma, J. Org. Chem., 2007, 72, 7420 CrossRef CAS; (f) Y. Ju and R. S. Varma, J. Org. Chem., 2006, 71, 135 CrossRef CAS; (g) Y. Ju, D. Kumar and R. S. Varma, J. Org. Chem., 2006, 71, 6697 CrossRef CAS.
- (a) M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2008, 8, 291 CrossRef CAS; (b) M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 686; (c) M. N. Nadagouda and R. S. Varma, Cryst. Growth Des., 2007, 7, 2582 CrossRef CAS; (d) H. Choi, Y. J. Kim, R. S. Varma and D. D. Dionysiou, Chem. Mater., 2006, 18, 5377 CrossRef CAS.
- (a) V. Polshettiwar, M. N. Nadagouda and R. S. Varma, Chem. Commun., 2008 10.1039/b814715a; (b) V. Polshettiwar, B. Baruwati and R. S. Varma, Green. Chem., 2008, 10, in press Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures; recyclability and Pd-leaching of catalyst, TEM, XRD of nano-ferrites; NMR and MS data of compounds. See DOI: 10.1039/b817669h |
| This journal is © The Royal Society of Chemistry 2009 |