Synthesis of functional molecular rod oligomers†
Casper S.
Andersen
and
Kurt V.
Gothelf
*
Danish National Research Foundation: Centre for DNA Nanotechnology, Department of Chemistry and Interdisciplinary Nanoscience Center (iNANO), Langelandsgade 140, 8000 Aarhus C, Denmark. E-mail: kvg@chem.au.dk; Web: http://www.cdna.dk Fax: +45 8619 6199; Tel: +45 89423907
First published on 5th November 2008
Abstract
We are presenting the synthesis of a phenylene ethynylene-based rod containing an Fmoc protected amino group and an activated acid which can be applied to peptide couplings. This linear amino acid analogue is applied to the synthesis of di-, tri- and pentamers. The pentamer has a molecular mass of 6.5 kDa and is characterized by 1H NMR spectroscopy and MALDI-TOF MS. The method has potential for the formation of long macromolecular oligomers.
During the last two decades it has been demonstrated that individual organic molecules can possess electronic properties very similar to those of silicon-based components.1 Wires, rectifiers, memory units, and transistors are among the molecular components that have been synthesized and tested.2 The major obstacle for the realization of electronic circuits constructed from individual molecules remains our lacking capability in assembling the molecular building blocks into predetermined networks. Tour et al. demonstrated the synthesis of molecular wires of up to 16 nm, which is impressive for a monodisperse organic molecule.3 New solution phase and solid phase strategies were developed to obtain these molecular wires. However, how to connect the wire to other components, junctions and/or electrodes remains an unsolved problem.
One of the most studied classes of compounds for molecular electronics—and in particular for wires—are the oligo(phenylene ethynylene)-based components.1,2 The commonly used method for preparation of oligo(phenylene ethynylene)s is the Sonogashira cross-coupling reaction between terminal acetylenes and arylhalides.4 These reactions most often proceed with yields ranging from 60 to 90%, accompanied by alkyne dimerization as the most common byproduct. However, compared to the efficiency of the single coupling cycles in oligonucleotide and peptide synthesis (often >99%), the yields obtained in Sonogashira reactions are not sufficiently high for the development of an iterative synthesis of phenylene ethynylene oligomers using higher numbers of units.
In previous studies we have applied the oligonucleotide-functionalized molecular rod assembly of oligomers,5–7 whereas here we present a method of assembling molecular rods by peptide synthesis. The key molecular building block 1 (Fig. 1) contains distinct features for application in peptide synthesis and possesses the potential for subsequent chemical reactions. It is based on a phenylene ethynylene backbone. Two dodecyloxy chains are attached to the central part of the aryl rod to improve the solubility of the intermediates and the macromolecular assemblies that we are pursuing. Furthermore, the module contains an Fmoc protected amino group and an activated ester for assembly by peptide couplings. The terminal groups are protected salicylaldehydes, which can potentially be deprotected after the first coupling for the formation of metal salen bridges between the modules. Here we demonstrate the synthesis of this compound and its application to the synthesis of di-, tri- and pentamers by peptide couplings.
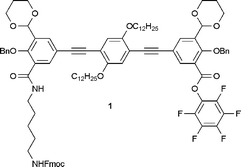 | ||
| Fig. 1 Structure of building block 1. | ||
For construction of the terminal aryl moiety in 1 consisting of a protected salicylaldehyde and a functional group for peptide couplings, compound 4 was synthesized as outlined in Scheme 1. According to a previously reported procedure,6 compound 2 was formylated in a Duff reaction followed by acetal formation. The resulting acid 3 is a hydrophilic and unstable compound, and was benzylated on the phenol and the carboxyl groups to give the stable compound 4 in an overall yield of 28% from 2. As an alternative strategy we also tested the introduction of MOM protective groups, but the resulting product was difficult to purify and the reactions containing MOM-protecting groups led to poor yields in the subsequent Sonogashira reactions.
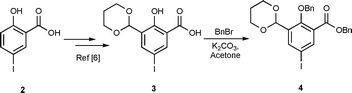 | ||
| Scheme 1 Synthesis of compound 4 in an overall yield of 28% from compound 2. | ||
The central 2,5-bis(dodecyloxy)-1,4-diethynylbenzene 5 was prepared from hydroquinone according to modified literature procedures.8 The Sonogashira reaction between 4 and 5, constructing the molecular backbone by the formation of 6, proceeded in 61% yield (Scheme 2). By the treatment of 6 with LiOH in THF, the diacid 7 was obtained quantitatively. In parallel studies we have formed the diacid of a compound similar to 7, but without the dodecyloxy groups; however, this compound was completely insoluble in any solvent and impossible to purify. Compound 7 was—on the contrary—soluble in THF and after treatment with dicyclohexylcarbodiimide (DCC) and pentafluorophenol, compound 8 was obtained in 77% yield after crystallization.
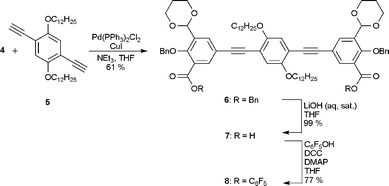 | ||
| Scheme 2 Formation of compound 8. | ||
The reaction of compound 8 with mono 1-N-Fmoc-1,5-diaminopentane with the aim of preparing 1 is a reaction which gives an almost 2:1 ratio of compounds 1 and 9 in 40% and 19% yields, respectively, along with recovery of 20% of the unreacted starting material (Scheme 3). The Fmoc protecting groups in diamide 9 were removed upon base treatment to give the diamine building block 10.
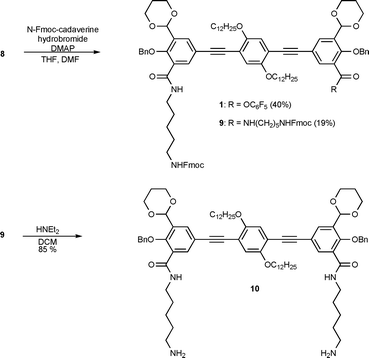 | ||
| Scheme 3 Synthesis of compounds 1 and 10. | ||
With the molecular building blocks 1 and 10 at hand we were ready to test their applicability for coupling into oligomeric structures.
By treatment of 2.0 equivalents of compound 1 with hexanediamine in THF/pyridine and in the presence of N,N-dimethylaminopyridine (DMAP) the dimeric structure 11 was formed and isolated in 94% yield (Scheme 4). Under similar conditions the trimer 12 was formed by mixing diamine 10 with 2.1 equivalents of 1 at rt in the presence of DMAP. The product was purified by precipitation and obtained in 60% yield. To obtain the pentamer the Fmoc protecting groups in 12 were removed by mild base treatment to yield diamine 13. Finally, reaction of 13 with two equivalents of 1 gave the pentamer 14 in 75% yield. The identity of pentamer 14 was verified by MALDI-TOF mass spectrometry and 1H-NMR spectroscopy.
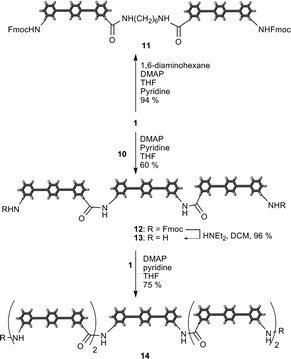 | ||
| Scheme 4 Formation of monodisperse oligomers (dimer 11, trimers 12 and 13 and pentamer 14). | ||
In summary we have demonstrated the synthesis of two conjugated molecular rods 1 and 10, both containing an oligo(phenylene ethynylene) backbone and functional headgroups containing protected salicylaldehydes and functionalities for peptide couplings. The building blocks have been applied to the formation of oligomers such as dimer 11, trimers 12 and 13 and pentamer 14. The pentamer is a macromolecular structure with a molecular mass of 6.5 kDa as verified by MALDI-TOF mass spectrometry. As we have previously demonstrated for the DNA-directed assembly of similar structures,5 the protected salicylaldehydes in the products can potentially be deprotected and applied to the formation of metal-salen complexes, which are coplanar, and furthermore such structures have potential as conducting molecular wires.
Notes and references
- J. M. Tour, Acc. Chem. Res., 2000, 33, 791 CrossRef CAS; J. M. Tour, A. M. Rawlett, M. Kozaki, Y. Yao, R. C. Jagessar, S. M. Dirk, D. W. Price and M. A. Reed, Chem. Eur. J., 2001, 7, 5118 CrossRef CAS; J. Chen, W. Wang, J. Klemic, M. A. Reed, B. W. Axelrod, D. M. Kaschak, A. M. Rawlett, D. W. Price, S. M. Dirk, J. M. Tour, D. S. Grubisha and D. W. Bennett, Ann. N. Y. Acad. Sci., 2002, 960, 69 CAS; N. Robertson and C. A. McGowan, Chem. Soc. Rev., 2003, 32, 96 RSC; J. M. Tour, Molecular Electronics: Commercial Insights, Chemistry, Devices, Architecture and Programming, World Scientific, New Jersey, 2003 Search PubMed.
- L. A. Bumm, J. J. Arnold, M. T. Cygan, T. D. Dunbar, T. P. Burgin, L. Jones II, D. L. Allara, J. M. Tour and P. S. Weiss, Science, 1996, 271, 1705 CrossRef CAS; M. A. Reed, C. Zhou, C. J. Muller, T. P. Burgin and J. M. Tour, Science, 1997, 278, 252 CrossRef CAS; D. K. James and J. M. Tour, Aldrichimica Acta, 2006, 39, 47 CAS; J. Chen, M. A. Reed, A. M. Rawlett and J. M. Tour, Science, 1999, 286, 1550 CrossRef CAS; Z. J. Donhauser, B. A. Mantooth, K. F. Kelly, L. A. Buum, J. D. Monnell, J. J. Stapleton, D. W. Price, Jr., A. M. Rawlett, D. L. Allara, J. M. Tour and P. S. Weiss, Science, 2001, 292, 2303 CrossRef CAS; S. Kubatkin, A. Danilov, M. Hjort, J. Cornil, J.-L. Bredas, N. S. Hansen, P. Hedegaard and T. Bjornholm, Nature, 2003, 425, 698 CrossRef CAS; F.-R. F. Fan, J. Yang, L. Cai, D. W. Price, Jr., S. M. Dirk, D. V. Kosynkin, Y. Yao, A. M. Rawlett, J. M. Tour and A. J. Bard, J. Am. Chem. Soc., 2002, 124, 5550 CrossRef CAS.
- S. Huang and J. M. Tour, J. Org. Chem., 1999, 64, 8898 CrossRef CAS; S. Huang and J. M. Tour, J. Am. Chem. Soc., 1999, 121, 4908 CrossRef CAS; S. Huang and J. M. Tour, Tetrahedron Lett., 1999, 40, 3447 CrossRef; L. Jones II, J. Schumm and J. M. Tour, J. Org. Chem., 1997, 62, 1388 CrossRef CAS; J. M. Tour, Chem. Rev., 1996, 96, 537 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef.
- K. V. Gothelf, A. H. Thomsen, M. Nielsen, E. Cló and R. S. Brown, J. Am. Chem. Soc., 2004, 126, 1044 CrossRef CAS; R. S. Brown, M. Nielsen and K. V. Gothelf, Chem. Commun., 2004, 1464 RSC; K. V. Gothelf and R. S. Brown, Chem. Eur. J., 2005, 11, 1062 CrossRef CAS; M. Nielsen, V. Dauksaite, J. Kjems and K. V. Gothelf, Bioconjugate Chem., 2005, 16, 681; P. Blakskjaer and K. V. Gothelf, Org. Biomol. Chem., 2006, 4, 3442 RSC.
- M. Nielsen, A. H. Thomsen, E. Cló, F. Kirpekar and K. V. Gothelf, J. Org. Chem., 2004, 69, 2240 CrossRef CAS.
- C. S. Andersen, H. Yan and K. V. Gothelf, Angew. Chem. Int. Ed., 2008, 67, 5569 CrossRef.
- Guorong Li, Xianhong Wang* and Fosong Wang, Tetrahedron Lett., 2005, 46, 8971 CrossRef CAS; González-Rojano Norma, Arias-Marín Eduardo, Navarro-Rodríguez Dámaso and Weidner Steffen, Synlett, 2005, 8, 1259.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, and NMR and MS characterization data. See DOI: 10.1039/b815099k |
| This journal is © The Royal Society of Chemistry 2009 |
