Ultra-sensitive detection of adipocytokines with CMOS-compatible silicon nanowire arrays†
Tze-Sian
Pui
ab,
Ajay
Agarwal
a,
Feng
Ye
b,
Zhi-Qiang
Tou
b,
Yinxi
Huang
b and
Peng
Chen
*b
aInstitute of Microelectronics, A*STAR (Agency for Science, Technology and Research), 11 Science Park Road, 117685 Singapore
bDivision of Bioengineering, Nanyang Technological University, 70 Nanyang Drive, 637457 Singapore. E-mail: chenpeng@ntu.edu.sg
First published on 13th August 2009
Abstract
Perfectly aligned arrays of single-crystalline silicon nanowires were fabricated using top-down CMOS-compatible techniques. We demonstrate that these nanowire devices are able to detect adipocytokines secreted by adipose cells with femtomolar sensitivity, high specificity, wide detection range, and ability for parallel monitoring. The nanowire sensors also provide a novel tool to reveal the poorly understood signaling mechanisms of these newly recognized signaling molecules, as well as their relevance in common diseases such as obesity and diabetes.
1. Introduction
Nanostructured materials such as nanoparticles,1 nanopores,2,3carbon nanotubes,4–6 and nanowires,7 are bringing unprecedented opportunities to biology. Their nanoscale dimensions allow them to interact intimately with, and spy on, the nanoscopic world of biomolecules. One-dimensional silicon nanowires (SiNWs) configured as field-effect transistors (FETs), in particular, have recently emerged as a novel class of nanoelectric biosensors for rapid and highly sensitive detection of various biospecies,8,9 including DNA,10protein biomarkers,11,12 viruses13 and toxins.14 In the present work, perfectly aligned arrays of single-crystal SiNWs were fabricated using top-down complementary metal–oxide–semiconductor (CMOS)-compatible fabrication techniques standardized in large-scale production of microelectronics. We demonstrate that these nanowire devices are able to detect adipocytokines secreted by the adipose cells using a simple detection scheme with femtomolar sensitivity, high specificity, wide detection range, and ability for parallel multiplexed monitoring. This development is of obvious scientific and clinical significance in terms of revealing the poorly understood signaling mechanisms of these newly recognized hormone molecules as well as their relevance in common diseases such as obesity, diabetes, cardiovascular disease, and so on.15–17Adipose tissue, which was previously regarded as a passive energy (fat) storage tissue, has recently been recognized as a highly active endocrine organ, secreting a range of signaling molecules called adipocytokines (including leptin, resistin, adiponectin, TNF-alpha, etc.).18 These molecules have widespread effects on health due to their important regulatory roles in energy homeostasis,19glucose and lipid metabolism,15,19 immune response,20 neuronal functions,21 neuroendocrine functions,22 and bone formation.23 But, the mechanisms that trigger secretion of these molecules, and how they act collaboratively in regulating many physiological functions are still poorly understood. Here, we study the secretion of leptin and resistin using a nanowire approach. Leptin, encoded by the obese gene, was discovered in 1994 as the first member of the growing family of adipocytokines.24 This 16 kDa protein is secreted in response to hyperinsulinemia after a meal or other stimuli, as a signal of nutritional status to the hypothalamus, thereby increasing insulin sensitivity, inhibiting food intake, enhancing energy expenditure and regulating body fat. Resistin (a 12.5 kDa adipocytokine discovered in 2001) is also regulated by nutritional status and is thought to cause insulin resistance in obesity-related type 2 diabetes mellitus.25 Although it is evident that leptin and resistin play distinct regulatory roles in metabolic activities, the interplay between their secretion and regulation are poorly studied.26
Developing effective assay methods for adipocytokines would certainly provide a great impetus to understand the multifaceted physiological roles of these molecules and their implications in pathogenesis. Current analytical methods for adipocytokines include immunoblotting, immunohistochemistry, immunoprecipitation, enzyme-linked immunosorbent assay (ELISA), and radio-immunoassay (RIA). These methods, however, are time-consuming, generally of low sensitivity, and require separation and/or concentration and/or labeling and/or signal amplification. Although RIA provides high sensitivity, the involvement of radioisotopes demands extra precautions and expensive facilities. Herein, we demonstrate that SiNW-based FETs offers a novel alternative to detect adipocytokines secreted from adipocytes.
2. Materials and methods
2.1 Molecules and chemicals
Polyclonal antibodies against murine leptin and murine resistin were purchased from Santa Cruz Biotechnology (USA). Murine resistin was purchased from Biovision (USA). Murine Leptin, 3-aminopropyltriethoxysilane (APTES), ethanolamine, glutaraldehyde and other chemicals were obtained from Sigma Aldrich (USA). The isoelectric points of leptin antibody and resistin antibody were determined by zeta-potential measurement using a Zetasizer (Malvern Instruments, UK).2.2 Sensor fabrication
An array of single-crystal SiNWs were fabricated on silicon-on-insulator (SOI) wafers using the CMOS-compatible top-down fabrication processes derived and improved from our previously developed method (see the process flow diagram in the ESI† ).27 SOI wafers with a 70 nm-thick silicon layer and 145 nm-thick buried silicon oxide layer were used. The resistivity of the silicon layer was adjusted by doping with n-type impurities (phosphorous) using an ion implanter to achieve a phosphorous ion concentration of ∼1 × 1018 cm−3. The dopants were activated by thermal annealing at 1000 °C for 10 s. Photoresist strips (∼160 nm wide) were patterned onto the silicon layer using deep-ultraviolet lithography followed by resist trimming to reach the dimension of ∼90 nm. The resist pattern was then transferred to the silicon layer by reactive-ion etching. The critical dimension of the resulting SiNWs was further reduced to ∼40 nm by oxidation at 900 °C and subsequent etching away of the silicon dioxide. The silicon contact pad structures at the ends of the NWs were selectively and heavily doped with phosphorous ions to a concentration >5 × 1020 cm−3 followed by aluminum metallization and alloying to establish Ohmic contacts with metal interconnections. The devices were subsequently passivated by silicon nitride (200 nm) and high-density-plasma (HDP) silicon dioxide (1500 nm) layers. The double passivation layers of silicon nitride and the thick dense silicon dioxide greatly reduced undesirable leakage currents and provided reliable protection during long-time wet etching. Finally, the metal pads and SiNWs were exposed through lithography and etching.2.3 SiNW functionalization
The SiNW arrays were soaked in 0.5% hydrofluoric acid solution for 7 min to etch away the thick layer of SiO2 and expose the fresh NWs. The SiNW chips were then left in ambient conditions for two days to allow the growth of a very thin native SiO2 layer (∼1 nm) to enable silanization. After cleaning with DI water and absolute ethanol, SiNW chips were immersed and silanized in 2% APTES (in absolute ethanol) for 30 min. They were then rinsed thoroughly with absolute ethanol and incubated in an oven at 150 °C for 1 h. The silanized SiNW chips were subsequently dipped in 5% glutaraldehyde (in DI water) at room temperature for 2 h followed by overnight incubation in the leptin or resistin antibody solution (2 µg mL−1 in 10 mM sodium bicarbonate, pH 9.0) at 4 °C. After rinsing with DI water, the SiNWs were treated with ethanolamine solution (titrated to pH 9.0) for 30 min. The SiNW chips were finally ready for use after washing with copious amounts of DI water and blow drying with nitrogen. The SiNW array was separated into two sensing regions by silicone rubber. The two regions were functionalized differently for parallel detection of leptin and resistin.2.4 Electrical measurements
Nanowire conductance was monitored using a patch-clamp amplifier (Multiclamp 700B, Axon Instruments) with a low filter of 1 kHz, sampling frequency of 4 kHz, and bias voltage (Vds) of 200 mV. A small volume (100 µL) of adipocytokine-containing sample solution (leptin or resistin in PBS, or 3T3-L1 adipocyte culture medium) was applied to a functionalized NW chip for 30 min incubation at 4 °C, followed by thorough rinsing with DI water. During the recordings, NWs were immersed in 1 mM PBS solution titrated to pH 7.0. This low ionic strength increases the Debye length of charge screening to ∼2.5 nm, thus enhancing the field-effect introduced by the biomolecules.2.5 Cell culture and differentiation
3T3-L1 Fibroblasts (American Type Culture Collection, USA) were cultured in DMEM (Gibco, USA) supplemented with 10% (v:v) heat-inactivated bovine calf serum (Gibco) and 1% penicillin-streptomycin (Gibco) at 37 °C in a humidified atmosphere of 5% CO2 : 95% air. 3T3-L1 Fibroblasts were induced to differentiate into adipocytes as previously described (see the ESI† ).28 Differentiation was confirmed by the morphological changes and appearance of fat droplets in the cells.3. Results and discussion
Hundreds of perfectly aligned NWs can be readily fabricated in one chip with a narrowly distributed electrical conductance. Measurements (in dry conditions) of 90 NWs on a typical chip gave an average conductance of 124.1 ± 3.2 nS , indicating uniformity in NW dimensions and properties. The cross-sectional dimension of the SiNWs can be controlled to different sizes, ranging from 20–100 nm, while its length may vary from a few to 100s of μm with precise control. The small diameter of the NWs ensures high sensitivity while the long length enables multi-copy detection (thus enhanced signal). In this study, each chip has 180, 100 µm-long NWs. As illustrated in Fig. 1(a), the SiNWs are grouped in clusters (five wires each) with a pitch distance of 200 µm. Such layout is suitable for multiplexed sensing as each cluster may serve as one sensing unit for a particular molecular target. The zoomed-in scanning electron micrograph depicts one such NW cluster, in which the NWs are uniformly spaced at 2 µm intervals, and each wire is electrically addressable by individual insulated metal contact lines. A typical transmission electron micrograph of a NW cross-section, prepared using a focused ion beam, indicates they have a width of 45 nm and a height of 60 nm [Fig. 1(a)]. The rounded top-edge, achieved by removing ∼50 nm of silicon dioxide in diluted hydrofluoric acid, avoids sharp-edge effects in the NW properties and non-specific interactions with biomolecules, while the foot at the base of the wire provides the necessary mechanical strength for the NWs to survive the solution-based processes.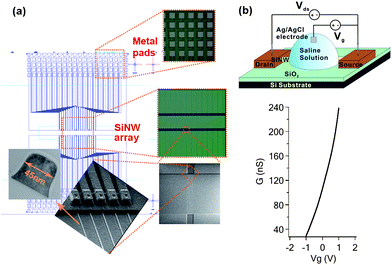 | ||
| Fig. 1 (a) Schematic illustration of a fabricated SiNW array, optical images of the metal pads and SiNW array, an SEM image and zoomed-in SEM image of a cluster of five SiNWs, and a TEM image of a SiNW cross-section are depicted in clockwise order. (b) Schematic illustration of a SiNW FET for biosensing. While a voltage bias (Vds) is applied across the SiNW, its drain–source current (Ids) can be continuously monitored. When a gate voltage (Vg) is applied to the solution bathing the SiNW, the current varies. As demonstrated in the bottom, with Vds = 200 mV, the conductance of the n-type SiNW (G) increased dramatically from 29.2 to 238.0 nS as Vg is swept from −1 to +1 V. The transconductance at Vg = 0 V is ∼0.1 nS mV−1. | ||
Compared with the bottom-up approaches involving nontrivial assembly process of pre-synthesized NWs,29 the top-down approach offers better scalability and integrability, and possibility for low-cost mass production. In contrast to polysilicon NWs27 whose conductance is significantly compromised by charge-scattering effects, single-crystal SiNWs have a higher conductivity and therefore larger transconductance (current response) in response to a given field-effect (from the biomolecules). Fig. 1(b) shows that our single-crystal SiNWs manifest n-type field-effect characteristics and are highly responsive to the Vg applied, via an Ag/AgCl electrode, to the saline solution bathing the SiNW. Specifically, its conductance varies from 29.2 to 238.0 nS while Vg changes from −1 to +1 V. This demonstrates that SiNWs are desirable for electrical biosensing because their conductance is highly sensitive to electrochemical perturbations at the NW surface, or its immediate vicinity, owing to their large surface-to-volume ratio.
Upon binding of charged biomolecules, the current that passes through a voltage-biased NW varies because of the electrostatic influence of the biomolecules. The specificity of such electronic transduction usually relies on the mediation of certain molecular recognition elements (e.g. antibodies) that are grafted onto the NW in order to selectively fish out the target molecules from the solution onto the NW surface. We cross-linked the antibodies against leptin or resistin onto SiNWs through a sequence of functionalization steps [Fig. 2(a)]. SiNWs were first silanized with APTES. As expected, the positively charged amino groups imparted onto the SiNW surface caused an increase in the NW conductance because of its n-type field-effect characteristics [Fig. 2(b)]. Subsequently, glutaraldehyde was conjugated covalently to the amino-modified SiNWs via one of its aldehyde groups. The positively charged amino groups on the SiNWs were quenched due to this reaction, leading to a reduction in SiNW conductance. Finally, the antibodies against leptin or resistin were immobilized onto the SiNWs by a covalent reaction between the amino groups on the antibodies and the free aldehyde groups from glutaraldehyde. As anticipated, conjugation of negatively charged leptin antibody and resistin antibody (whose isoelectric points are pH 4.1 and pH 4.4, respectively, as determined by zeta-potential measurements) both decreased the NW conductance. These measurements indicate clearly that each functional step is successful and NW conductance can be sensitively modulated by the electrostatic influence from the attached molecules. To avoid interference from unwanted binding of other proteins or molecules, the remaining free aldehyde groups on the SiNWs were passivated with ethanolamine.
 | ||
| Fig. 2 (a) Schematic illustration of a SiNW chip functionalized with leptin antibodies and resistin antibodies in two separate sensing regions. (b) The change in SiNW conductance relative to the conductance of the bare SiNW (‘1. Reference’), ΔG, resulting from each functionalization step (2, 3, and 4). | ||
Physiologically, leptin is negatively charged, as its isoelectric point is pH 5.7. When leptin molecules bind to a leptin-antibody functionalized SiNW, a decrease in SiNW conductance should be observed. The amplitude of such a decrease should depend on the number of leptin–antibody pairs on the SiNW at equilibrium, which is determined by the leptin concentration. As demonstrated in Fig. 3(a), this is indeed the case. The SiNWs produced consistent responses (current decrease) to leptin concentration as low as 10 fM (or 160 fg mL−1). The responding amplitude is logarithmically proportional to the leptin concentration, until it saturates at a leptin concentration of ∼500 pM, and the detection can be realized over a wide range of concentrations that exceeds four orders of magnitude. In contrast to leptin, binding of resistin molecules to a resistin-antibody functionalized SiNWs enhanced the SiNW conductance because resistin is positively charged due to its high isoelectric point (pH 7.7). As demonstrated in Fig. 3(b), a wide detection range (four orders of concentration) and even higher sensitivity were achieved for resistin. The increase in SiNW conductance is logarithmically proportional to the resistin concentration below the concentration of ∼500 pM. Even at resistin concentrations as low as 10 fM (or 101 fg mL−1), the SiNW response (220.0 pA), is about 10 times larger than the measurement variation (26.5 pA, n = 30 NWs) and 100 times larger than the SiNW noise (2.3 pA). Despite the fact that the isoelectric point of resistin is closer to physiological pH than that of leptin, resistin gave rise to a larger response, probably because it carries more net charge or because its charged group is physically closer to the NW when it binds with its antibody . The detection sensitivity for both leptin and resistin is comparable to the most sensitive RIA assay . While functionalized SiNWs are highly sensitive to the presence of leptin or resistin, they are not responsive to bovine serum albumin (100 µg mL−1 or 1.4 µM in PBS solution) and bovine serum (10% v:v in DMEM) which contains a variety of protein species (see the ESI† ), demonstrating the high specificity of the SiNW detection.
 | ||
| Fig. 3 Change in SiNW conductance vs. the concentration of leptin (a) and resistin (b). Each data point was averaged from the measurements of 30 SiNWs on three different chips functionalized with both leptin and resistin antibodies. The error bars indicate the standard deviations. The fittings (solid lines) indicate that the change in NW conductance is logarithmically proportional to the adipocytokine concentration. | ||
Adipocytes differentiated from 3T3-L1 fibroblasts are a popular cell model to study the physiology of adipocytes [Figs. 4(a) and (b)]. Using our SiNW chips, the kinetics of leptin and resistin secretion from 3T3-L1 adipocytes was monitored simultaneously on the same chip, under resting conditions or in presence of stimuli [Figs. 4(c) and (d)]. The flask of 3T3-L1 culture was first replenished with fresh culture medium (t = 0 min, free of leptin and resistin). Subsequently, the culture medium (containing both secreted leptin and resistin) was collected every 30 min for determination of leptin and resistin concentrations over a period of several hours using a SiNW chip that had been functionalized with both leptin and resistin antibodies in two separate sensing regions. The protocol of sample collection was repeated for the same flask of cells but with the addition of a stimulation cocktail containing 25 mM glucose, 0.25 µM insulin and 1 mM pyruvate (GPI stimulation) to the culture medium. In parallel with the measurements on the control samples, this set of samples was assayed in another dually functionalized SiNW chip.
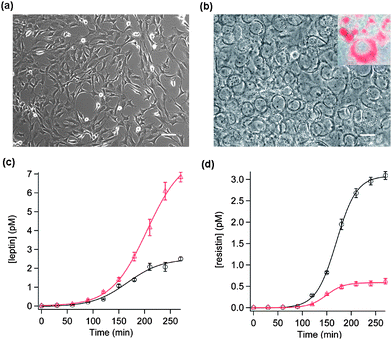 | ||
| Fig. 4 Phase-contrast TEM images of 3T3-L1 cells before (a) and after (b) differentiation into adipocytes (the white scale bars correspond to 100 µm). The inset in (b) shows the oil red-staining of the fat droplets inside the adipocytes. The kinetics of leptin (c) and resistin (d) secretion from 3T3-L1 adipocytes are shown. Two dually functionalized SiNW chips were used to analyze the aliquots of culture medium collected from a flask of cell culture over a time period of 4.5 h with (red triangles) and without (black circles) GPI stimulation, respectively. Each data point is an average of measurements taken from five SiNWs on the same chip. The error bars represent the standard deviations. Leptin and resistin concentrations were determined based on the changes in SiNW conductance and the calibration curves presented in Fig. 3. Each data set is fitted to a sigmoidal function: Csat/(1 + exp((thalf − t)/τ)) to obtain the saturation concentration (Csat), half-time (thalf), and time constant (τ). For basal leptin secretion, Csat = 2.54 pM, thalf = 166.4 min, and τ = 33.5 min. For GPI-stimulated leptin secretion, Csat = 7.98 pM, thalf = 204.5 min, and τ = 34.7 min. For basal resistin secretion, Csat = 3.09 pM, thalf = 169.6 min and τ = 20.6 min. For GPI-stimulated resistin secretion, Csat = 0.59 pM, thalf = 149.3 min, and τ = 17.8 min. | ||
In a typical experiment, demonstrated in Fig. 4(c), the leptin concentration steadily increases over time, even under resting conditions. This suggests that adipocytes constitutively secrete leptin molecules without the need of stimulation. Presumably, this is important in maintaining the basal circulating leptin concentration in blood to suppress appetite (or to avoid constant hunger). The constitutive secretion of leptin reached an apparent plateau sigmoidally. The sigmoidal fitting of the data points indicates a saturation concentration of ∼2.54 pM and a time constant of secretion of ∼33.5 min. It seems that there is certain self-limiting (auto-inhibition) mechanism to control the basal leptin level, likely through the leptin receptors expressed in adipocytes themselves.30 It has been shown that GPI can potently stimulate leptin secretion from adipocytes.31 This is confirmed by our observation that GPI caused a significant increase in leptin secretion (3.36 ± 1.28 fold in 3 h, 3 cell cultures). In the example shown in Fig. 4(c), leptin secretion was enhanced 2.73-fold by GPI. Our data agrees well with the kinetics of leptin secretion reported previously using RIA31 and immunoblotting.32
The secretion of resistin has been even less investigated as compared to that of leptin. Fig. 4(d) presents quantitatively, likely for the first time, the kinetics of resistin secretion. As shown, 3T3-L1 adipocytes also constitutively secreted resistin and the concentration of resistin increased sigmoidally to a saturation level of ∼3.09 pM with a time constant of ∼20.6 min. Similar to leptin secretion, resistin secretion appeared to be self-limiting although resistin receptors have not been found in adipocytes. Interestingly, we discovered that, in contrast to its stimulatory effect on leptin secretion, GPI significantly inhibited the secretion of resistin (to 43.5 ± 19.4% in 3 h, 3 cell cultures). As leptin and resistin have different effects on insulin sensitivity, it is perhaps not surprising that insulin regulates their secretion oppositely. Despite the fact that GPI was influential on the extent of leptin and resistin secretion, it did not significantly affect their secretion kinetics (i.e., the time constant and half-time of secretion were not significantly altered by GPI treatment as shown in Figs. 4(c) and (d)).
4. Conclusions
In summary, we have demonstrated a SiNW approach to detect adipocytokines with femtomolar sensitivity, adding a novel tool to study adipobiology. Electronic detection realized by SiNWs is simple, fast, and label-free. Moreover, given the small size of the sensing element (the NW), this approach requires only a tiny amount of sample for measurement. The array format of our SiNW chips enables parallel multiplexed sensing for multiple species and experimental protocols. For our specific application, in principle, secretion of multiple adipocytokines in response to various stimuli can be assayed simultaneously on one chip. This would help to understand the interplay between these adipocytokines: how they play regulatory roles in concert for a physiological function, and how a metabolic syndrome results from (or causes) alteration of the adipocytokine profile. As the SiNW array was made using standardized CMOS-compatible techniques, large-scale and low-cost production, easy integration with electronics can be realized. Using this SiNW approach, the secretion kinetics of leptin and resistin from 3T3-L1 adipocytes was revealed quantitatively, and it was discovered that, in contrast to its stimulatory regulation on leptin secretion, insulin inhibits the secretion of resistin.Acknowledgements
We acknowledge the support from an A*Star SERC grant (072 101 0020) and an AcRF tier 2 grant (T206B3220) to PC.References
- X. L. Luo, A. Morrin, A. J. Killard and M. R. Smyth, Electroanalysis, 2006, 18, 319 CrossRef CAS.
- P. Chen and C. M. Li, Small, 2007, 3, 1204 CrossRef CAS.
- Y. R. Kim, C. M. Li, Q. Wang and P. Chen, Front. Biosci., 2007, 12, 2978 CrossRef CAS.
- Y. Huang, H. G. Sudibya, D. Fu, R. Xue, X. Dong, L.-J. Li and P. Chen, Biosens. Bioelectron., 2009, 24, 2716 CrossRef CAS.
- J. Zhang, D. L. Fu, M. B. Chan-Park, L. J. Li and P. Chen, Adv. Mater., 2009, 21, 790 CrossRef CAS.
- H. G. Sudibya, J. Ma, X. Dong, S. Ng, L. J. Li, X. Liu and P. Chen, Angew. Chem., Int. Ed., 2009, 48, 2723 CrossRef CAS.
- B. He, T. J. Morrow and C. D. Keating, Curr. Opin. Chem. Biol., 2008, 12, 522 CrossRef CAS.
- F. Patolsky, G. Zheng and C. M. Lieber, Nanomedicine, 2006, 1, 51.
- Y. Cui, Q. Q. Wei, H. K. Park and C. M. Lieber, Science, 2001, 293, 1289 CrossRef CAS.
- Z. Li, Y. Chen, X. Li, T. I. Kamins, K. Nauka and R. S. Williams, Nano Lett., 2004, 4, 245 CrossRef CAS.
- E. Stern, J. F. Klemic, D. A. Routenberg, P. N. Wyrembak, D. B. Turner-Evans, A. D. Hamilton, D. A. LaVan, T. M. Fahmy and M. A. Reed, Nature, 2007, 445, 519 CrossRef CAS.
- G. F. Zheng, F. Patolsky, Y. Cui, W. U. Wang and C. M. Lieber, Nat. Biotechnol., 2005, 23, 1294 CrossRef CAS.
- F. Patolsky, G. F. Zheng, O. Hayden, M. Lakadamyali, X. W. Zhuang and C. M. Lieber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14017 CrossRef CAS.
- N. N. Mishra, W. C. Maki, E. Cameron, R. Nelson, P. Winterrowd, S. K. Rastogi, B. Filanoski and G. K. Maki, Lab Chip, 2008, 8, 868 RSC.
- M. Faraj, H. L. Lu and K. Cianflone, Biochem. Cell Biol., 2004, 82, 170 Search PubMed.
- M. W. Rajala and P. E. Scherer, Endocrinology, 2003, 144, 3765 CrossRef CAS.
- A. R. Nawrocki and P. E. Scherer, Drug Discovery Today, 2005, 10, 1219 CrossRef CAS.
- J. L. Miner, J. Anim. Sci., 2004, 82, 935 Search PubMed.
- E. D. Rosen and B. M. Spiegelman, Nature, 2006, 444, 847 CrossRef CAS.
- F. Lago, C. Dieguez, J. Gomez-Reino and O. Gualillo, Nat. Clin. Pract. Rheumatol., 2007, 3, 716 Search PubMed.
- J. Harvey, Curr. Opin. Pharmacol., 2007, 7, 643 CrossRef CAS.
- R. S. Ahima, D. Prabakaran, C. Mantzoros, D. Q. Qu, B. Lowell, E. MaratosFlier and J. S. Flier, Nature, 1996, 382, 250 CrossRef CAS.
- T. A. Cock and J. Auwerx, Lancet, 2003, 362, 1572 CrossRef CAS.
- Y. Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold and J. M. Friedman, Nature, 1994, 372, 425 CrossRef CAS.
- C. M. Steppan, S. T. Bailey, S. Bhat, E. J. Brown, R. R. Banerjee, C. M. Wright, H. R. Patel, R. S. Ahima and M. A. Lazar, Nature, 2001, 409, 307 CrossRef CAS.
- R. Burcelin, Endocrinology, 2007, 149, 443 CrossRef.
- T. S. Pui, A. Agarwal, F. Ye, N. Balasubramanian and P. Chen, Small, 2009, 5, 208 CrossRef CAS.
- D. L. Shao and M. A. Lazar, J. Biol. Chem., 1997, 272, 21473 CrossRef CAS.
- F. Patolsky, G. F. Zheng and C. M. Lieber, Nat. Protoc., 2006, 1, 1711 Search PubMed.
- I. Aprath-Husmann, K. Rohrig, H. Gottschling-Zeller, T. Skurk, D. Scriba, M. Birgel and H. Hauner, Int. J. Obes., 2001, 25, 1465 CrossRef CAS.
- J. R. Levy and W. Stevens, Endocrinology, 2001, 142, 3558 CrossRef CAS.
- V. A. Barr, D. Malide, M. J. Zarnowski, S. I. Taylor and S. W. Cushman, Endocrinology, 1997, 138, 4463 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Process diagram of nanowire fabrication; specificity of nanowire detection; induced differentiation of 3T3-L1 cells. See DOI: 10.1039/b9nr00092e |
| This journal is © The Royal Society of Chemistry 2009 |
