Label-free SERS detection of relevant bioanalytes on silver-coated carbon nanotubes: The case of cocaine†
Marcos
Sanles-Sobrido
a,
Laura
Rodríguez-Lorenzo
a,
Silvia
Lorenzo-Abalde
b,
África
González-Fernández
b,
Miguel A.
Correa-Duarte
*a,
Ramón A.
Alvarez-Puebla
*a and
Luis M.
Liz-Marzán
a
aDepartamento de Química Física and Unidad Asociada CSIC, Universidade de Vigo, 36310 Vigo, Spain. E-mail: macorrea@uvigo.es; ramon.alvarez@uvigo.es
bGrupo de Inmunología, Universidade de Vigo, 36310 Vigo, Spain
First published on 13th August 2009
Abstract
Surface-enhanced Raman scattering (SERS) spectroscopy can be used for the label-free determination and quantification of relevant small biometabolites that are hard to identify by conventional immunological methods, in the absence of labelling. In this work, detection is based on monitoring the vibrational changes occurring at a specific biointerface (a monoclonal antibody , mAb) supported on silver-coated carbon nanotubes (CNT@Ag). Engineered CNT@Ag play a key role, as they offer a stable substrate to support the biointerface, with a high density of hot spots. Proof of concept is demonstrated through the analysis and quantification of the main cocainemetabolitebenzoylecgonine. These results open a new avenue toward the generation of portable sensors for fast ultradetection and quantification of relevant metabolites. The use of discrete particles (CNT@Ag@mAb) rather than rough films, or other conventional SERS supports, will also enable a safe remote interrogation of highly toxic sources in environmental problems or in biological fluids.
Introduction
Surface-enhanced Raman scattering (SERS) spectroscopy is one of the most powerful analytical techniques with capacity for single-molecule detection.1,2 Conventional SERS experiments rely on the interaction of a given analyte with a nanosized gold or silver structure, usually as a colloidal suspension or a nanostructured film,3,4 which is able to generate a strong electromagnetic field when excited with visible or infrared light.5,6 However, the applicability of this approach is sometimes limited, especially when complex mixtures are to be analysed. In such cases, the target analyte’s vibrational features are very often concealed by signals from other species present in the sample, in particular when the analyte is very dilute. Some strategies have been devised to overcome this concealing effect, such as using codified SERS labels7–9 or coupling the SERS experiment to a prior purification step by using, for example, chromatography or electrophoresis.10–12 These strategies are powerful and reliable. However, the former requires the analyte to be able to complex to two different antibodies (for capture and detection), and thus is only useful for medium- to high-molecular-weight analytes, while the second is time consuming and requires the design of sophisticated specific instrumentation.Other possibilities, such as the use of biointerfaces that may specifically bind certain moieties (i.e.antibodies and nucleic acids), are now being studied by several groups.13–15 By using SERS together with this strategy, we aim to monitor changes in the enhanced vibrational signal of the receptor, as a result of a specific binding event with the analyte of interest. In line with this, SERS spectroscopy would be the method of choice as it brings together the sensitivity of Raman scattering spectroscopy to small changes in the structure of macromolecules,16–19 magnified, in this case, by the surface selection rules,20,21 with the ability of SERS to detect minute amounts of analyte. To succeed in this approach, however, it is essential to design hybrid materials that are able to covalently support large macromolecules such as antibodies on optically active nanoparticles, without affecting their optical properties, and that can be easily separated and cleaned from the biological fluid of interest.22
Results and discussion
We report in this article the design and fabrication of an optically stable SERS substrate comprising silver-coated carbon nanotubes (CNT@Ag), with activity in a wide window of excitation wavelengths, ranging from the visible to the near-infrared (NIR). These materials are easily resuspended in solution by sonication without any change of their optical properties.23 A proof of concept of the suitability of this substrate for the label-free indirect ultradetection of relevant bioanalytes is demonstrated by coupling a specific monoclonal antibody (mAb) directed against benzoylecgonine (BCG), a major cocainemetabolite, which is expressed in biological fluids such as saliva, blood, or sweat24 and, which can therefore be used as an indicator of drug abuse.25Comparison of the results with those obtained from the direct SERS detection of BCG on the same substrate reveals that, whereas direct detection offers a better detection limit than the specific indirect method, label-free indirect detection offers quantitative results26–28 at physiological levels, so that not only can drug abuse be demonstrated, but the consumption can also be quantified with a portable, non-invasive and fast technique.
CNTs were selected as useful templates where the controlled deposition of Ag nanoparticles can be achieved,29 as required for an optimised SERS substrate. Additionally, CNTs possess a high physical and chemical stability, which together with a high aspect ratio have also led us to anticipate their suitability for the preparation of advanced SERS substrates.22 The fabrication of the CNT@Ag substrates used in this work comprised the homogeneous electrostatic adsorption of small silver nanoparticles (2 nm diameter) onto polyallylamine hydrochloride (PAH)-coated CNTs.30,31 The small Ag nanoparticles were used as seeds and further grown with the intention of both increasing the effective cross-section and obtaining silver nanoparticles in close contact for an increased number of hot spots (sites with a large electromagnetic field, where SERS is most efficient). The colloidal stability of these modified CNTs (Fig. 1a) is crucial to allow adsorption of the target analytes and antibodies onto the metal nanoparticles and though they slowly sediment over time, redispersion can be achieved by simply shaking the solution. The profiles of the corresponding UV–Vis spectraconfirm the retention and high coverage of the Ag nanoparticles on the CNTs (Fig. 1b). While a colloid containing silver nanoparticles with similar size and surface properties presents a plasmon band centred at 412 nm, the particles grown on the CNT external walls show a very broad surface plasmon band over the visible and NIR, similar to that of evaporated silver films.22,32 The SERS-enhancing properties of the composite nanotubes were studied with SERS (Fig. 1c). To this end, dilute solutions of 1-naphthalenethiol (1NAT, 10 µL 10−5 M) were added to 0.5 mL aliquots of CNT@Ag (0.011 mg mL−1). After allowing 30 min for thermodynamic equilibrium to be reached, 10 µL aliquots were cast and air-dried on glass slides and their SERS-enhancing properties were studied by excitation with three different laser lines: 532, 633 and 785 nm. The spectra obtained present strong SERS signals corresponding to 1NAT: ring stretching (1553, 1503, and 1368 cm−1), CH bending (1197 cm−1), ring breathing (968 and 822 cm−1), ring deformation (792, 664, 539, and 517 cm−1), and CS stretching (389 cm−1).32 Interestingly, a high signal-to-noise ratio was achieved for all three excitation laser lines, which is related to the broad plasmon band of the composite colloid and confirms the formation of a high density of hot spots on the coated CNT surface.33–35
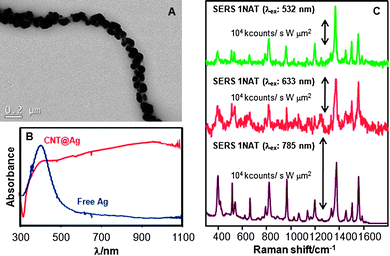 | ||
| Fig. 1 (A) Representative TEM image of CNT@Ag. (B) Normalized UV–Vis spectra of silver nanoparticles assembled on PAH-coated CNTs and of a colloid of Ag nanoparticles with similar size. (C) SERS spectra of 1NAT on CNT@Ag measured upon excitation with three different laser lines. | ||
The selected receptor, a mouse mAb anti-BCG, is highly specific (affinity constant 5.8 × 109) to cocaine and to its main metabolite, BCG, but shows very low cross-reactivity with other degradation metabolites of cocaine such as ecgonine or ecgonine methylester (recognition of 3.2% and 0.1%, respectively). The mAb anti-BCG was conjugated with the nanostructured silver surfaces by means of standard carbodiimide chemistry (see the Experimental section for details) at low concentration. Small amounts of BCG (10 µL 10−5 M) were added to 0.1 mL solutions of CNT@Ag (0.011 mg mL−1) with and without antibody , as illustrated in Fig. 2. Aliquots of the different mixtures (10 µL) were then cast and air-dried on glass slides and SERS spectra were collected using a NIR (785 nm) laser line for excitation, so as to avoid inducing damage on the samples.
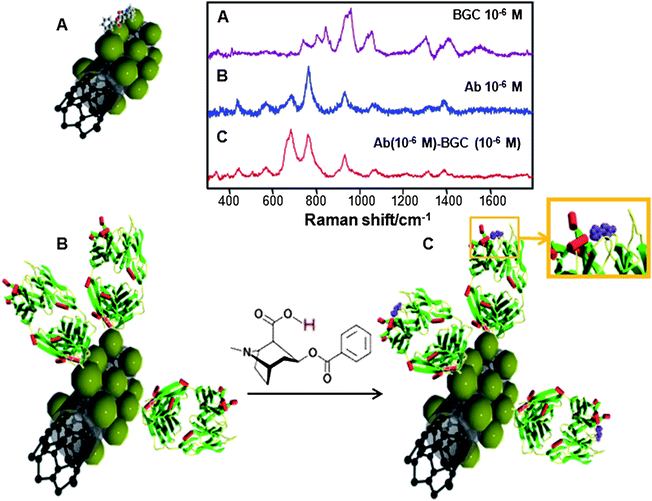 | ||
| Fig. 2 Schematic representation (green spheres represent Ag NPs on which the analytes can be retained) and SERS spectra of the direct (A) and the label-free specific indirect detection of BCG on CNT@Ag (B and C). (B) corresponds to the Fab mAb fragments adsorbed on the CNT@Ag substrate whereas (C) depicts the complexed mAb–BCG system.29 | ||
The SERS spectrum of BCG (Fig. 2A) is dominated by bands at 1581 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching), 1440 cm−1 (C–H bending), 1313 cm−1 (NC–H stretching), 1031cm−1 (C–N stretching), 968 cm−1 (ring breathing), 866 cm−1 (pyrrolidine C–C stretching), 812 cm−1 (C–H out-of-plane deformation), and 764 cm−1 (piperidine C–C stretching). The SERS spectrum of the antibody (Fig. 2B) is dominated by 1343 cm−1 (C–H deformation), 1307 cm−1 (amide III), 1085 and 924 cm−1 (C–C stretching), 764 cm−1 (C–C bending), and 693 cm−1 (C–H bending).36 Notably, the spectrum of the mAb–BCG complex is similar to that of the free antibody but displays significant and consistently reproducible differences. The main difference is a drastic enhancement of the band at 693 cm−1 (C–H bending) for the mAb–BCG complex.
C stretching), 1440 cm−1 (C–H bending), 1313 cm−1 (NC–H stretching), 1031cm−1 (C–N stretching), 968 cm−1 (ring breathing), 866 cm−1 (pyrrolidine C–C stretching), 812 cm−1 (C–H out-of-plane deformation), and 764 cm−1 (piperidine C–C stretching). The SERS spectrum of the antibody (Fig. 2B) is dominated by 1343 cm−1 (C–H deformation), 1307 cm−1 (amide III), 1085 and 924 cm−1 (C–C stretching), 764 cm−1 (C–C bending), and 693 cm−1 (C–H bending).36 Notably, the spectrum of the mAb–BCG complex is similar to that of the free antibody but displays significant and consistently reproducible differences. The main difference is a drastic enhancement of the band at 693 cm−1 (C–H bending) for the mAb–BCG complex.
It is well-known that when an antibody binds specifically to its ligand, the interaction modifies the structure of the whole antibody .37 The conformational change especially affects the fragment crystallizable (Fc) region, which includes all heavy-chain constant domains except CH1. In the case of a mAb anti-BCG, modifications in the fragment antigen binding (Fab) region after the interaction have been studied by single-crystal X-ray diffraction and computational modelling (see the resulting molecular structure in Fig. 3A).38 Notably, analysis (Fig. 3B) of the overlapped structures before (green) and after (red) the interaction between the Fab and BCG, shows that the positions of a significant number of atoms do not match between both structures.
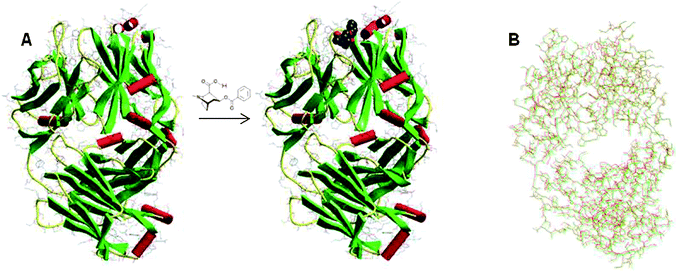 | ||
| Fig. 3 (A) Molecular structure of the Fab mAb fragment directed against BCG before and after conjugation with BCG. (B) Overlapped molecular structure showing the slight structural differences between the free Fab antibody fragments (green) and the conjugated system (red). These structures can be accessed free of charge in the Protein Data Bank (access numbers: 1RFD and 1QYG).34 | ||
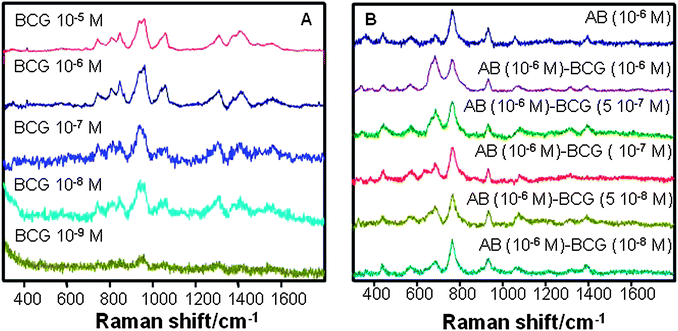
Although the detection mechanism is conceptually different, indirect label-free detection, based on changes in the coupled antibody SERS signal (Fig. 4B), yields detection limits of about the same order of magnitude. However, specific indirect detection presents several important advantages over the direct approach. First of all and most importantly, it is selective and it could be carried out within complex biological fluids such as saliva or urine, by simply allowing some time for the BCG coupling onto the antibody receptor and then removing the remaining components of the sample by just washing the CNT@Ag@antibody tubes for further analysis. Second, the SERS spectra of the coupled system contain one intense band (764 cm−1; C–C in-plane bending) that hardly changes upon conjugation of BCG, as compared with changes recorded in the out-of-plane C–H bending (693 cm−1). This different behaviour is very likely related to smaller changes in the orientation of the carbon chain as compared with that of the substituted hydrogen atoms and could be interpreted as follows. If we imagine the protein carbon chain extending perpendicular to the nanoparticle surface, the hydrogen atoms that saturate this chain are almost parallel to the surface. Thus, a small change in the antibody skeleton will yield a small orientational change in the carbon chain but a much more extended orientational change in the C–H bonds, with respect to the induced dipole normal to the surface. In full agreement with the SERS selection rules,20,39 vibrations with a deeper orientational change with respect to the surface will be more affected than those with a lesser orientational change. This indirect detection can also be exploited to obtain quantitative information regarding the concentration of BCG in the sample by simply considering the band that remains basically constant as an internal standard (Fig. 5). The plot of the ratio between the areas under the bands at 763 and 693 cm−1vs. molar concentration of BCG in the sample gives rise to a linear correlation, with an impressive r2 value of 0.9896 (Fig. 5B), thus demonstrating the quantitative nature of this method of analysis.
 | ||
| Fig. 4 Direct (A) and label-free indirect specific (B) SERS detection of BCG as a function of sample concentration. | ||
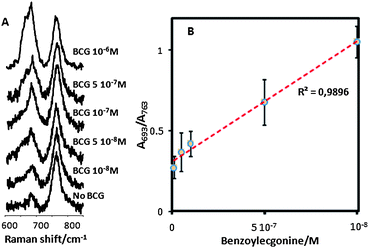 | ||
| Fig. 5 (A) SERS spectra for free anti-BCG antibody and in the presence of decreasing concentrations of BCG. (B) Variation of the relative contribution of the C–C and C–H bending bands (ratio between corresponding areas) with BCG concentration. The red dotted line is a linear fit to the data. | ||
The specificity of these changes was confirmed by carrying out the same experiments but using polyclonal anti-BCG antibodies (see the ESI† ). Notably, no neat spectral changes were detected upon extended exposure to BCG. These may be explained in light of the nature of monoclonal and polyclonal antibodies . While monoclonal antibodies are monospecific and identical because they are produced by a single hybrid cell, the latter come from different B cells. Thus polyclonal antibodies are actually a mixture of different immunoglobulin molecules secreted against a specific antigen, each one recognising a different epitope . This diversity in the molecular structures that constitute the polyclonal antibodies , renders its SERS spectra much more complex. They exhibit lower intensity at the same concentration than the mAb, and are not suitable for recording changes upon exposure to the antigen, because these will be much weaker and delocalised.
Finally, it is interesting to compare the performance of the described method with other detection tools that have been developed for detection of cocaine and its metabolites. The detection limits for a variety of methods based on either separation (HPLC, LC or solid-phase extraction, coupled to fluorescence or mass detection),40–44 or immunological methods (ELISA, FIA or RIA)45–48 are in the range of 1 ng mL−1 (∼1 nM), i.e. of the same order of the method presented here. However, one needs to bear in mind that the former (separation methods) require extensive sample preparation and are time consuming and not suitable for in situ detection, while the latter (immunological methods) require secondary detection antibodies , which not only notably increase the cost, but also reduce the reliability of the methods for the determination of small molecules, as the target structure needs to be bound by two different sites.
Conclusions
In summary, we have demonstrated the advantages of using selective monoclonal antibodies covalently bound to engineered silver-coated carbon nanotubes for the determination of relevant small bioanalytes. This system profits from both the sensitivity of SERS spectroscopy to small changes in the conformation of macromolecules and from the surface selection rules to evidence the changes taking place at the biointerface (the monoclonal selective antibody ) upon complexation with the antigen (drug metabolite). This label-free, indirect detection method offers quantitative results at physiological levels as the biointerface can also behave as an internal standard. The results presented pave the way toward the generation of portable sensors for fast ultradetection and quantification of relevant metabolites, which are important in diagnosis and detection. We see the use of discrete particles (CNT@Ag@mAb) instead of metal films or other conventional SERS supports as the basis for the safe, remote interrogation of highly toxic sources in the environment or in biological fluids.Methods
All the chemicals were purchased from Aldrich unless otherwise stated.Silver-coated carbon nanotubes (CNT@Ag)
Silver seeds were produced by mixing 5 mL of 2.5 mM trisodium citrate, 0.25 mL of 500 mg L−1 polystyrene sodium sulfonate (PSS, Mw ≈ 100![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000), and 0.3 mL of 10 mM NaBH4, and then adding drop-wise 5 mL of 0.5 mM AgNO3 under stirring.49 Seeds were assembled onto poly(allylamine hydrochloride) (PAH; Mw ≈ 70
000), and 0.3 mL of 10 mM NaBH4, and then adding drop-wise 5 mL of 0.5 mM AgNO3 under stirring.49 Seeds were assembled onto poly(allylamine hydrochloride) (PAH; Mw ≈ 70![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000) coated carbon nanotubes (NanoLab) by drop-wise addition of 15 mL of a suspension containing 7.5 × 10−2 mg mL−1 of PAH-coated multiwalled CNTs (Nanolab; 15 ± 5 nm diameter, 5–20 µm length, and 95% purity) to the seed colloid under sonication.30,31 The silver seeds were subsequently grown in situ on the nanotubes (2.0 mL, 0.011 mg mL−1) by adding 5.0 mL of distilled water, 75 µL of 10 mM ascorbic acid, 3.0 mL of 0.5 mM AgNO3, and 0.5 mL of 25 mM sodium citrate.
000) coated carbon nanotubes (NanoLab) by drop-wise addition of 15 mL of a suspension containing 7.5 × 10−2 mg mL−1 of PAH-coated multiwalled CNTs (Nanolab; 15 ± 5 nm diameter, 5–20 µm length, and 95% purity) to the seed colloid under sonication.30,31 The silver seeds were subsequently grown in situ on the nanotubes (2.0 mL, 0.011 mg mL−1) by adding 5.0 mL of distilled water, 75 µL of 10 mM ascorbic acid, 3.0 mL of 0.5 mM AgNO3, and 0.5 mL of 25 mM sodium citrate.
Characterization
Optical characterization was carried out by UV–Vis–NIR spectroscopy with an Agilent 8453 spectrophotometer, using a 10 mm path length quartz cuvette. Transmission electron microscopy (TEM) images were obtained with a JEOL JEM 1010 transmission electron microscope operating at an acceleration voltage of 100 kV.Antibody biocoupling onto CNT@Ag
100 µL of thioglycolic acid was added to 1.0 mL of CNT@Ag and left for 12 h so that the thiol was adsorbed on the silver surface. The thiol-functionalized CNT@Ag were centrifuged twice at 3500 rpm for 15 min and redispersed in phosphate-buffered saline (PBS). Coupling of the monoclonal mouse anti-benzoylecgonine (ref: B1077-08; US Biological) or the polyclonal sheep anti-benzoylecgonine antibodies (ref: ab31025; Abcam) to thioglycolic acid-functionalized CNT@Ag was achieved using carbodiimide chemistry to conjugate the primary amines of the antibody (Ab) with the carboxyl groups of thioglycolic acid. Briefly, the thioglycolic acid-functionalized CNTs@Ag (1 mL) and 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide (EDAC) conjugation buffer (50 µL, 2% w/v EDAC, 3% w/v N-hydroxysuccinimide in PBS, pH 7.2) were mixed in an orbital shaker for 15 min at room temperature. The Ab (1 to 10 µg) was added to the reaction mixture containing the CNT@Ag and incubated for 2 h with stirring, at room temperature. After 2 h, the reaction was quenched with 10 µL of 1 M hydroxylamine to regenerate the original, non-reacted carboxyl groups. The antibody -conjugated CNT@Ag were centrifuged at 3500 rpm for 15 min and washed three times with 0.5 mL PBS.Surface-enhanced Raman scattering (SERS) spectroscopy
The inelastic scattered radiation was collected with a LabRam HR system (Horiba-Jobin Yvon), equipped with Peltier charge-coupled device (CCD) detectors and an Olympus microscope. The spectrographs have 1800 g mm−1 gratings with additional band-pass filter optics. Samples were excited with three different laser lines at 532 (Nd:Yag), 633 (He–Ne) and 785 nm (diode). The power at the sample was set at 100 µW, with accumulation times of 10 s. The laser line was focused onto the sample in backscattering geometry using a 50× objective (numerical aperture 0.75) providing scattering areas of ca. 1 µm2.The SERS optical activity of the prepared CNT@Ag was tested with 1-naphthalenethiol (1NAT, Acros Organics), a well-studied SERS probe. Samples were prepared by adding 10 µL 1NAT 10−5 M solutions to 500 µL of CNT@Ag (0.011 mg mL−1). After 30 min (to reach thermodynamic equilibrium), 10 µL aliquots were cast and air-dried on glass slides and studied with three laser lines: 532, 633 and 785 nm.
For BCG detection, small amounts of BCG (10 µL from 10−5 to 10−9 M) were added to 100 µL of CNT@Ag (0.011 mg mL−1) with and without antibody . Aliquots of the different samples (10 µL) were cast and air-dried on glass slides and SERS spectra were collected, exciting with a NIR (785 nm) laser line to avoid damaging the samples.
Acknowledgements
R.A.A.-P. and M.A.C.-D. acknowledge the RyC (MEC, Spain) and IPP (Xunta de Galicia) programs. S.R.-L. acknowledges the Torres Quevedo program (MEC, Spain). This work has been funded by the Spanish Ministerio de Ciencia e Innovación (Grants MAT2007-62696, MAT2008-05755, Consolider Ingenio 2010- CSD2006-12) and the Xunta de Galicia (Grants PGIDIT06TMT31402PR, 08TMT008314PR and 2008/077).References
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957 CrossRef CAS.
- R. Aroca, Surface-enhanced Vibrational Spectroscopy, John Wiley & Sons, Chichester, 2006 Search PubMed.
- R. A. Tripp, R. A. Dluhy and Y. Zhao, Nano Today, 2008, 3, 31 CrossRef CAS.
- M. J. Banholzer, J. E. Millstone, L. Qin and C. A. Mirkin, Chem. Soc. Rev., 2008, 37, 885 RSC.
- N. P. W. Pieczonka and R. F. Aroca, ChemPhysChem, 2005, 6, 2473 CrossRef CAS.
- R. F. Aroca, R. A. Alvarez-Puebla, N. Pieczonka, S. Sanchez-Cortez and J. V. Garcia-Ramos, Adv. Colloid Interface Sci., 2005, 116, 45 CAS.
- M. Y. Sha, H. Xu, M. J. Natan and R. Cromer, J. Am. Chem. Soc., 2008, 130, 17214 CrossRef CAS.
- S. P. Mulvaney, M. D. Musick, C. D. Keating and M. J. Natan, Langmuir, 2003, 19, 4784 CrossRef.
- M. Sanles-Sobrido, W. Exner, L. Rodríguez-Lorenzo, B. Rodríguez-González, M. A. Correa-Duarte, R. A. Alvarez-Puebla and L. M. Liz-Marzán, J. Am. Chem. Soc., 2009, 131, 2699 CrossRef CAS.
- B. J. Kennedy, R. Milofsky and K. T. Carron, Anal. Chem., 1997, 69, 4708 CrossRef CAS.
- R. Sheng, F. Ni and T. M. Cotton, Anal. Chem., 1991, 63, 437 CrossRef CAS.
- L. He, M. J. Natan and C. D. Keating, Anal. Chem., 2000, 72, 5348 CrossRef CAS.
- B. R. Baker, R. I. Lai, M. S. Wood, E. H. Doctor, A. J. Heeger and K. W. Plaxco, J. Am. Chem. Soc., 2006, 128, 3138 CrossRef CAS.
- J. Elbaz, R. Tel-Vered, R. Freeman, H. B. Yildiz and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 133 CrossRef CAS.
- J. Chen, J. Jiang, X. Gao, G. Liu, G. Shen and R. Yu, Chem.–Eur. J., 2008, 14, 8374 CrossRef CAS.
- A. J. Bonham, G. Braun, I. Pavel, M. Moskovits and N. O. Reich, J. Am. Chem. Soc., 2007, 129, 14572 CrossRef CAS.
- E. S. Grabbe and R. P. Buck, J. Am. Chem. Soc., 1989, 111, 8362 CrossRef CAS.
- R. A. Alvarez-Puebla, J. P. Bravo-Vasquez, B. Cui, T. Veres and H. Fenniri, ChemMedChem, 2007, 2, 1165 CrossRef CAS.
- R. A. Alvarez-Puebla, J. J. Garrido and R. F. Aroca, Anal. Chem., 2004, 76, 7118 CrossRef CAS.
- M. Moskovits and J. S. Suh, J. Phys. Chem., 1984, 88, 5526 CrossRef CAS.
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783 CrossRef CAS.
- P. Taladriz-Blanco, L. Rodriguez-Lorenzo, M. Sanles-Sobrido, P. Herves, M. A. Correa-Duarte, R. A. Alvarez-Puebla and L. M. Liz-Marzan, ACS Appl. Mater. Interfaces, 2009, 1, 56 Search PubMed.
- M. Spuch-Calvar, L. Rodriguez-Lorenzo, M. P. Morales, R. A. Alvarez-Puebla and L. M. Liz-Marzan, J. Phys. Chem. C, 2009, 113, 3373 CrossRef CAS.
- O. H. Drummer, Clin. Biochem. Rev., 2006, 27, 147 Search PubMed.
- W. Schramm, P. A. Craig, R. H. Smith and G. E. Berger, Clin. Chem., 1993, 39, 481 CAS.
- S. E. J. Bell, J. N. Mackle and N. M. S. Sirimuthu, Analyst, 2005, 130, 545 RSC.
- S. E. J. Bell and N. M. S. Sirimuthu, Analyst, 2004, 129, 1032 RSC.
- S. E. J. Bell and N. M. S. Sirimuthu, Chem. Soc. Rev., 2008, 37, 1012 RSC.
- M. A. Correa-Duarte and L. M. Liz-Marzan, J. Mater. Chem., 2006, 16, 22 RSC.
- M. A. Correa-Duarte, N. Sobal, L. M. Liz-Marzan and M. Giersig, Adv. Mater., 2004, 16, 2179 CrossRef CAS.
- M. Grzelczak, M. A. Correa-Duarte, V. Salgueirino-Maceira, B. Rodriguez-Gonzalez, J. Rivas and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2007, 46, 7026 CrossRef CAS.
- R. A. Alvarez-Puebla, D. S. dos Santos Jr. and R. F. Aroca, Analyst, 2004, 129, 1251 RSC.
- P. Etchegoin, L. F. Cohen, H. Hartigan, R. J. C. Brown, M. J. T. Milton and J. C. Gallop, J. Chem. Phys., 2003, 119, 5281 CrossRef CAS.
- S. J. Lee, Z. Guan, H. Xu and M. Moskovits, J. Phys. Chem. C, 2007, 111, 17985 CrossRef CAS.
- G. Braun, I. Pavel, A. R. Morrill, D. S. Seferos, G. C. Bazan, N. O. Reich and M. Moskovits, J. Am. Chem. Soc., 2007, 129, 7760 CrossRef CAS.
- R. Tuma, J. Raman Spectrosc., 2005, 36, 307 CrossRef CAS.
- D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, W.H. Freeman and Company, cop., New York, 2008 Search PubMed.
- P.I.R. a. 1QYG; E. Pozharski, A. Moulin, A. Hewagama, A. B. Shanafelt, G. A. Petsko and D. Ringe, J. Mol. Biol., 2005, 349, 570 Search PubMed.
- J. K. Sass, H. Neff, M. Moskovits and S. Holloway, J. Phys. Chem., 1981, 85, 621 CrossRef CAS.
- E. I. Miller, F. M. Wylie and J. S. Oliver, J. Anal. Toxicol., 2008, 32, 457–469 CAS.
- F. E. Dussy, C. Berchtold, T. A. Briellmann, C. Lang, R. Steiger and M. Bovens, Forensic Sci. Int., 2008, 177, 105 CrossRef CAS.
- C. Moore, C. Coulter and K. Crompton, J. Chromatogr. B, 2007, 859, 208 CrossRef CAS.
- E. J. Rook, M. J. X. Hillebrand, H. Rosing, J. M. Van Ree and J. H. Beijnen, J. Chromatogr. B, 2005, 824, 213 CAS.
- E. Cognard, S. Rudaz, S. Bouchonnet and C. Staub, J. Chromatogr. B, 2005, 826, 17 CrossRef CAS.
- C. Kollias-Baker, L. Maxwell, S. Stanley and T. Boone, J. Vet. Pharmacol. Ther., 2003, 26, 429 CrossRef CAS.
- I. Kim, A. J. Barnes, R. Schepers, E. T. Moolchan, L. Wilson, G. Cooper, C. Reid, C. Hand and M. A. Huestis, Clin. Chem., 2003, 49, 1498 CrossRef CAS.
- D. A. Kidwell, J. D. Kidwell, F. Shinohara, C. Harper, K. Roarty, K. Bernadt, R. A. McCaulley and F. P. Smith, Forensic Sci. Int., 2003, 133, 63 CrossRef CAS.
- D. E. Moody, A. C. Spanbauer, J. L. Taccogno and E. K. Smith, J. Anal. Toxicol., 2004, 28, 86–93 CAS.
- D. Aherne, D. M. Ledwith, M. Gara and J. M. Kelly, Adv. Funct. Mater., 2008, 18, 2005 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SERS spectra of a polyclonal antibody supported on CNT@Ag before and after exposure to BCG. See DOI: 10.1039/b9nr00059c |
| This journal is © The Royal Society of Chemistry 2009 |
