Synthesis of highly luminescent cobalt(II)-bis(8-hydroxyquinoline)nanosheets as isomeric aromatic amine probes†
Haibing
Li
* and
Yuling
Li
Key Laboratory of Pesticide & Chemical Biology (CCNU), Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, PR China. E-mail: lhbing@mail.ccnu.edu.cn; Tel: +86-27-67866423
First published on 28th August 2009
Abstract
Highly luminescent and water-soluble cobalt(II)-bis(8-hydroxyquinoline) (CoQ2) nanosheets have been successfully synthesized via a simple, rapid sonochemical method. The water-soluble CoQ2nanosheets were characterized by luminescence spectroscopy, UV–vis spectroscopy, FT-IR spectroscopy and transmission electron microscopy (TEM). The CoQ2nanosheets allow highly sensitive and selective determination of p-nitroanilinevia fluorescence quenching. Under optimal conditions, the relative fluorescence intensities of nanosheets decreased linearly with increasing p-nitroaniline. However, the sensitivity of CoQ2nanosheets toward other aromatic amines including o-diaminobenzene, m-diaminobenzene, p-diaminobenzene, p-toluidine, o-nitroaniline, m-nitroaniline, p-chloroaniline and aniline is negligible. It is found that p-nitroaniline can quench the luminescence of CoQ2nanosheets in a concentration-dependent manner that is best described by a Stern–Volmer-type equation. The possible underlying mechanism is discussed.
1. Introduction
Aromatic amines (AAMs) are widely used as raw materials or intermediates in the manufacturing of dyes, pesticides, medicines and pharmaceuticals.1 But, aromatic amines are highly toxic materials that can easily permeate through soil and contaminate groundwater and enter the body when people consume food or water contaminated with them. Currently, the International Agency for Research on Cancer (IARC) has classified six aromatic amines as carcinogenic or probably carcinogenic to humans, accordingly, the remnants of aromatic amines in environment has raised a great concern.2,3 As a consequence, aromatic amines are suspected to be harmful to humans and need to be monitored regularly, and the determination method must be simple, rapid and effective.Several analytical methods have been reported for the determination of aromatic amines. Among them, the most commonly employed techniques are gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC).4–6 Nowadays, as a useful analytical technique, fluorescence (FL) detection has been extensively employed with high sensitivity. Many organic dyes and special inorganic nanomaterials have been reported as fluorescent probes.5–12 For instance, Danielsona and coworkers have developed an N-alkyl acridine orange dye as a fluorescence probe for the determination of cardiolipin.13Calixarene-modified CdTe, synthesized by our group, allowed a highly sensitive determination of polycyclic aromatic hydrocarbons14 and pesticides.15 In comparison to traditional organic dyes, inorganic nanomaterials such as semiconductor quantum dots (QDs) have attracted great interest in the past decade due to their unique optical properties including a narrow, tunable, symmetric emission and photochemical stability.14,15 However, in general, QDs, as sensors, have to be surface modified before use.
Also, metal complexes have been widely used as optoelectronic devices because of their unique electronic and optical properties.16 For the past few years, luminescent metal 8-hydroxyquinoline (MQn) chelates have attracted considerable interest owing to their various applications in photoluminescence, electroluminescence and field emission.17 Due to the unique optoelectronic properties of nanomaterials, more attention is attracted to nanostructured MQn chelates. AlQ3 and many other MQn chelates have also been demonstrated to be useful emitter materials, and some of them have been widely used in organic light-emitting devices (OLEDs).18,19 For instance, AlQ3 nanostructures, such as nanowires, nanorods, and nanometre-scale crystalline films, exhibited field emission with a relatively low turn-on voltage.20 In comparison with their extensive applications in OLEDs, only a few metal 8-hydroxyquinoline (MQn) nanomaterials have been employed as fluorescent analytical assays . Recently, fluorescent sensors based on MQn nanomaterials have attracted increasing attention, due to their high quantum yields and their multifunctional groups provide affinity sites for the binding of biomolecules.21 For example, Zhu and coworkers have reported an optical strategy based on the ZnQ2 nanorods for protein sensing.22 Cobalt complexes, which are important in vitamin-B12 model chemistry, are also known as catalysts for the reduction of CO2.23 However, the potential applications of cobalt 8-hydroxyquinoline complex-based nanomaterials in environmental pollution analysis are still at an early stage. To our knowledge, the use of cobalt complex-based nanomaterials as selective probes for the fluorescent determination of aromatic amines is almost unexplored.
In this work, we synthesized water-soluble, stable and highly fluorescent cobalt(II)-bis(8-hydroxyquinoline) complex nanosheets by a very simple sonochemical method and investigated their potential application as a selective fluorescent probe for p-nitroaniline.
2. Experimental section
2.1 Materials
All chemicals used were of analytical grade or of the highest purity available. 8-Hydroxyquinoline was purchased from Beijing Corp. (Beijing, China). Cobalt chloride and AAMs (o-diaminobenzene, m-diaminobenzene, p-diaminobenzene, p-toluidine, o-nitroaniline, m-nitroaniline, p-nitroaniline, p-chloroaniline, aniline) were obtained from Beijing Chemical Corp. (Beijing, China). All AAM standards were of 98–99% purity and were dissolved in 50% (v/v) ethanol–water solution.2.2 Preparation of cobalt(II)-bis(8-hydroxyquinoline) nano-complexes
Cobalt(II)-bis(8-hydroxyquinoline) nano-complexes were synthesized using a sonochemical method combined with a microemulsion technique,22 although some slight modifications were made here. Briefly, a water-in-oil (W/O) microemulsion was prepared by mixing TX-100 (30 mL), cyclohexane (55 mL), n-hexanol (15 mL), 0.2 M cobalt chloride aqueous solution (2.5 mL), and ethanol (2.5 mL). 8-Hydroxyquinoline (1.5 mmol, 0.2175 g) was dissolved in an ethanol–water solution (50%, v/v, 5 mL) and then added into the microemulsion. The mixture solution was exposed to ultrasound irradiation under ambient air for 45 min. When the reaction was finished, a yellowish-green precipitate was obtained. After cooling to room temperature, the precipitate was separated by centrifuging at a rotation rate of 9000 rounds per min. It was purified further by repeated cycles of centrifugation and dispersing in ethanol and then dried in air at room temperature. The final products were redispersed in 50% (v/v) ethanol–water solution for further usage.2.3 Characterization
UV–vis absorption spectra were acquired on a TU-1901 UV–vis spectrometer (Beijing Purkinje General Instrument Co. Ltd). Fluorescence spectra were taken on a Fluoromax-P luminescence spectrometer (HORIBA JOBIN YVON INC.). IR spectra were measured with a NEXUS FT/IR spectrometer (Thermo Nicolet Co.). Transmission electron microscopy (TEM) was recorded by a JEOL-JEM 2010 electron microscope operating at 200 kV. X-Ray diffraction (XRD) was carried out with a Shimadzu labx XRD-6000. Elemental analysis (EA) was carried out using a Heraeus CHN–O Rapid instrument.3. Results and discussion
3.1 Spectra characterizations of cobalt(II)-bis(8-hydroxyquinoline) nano-complexes
The EA of the sample shows that the content (%) of C, H and N is 62.14, 3.51 and 8.10, respectively. The values are consistent with the calculated values (C: 62.26%; H: 3.48%; N: 8.07%) and the product can be confirmed to be cobalt(II)-bis(8-hydroxyquinoline) (CoQ2). The X-ray diffraction (XRD) pattern of the as-prepared product is shown in Fig. S1 (ESI† ). The diffraction peaks can be indexed to be CoQ2.22,24 The component of the nanostructures was further identified with an FT-IR spectrum. As indicated in Fig. 1, the water of hydration in the samples was readily identified by the presence of a broad infrared absorption band in the region from 3000 to 3400 cm−1. The intensity ratio of the 3431 cm−1 band to the 1126 cm−1 band is commonly used to study the water molecule number in metal–quinoline chelates. Similar to the congeneric compounds,22 the bands of 1600 cm−1 should correspond to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration in the quinoline group. The bands at 1489 and 1470 cm−1 are assigned to CC/CN stretching and CH bending vibration of the pyridyl and phenyl groups in CoQ2.25 The bands observed in the spectrum with peak positions at 1264, 1210, and 1033 cm−1 are attributed to a CH/CCN bending and C–N/C–O stretching vibrations. Peaks at 664, 603, and 559 cm−1 should correspond to Co–O stretching vibrations, and the band at 502 cm−1 is attributed to the Co–N stretching vibrations.26
C stretching vibration in the quinoline group. The bands at 1489 and 1470 cm−1 are assigned to CC/CN stretching and CH bending vibration of the pyridyl and phenyl groups in CoQ2.25 The bands observed in the spectrum with peak positions at 1264, 1210, and 1033 cm−1 are attributed to a CH/CCN bending and C–N/C–O stretching vibrations. Peaks at 664, 603, and 559 cm−1 should correspond to Co–O stretching vibrations, and the band at 502 cm−1 is attributed to the Co–N stretching vibrations.26
 | ||
| Fig. 1 IR spectrum of the CoQ2nanosheets. | ||
The morphologies and sizes of the samples were characterized by TEM, and the results are shown in Fig. 2. With the ultrasound proceeding, the formed CoQ2 nanoparticles underwent fusion to form small sheets composed of small particles. Further ultrasound irradiation led to the continuous growth of CoQ2nanosheets. A possible formation mechanism is proposed in Fig. 3. These nanosheets have regularly quadrate morphologies with a width 100–150 nm and a length 2–5 µm. Microcrystals with large dimensions could not be observed, which suggests that microcrystals of CoQ2 are destroyed under ultrasound irradiation for long reaction times, and these microcrystals are changed into nanosheets due to weak bonding interactions between 2D coordination polymers.27
 | ||
| Fig. 2 TEM images of the CoQ2nanosheets. Scale bars: 500 nm. | ||
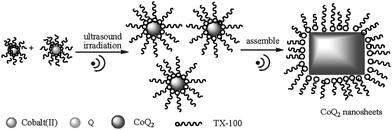 | ||
| Fig. 3 Schematic diagram showing the formation of CoQ2nanosheets. | ||
The photophysical properties of the CoQ2nanosheets have been investigated in an ethanol–water solution. Fig. S2 (ESI† ) shows the UV–vis absorption spectra of 8-hydroxyquinoline (Q), CoQ2 and CoQ2nanosheets. CoQ2 exhibits two resolved absorption bands at 310 nm and 356 nm, which is attributed to the transition of phenyl rings and the n–π transition, respectively.28 The absorption of CoQ2nanosheets is different from that regular CoQ2. There is an approximate 30 nm red-shift, which is due to the π–π stacking of the nanosheets (geometry change). Photoluminescence is a very important characteristic for the 8-hydroxyquinoline metal chelates. The difference between the CoQ2nanosheets and CoQ2 has also been monitored by fluorescence spectra. It can be observed that the fluorescence spectra of the CoQ2nanosheets exhibits a band centered at 450 nm. There is distinct red-shift in the peak position compared with that of the CoQ2, and the luminescence intensity increased with the formation of CoQ2nanosheets (Fig. S3, ESI† ). Ligand Q of CoQ2 exhibits free intramolecular rotation in the single complex molecule, but the rotation is inhibited in the aggregated nanosheet state. The inhibition of intramolecular rotation could be an effective mechanism for fluorescence enhancement, therefore, the CoQ2nanosheets show an aggregation-induced emission enhancement (AIEE) characteristic. The quantum yields (QY) of CoQ2 and CoQ2nanosheets in ethanol are measured in comparison with the value of rhodamine B (QY = 89%, EtOH) at room temperature, and are about 3.5% and 6.4%, respectively.
3.2 Effect of pH on the luminescence response
The effect of pH in the range from 1 to 13 was studied and is shown in Fig. 4. The results show an obvious decrease in the luminescence intensity of CoQ2nanosheets in a pH medium below 3 and beyond 8, meanwhile in the interval 4.0–6.0, it increases and is considered stable. Clearly, at low pH the ligand is dissolved and creates surface defects. At high pH the base can nucleophilically attack the surface, displacing the ligand creating surface defects. Finally, a pH of 6.0 is selected in the following biology assays . | ||
| Fig. 4 Effect of pH on luminescence response of the CoQ2nanosheets. | ||
3.3 Detection of aromatic amines
Fig. 5 shows the FL response of CoQ2nanosheets to 10−4 M aromatic amines including p-nitroaniline, o-diaminobenzene, m-diaminobenzene, p-diaminobenzene, p-toluidine, o-nitroaniline, m-nitroaniline, p-chloroaniline and aniline. It is shown that the p-nitroaniline can quench the luminescence of CoQ2nanosheets selectively, as indicated by the fluorescence photographs. From the fluorescence photographs, the fluorescence of the CoQ2nanosheets was quenched after p-nitroaniline was added. However, the sensitivity of the CoQ2nanosheets towards other aromatic amines including o-diaminobenzene, m-diaminobenzene, p-diaminobenzene, p-toluidine, o-nitroaniline, m-nitroaniline, p-chloroaniline and aniline is negligible. | ||
| Fig. 5 (a) Fluorescence spectra of CoQ2nanosheets with the relevant AAMs, (b) effect of 10−4 M relevant AAMs on the FL intensity of CoQ2nanosheets, (from 1 to 9: p-nitroaniline, o-diaminobenzene, m-diaminobenzene, p-diaminobenzene, p-toluidine, o-nitroaniline, m-nitroaniline, p-chloroaniline, aniline. Inset: fluorescence photographs of (I) CoQ2nanosheets and (II) CoQ2nanosheets and p-nitroaniline (under λ = 365 nm UV light irradiation). | ||
Fig. 6a show the effect of increasing concentrations of p-nitroaniline on the fluorescence of the nanosheets. It is found that p-nitroaniline quenches the fluorescence of CoQ2nanosheets in a concentration dependence that is best described by a Stern–Volmer type equation:
| Imax/I = 1 + KSV [S] |
 | ||
| Fig. 6 (a) Fluorescence spectra of the CoQ2 with increasing concentrations of p-nitroaniline. (b) Effect of p-nitroaniline concentration on the fluorescence of CoQ2nanosheets showing decreasing emission with increasing p-nitroaniline concentration. Inset: Stern–Volmer plot of p-nitroaniline concentration dependence on the FL intensity with a 0.997 correlation coefficient. | ||
To explore this method further it was used for more complex samples. Competition experiments were also performed for CoQ2nanosheets in a mixture of p-nitroaniline and background ions such as Li+, Na+, K+, Mg2+, Ca2+, Ba2+, Cl−, NO3−, Ac−, H2PO4−, HPO42−, C2O42− and SO32−. As shown in Fig. 7, other ions resulted in nearly no disturbance to the selective sensing of CoQ2nanosheets toward p-nitroaniline.
 | ||
| Fig. 7 Fluorescence spectra of CoQ2 in the presence of the p-nitroaniline and miscellaneous ions X including Li+, Na+, K+, Mg2+, Ca2+, Ba2+, Cl−, NO3−, AcO−, H2PO4−, HPO42−, C2O42− and SO32− (10 µM, excitation wavelength 320 nm). All spectral data were recorded at 10 min after p-nitroaniline addition. | ||
Fluorescence quenching can be static (e.g., complex formation) or dynamic (e.g., collision quenching).29 The complex formation between p-nitroaniline molecules and CoQ2 can be the main reason for the red-shift and the fluorescence quenching. From Fig. 6a, the wavelength of fluorescence red-shifts with increasing concentrations of p-nitroaniline, due to p-nitroaniline coordinating with CoQ2 to form a new product CoQ2Xn (X = p-nitroaniline) chelate as shown in Fig. 8. In addition, the electron-deficient nitroaromatics are strong quenchers to the electron-rich chromophores via an electron transfer mechanism for various photoluminescence materials, which results in the FL intensity of the new formation complex decreasing with increasing concentrations of p-nitroaniline.29,30 The p-nitroaniline isomer easily forms complexes with the Co ion due to a lack of steric hindrance (linear configuration of p-nitroaniline), which results in the quenching by p-nitroaniline being larger than for m-nitroaniline and o-nitroaniline.
 | ||
| Fig. 8 Schematic illustration of a possible formation mechanism of CoQ2Xn (X = p-nitroaniline). | ||
3.4 Analysis of water samples
Surface river water samples were collected from local rivers. The samples were filtered through 0.45 µm Supor filters and stored in precleaned glass bottles. As no AAMs in the collected water samples were detectable by the proposed method, a recovery study was carried out on the samples spiked with 2.5–0.1 µM AAMs to evaluate the developed method.To further demonstrate the practicality of the proposed method, the recovery test was studied by adding different amounts of p-nitroaniline into the water samples. The results were summarized in Table 1. The recoveries were from 95.6% to 105%. These results demonstrated that it was a promising approach and highly accurate, precise and reproducible. It can be used for the direct analysis of relevant samples.
| Spiked concentration/µM | Found concentration/µM | Recovery (%) | |
|---|---|---|---|
| p-Nitroaniline | 0.1 | 0.105 | 105.0 |
| p-Nitroaniline | 0.5 | 0.478 | 95.6 |
| p-Nitroaniline | 0.75 | 0.742 | 98.9 |
| p-Nitroaniline | 1 | 1.02 | 102.0 |
| p-Nitroaniline | 2.5 | 2.47 | 98.8 |
Conclusions
Cobalt(II)-bis(8-hydroxyquinoline) nanosheets have been successfully synthesized via a simple sonochemical method to develop a novel and highly sensitive luminescence probe for the optical recognition of AAMs. The synthesized CoQ2nanosheets allow the detection of p-nitroaniline as low as 9.38 ng L−1, thus affording a very sensitive detection system for AAMs analysis. Future studies will investigate obtaining more sensitive and selective metal complex-based nanosensors for the determination of AAMs in environmental samples.References
- P. F. Vogt and J. J. Geralis, Ullmann's Encyclopedia of Industrial Chemistry, ed. W. Gerhartz, Y. S. Yamamoto, F. T. Campbell, R. Pfefferkorn and J. F. Rounsaville, VCH, Weinheim, 5th edn, 1985, 235 Search PubMed.
- T. Zimmermann, W. J. Ensinger and T. S. Schmidt, Anal. Chem., 2004, 76, 1028–1031 CrossRef CAS.
- W. Leithe, The Analysis of Organic Pollutant s in Water and Wastewater. Ann Arbor Michigan, 1973 Search PubMed.
- T. Weiss and Angerer, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2002, 778, 179–192 CrossRef CAS.
- V. Singh, M. Gupta, A. Jain and K. K. Verma, J. Chromatogr., A, 2003, 1010, 243–253 CrossRef CAS.
- E. Marengo, M. C. Gennaro, V. Gianotti and E. Prenesti, J. Chromatogr., A, 1999, 863, 1–11 CrossRef CAS.
- E. Caudron, J. Y. Zhou, P. Chaminade, A. Baillet and P. Prognon, J. Chromatogr., A, 2005, 1072, 149–157 CrossRef CAS.
- H. A. Clark, M. Hoyer, M. A. Philbert and R. Kopelman, Anal. Chem., 1999, 71, 4831–4836 CrossRef CAS.
- E. J. Park, M. Brasuel, C. Behrend, M. A. Philbert and R. Kopelman, Anal. Chem., 2003, 75, 3784 CrossRef CAS.
- R. Bakalova, Z. Zhelev, H. Ohba and Y. Baba, J. Am. Chem. Soc., 2005, 127, 11328–11335 CrossRef CAS.
- C. Zhang and W. L. Johnson, J. Am. Chem. Soc., 2006, 128, 5324–5325 CrossRef CAS.
- C. Y. Chen, C. T. Cheng, C. W. Lai, P. W. Wu, K. C. Wu, P. T. Chou, Y. H. Choub and H. T. Chiu, Chem. Commun., 2006, 263–265 RSC.
- P. Kaewsuya, J. D. Miller, N. D. Danielson, J. Sanjeevi and P. F. James, Anal. Chim. Acta, 2008, 626, 111–118 CrossRef CAS.
- H. B. Li and F. G. Qu, J. Mater. Chem., 2007, 17, 3536–3544 RSC.
- H. B. Li and F. G. Qu, Chem. Mater., 2007, 19, 4148–4154 CrossRef CAS.
- H. B. Fu, D. B. Xiao, J. N. Yao and G. Q. Yang, Angew. Chem., Int. Ed., 2003, 42, 2883–2886 CrossRef CAS.
- J. J. Chiu, C. C. Kei, T. P. Perng and W. S. Wang, Adv. Mater., 2003, 15, 1361–1364 CrossRef CAS.
- L. S. Sapochak, F. E. Benincasa, R. S. Schofield, J. L. Baker, K. K. C. Riccio, D. Fogarty, H. Kohlmann, K. F. Ferris and P. E. Burrows, J. Am. Chem. Soc., 2002, 124, 6119–6125 CrossRef CAS.
- Y. S. Zhao, C. Di, W. Yang, G. Yu, Y. Liu and J. Yao, Adv. Funct. Mater., 2006, 16, 1985–1991 CrossRef CAS.
- J. S. Hu, H. X. Ji, A. M. Cao, Z. X. Huang, Y. Zhang, L. J. Wan, A. D. Xia, D. P. Yu, X. M. Meng and S. T. Lee, Chem. Commun., 2007, 3083–3085 RSC.
- G. Farruggia, S. Iotti, L. Prodi, M. Montalti, Zaccheroni, P. B. Savage, V. Trapani, P. Sale and F. I. Wolf, J. Am. Chem. Soc., 2006, 128, 344–350 CrossRef CAS.
- H. C. Pan, Fu. P. Liang, C. J. Mao, J. J. Zhu and H. Y. Chen, J. Phys. Chem. B, 2007, 111, 5767 CrossRef CAS.
- A. G. M. M. Hossain, T. Nagaoka and K. Ogura, Electrochim. Acta, 1997, 42, 2577–2585 CrossRef.
- S. C. Nayak, P. K. Das and K. K. Sahoo, J. Anal. Appl. Pyrolysis, 2003, 70, 699–709 CrossRef CAS.
- J. P. Phillips and J.F., Anal. Chim. Acta, 1957, 17, 231–233 CrossRef CAS.
- J. E. Tackett and D. T. Sawyer, Inorg. Chem., 1964, 3, 692–696 CrossRef CAS.
- Z. Q. Li, L. G. Qiu, W. Wang, T. Xu, Y. Wu and X. Jiang, Inorg. Chem. Commun., 2008, 11, 1375–1377 CrossRef CAS.
- L. Y. Wang, Q. W. Chen, G. H. Zhai, Z. Y. Wen and Z. X. Zhang, Dyes Pigm., 2007, 72, 357–362 CrossRef CAS.
- S. Zahn and T. M. Swager, Angew. Chem., Int. Ed., 2002, 41, 4225–4230 CrossRef.
- S. Yamaguchi and T. M. Swager, J. Am. Chem. Soc., 2001, 123, 12087–12088 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern of the CoQ2nanosheets, the UV–vis absorption spectra and fluorescence spectra of ligand Q, CoQ2 and CoQ2 nanosheets. See DOI: 10.1039/b9nr00019d |
| This journal is © The Royal Society of Chemistry 2009 |
