Synthesis of highly-ordered mesoporous carbon/silica nanocomposites and derivative hierarchically mesoporous carbon from a phenyl-bridged organosiloxane
Zhiwang
Wu
*a,
Jiebin
Pang
a and
Yunfeng
Lu
*ab
aDept. of Chemical & Biomolecular Engineering, Tulane University, New Orleans, LA 70118, USA. E-mail: zhiwang.wu@gmail.com; Fax: +1 504 8656744; Tel: +1 504 7824894
bDept. of Chemical & Biomolecular Engineering, University of California, Los Angeles, CA 90095, USA. E-mail: luucla@ucla.edu; Fax: +1 310 2064107; Tel: +1 310 7947238
First published on 13th August 2009
Abstract
Mesoporous carbon/silica nanocomposites and derivative hierarchically mesoporous carbon have been prepared using 1,4-bis(triethoxysilyl)benzene (BTEB) as a precursor for a carbon/silica network and Pluronic P123 (HO(CH2CH2O)20(CH2CH(CH3)O)70(CH2CH2O)20H) as a template for highly-ordered hexagonal pores. Co-assembly of BTEB and P123 and subsequent carbonization results in a mesoporous carbon/silica nanocomposite with hexagonally oriented pores. Removal of the silica component in the carbon/silica network creates a second porosity in the network and results in hierarchically mesoporous carbon. The mesostructure of these materials was characterized by transmission electron microscopy (TEM), field-emission scanning electron microscopy (FE-SEM), powder X-ray diffraction (PXRD), and N2 sorption.
1. Introduction
Mesoporous silica has attracted a great deal of attention since the 1990s, because of its unique properties.1–5 In this area, self-assembly has been demonstrated as one of the most promising and versatile techniques for the precise control of mesostructure. Soon after its inception, the multi-component self-assembly synthesis approach was extended to create a large variety of mesostructures and nanocomposites. Functionalization of mesoporous silica, a process that endows the internal pore surface, external particle surface, or pore wall with functionality, is necessary to turn inert silica into a functional material.6–8 The functionalization methods reported include post-grafting,9,10 direct-synthesis,11–13 and the use of functional surfactants.14 Among them, direct-synthesis provides the silica with functionality by co-assembly of surfactants with organosiloxanes which contain unhydrolyzable functional groups such as thiol, amine, epoxide, imidazole or allyl.10–13,15–21 However, current research in this area is limited in the synthesis of functionalized silica with organic or metallic moieties,8 and few nanoporous carbon/silica composites have been reported.22Highly nanoporous carbon materials have recently received much attention due to their potential applications in catalysis, hydrogen storage, column packing, and as supercapacitors.23 One of the most common techniques for synthesizing highly-ordered nanoporous carbon is a two-step templating approach by infiltration of a carbon precursor (e.g.sucrose) into preformed highly-ordered frameworks (e.g. mesoporous silica), carbonization of the precursor, and removal of the silica template.24 Although highly-ordered mesoporous structures with high surface areas (e.g. 1192 m2 g−1) can be obtained, there are several disadvantages to this two-step approach, which is tedious and time consuming. As an alternative, several easier one-step techniques have also been reported. For example, highly mesoporous carbon particles with a maximum surface area of 1219 m2 g−1 and irregular pore sizes of 20–30 nm were obtained by mixingsucrose and colloidal silica as precursors in an aerosol process, followed by carbonization and silica removal.23 Monolithic carbon aerogels have also been prepared by carbonization of polymeric aerogels using a supercritical CO2 drying technique.25
Herein, we report a one-step synthesis for highly-ordered mesoporous carbon/silica composites (denoted as MCS) with pore walls that are composed of periodically and molecularly integrated silica/carbon units. As illustrated in Fig. 1, MCS is synthesized by the co-assembly of the triblock copolymer Pluronic P123 (HO(CH2CH2O)20(CH2CH(CH3)O)70(CH2CH2O)20H) with 1,4-bis(triethoxysilyl)benzene (BTEB)26 followed by carbonization of the resulting phenylene/silica/surfactant composite (denoted as PSS), that converts the phenylene moieties into carbon and decomposes the surfactant. Further removal of silica from the MCS by NaOH solution results in hierarchically mesoporous carbon (denoted as HMC) with secondary pores formed during decomposition of silica walls. Compared to most preparation methods for mesoporous carbon, which make mesoporous carbon by infiltrating a carbon precursor into mesoporous silica pores,27carbonization of silica/carbon precursors, and final removal of silica, this is a simpler method. Mesoporous carbon can be used for hydrogen storage, catalysis, adsorption, energy storage and electrochemistry, etc.27
 | ||
| Fig. 1 Schematic illustration of a synthesis route from PSS to MCS and HMC. | ||
2. Experimental
The synthesis of PSS was based on the literature,26 with modification. In a typical synthesis, 0.99 g P123 was dissolved in 0.2 mL 37% HCl and 36.1 g DI H2O with heating and stirring until the solution was clear. Then 1.10 g of BTEB was added to the P123 solution with stirring at 0 °C bath for 1 h. Then the bath temperature was increased to 40 °C and the solution was stirred for another 20 h. After that, the solution was removed to an autoclave and treated at 100 °C for 24 h. As-synthesized white precipitated PSS powders were cooled to room temperature, recovered by filtration, washed with DI H2O, and dried at 70 °C overnight. The PSS powders were subsequently carbonized at 900 °C for 4 h in zero-grade nitrogen gas with a flow rate of 10 cm3 min−1 resulting in MCS. To better understand the mesostructure formation, surfactant was also removed from PSS by ethanol extraction for 3 days to get MPS, for comparison. MCS was washed with 5 M NaOH with vigorous stirring at 80 °C for 2 days to remove silica to get HMC. NaOH or HF solutions were used to remove the silica components from composites as reported in our previous papers,23,24,27,28 and energy dispersive X-ray spectroscopy (EDX, not shown here) confirmed that less than 2.5% silica remained after the treatments. All chemicals used in this study were AR-grade and purchased from Sigma-Aldrich (St. Louis, MO) without further purification. Water was purified by distillation and deionization (18 MΩ).Powder X-ray diffraction (PXRD) patterns were recorded on a Siemens D500 diffractometer operated at 40 kV, 30 mA (Cu Kα radiation, λ = 0.15406 nm). N2 adsorption–desorption isotherms were measured at 77 K by a micromeritics ASAP 2010 analyzer. Transmission electron microscopy (TEM) images were taken using a JEOL 2010 microscope operated at 200 kV. Field-emission scanning electron microscopy (FE-SEM) images were obtained using a Hitachi S-4800 FE-SEM operating at 10 kV and the specimen was mounted on a SEM holder using conductive carbon double-sided sticky tape. Thermogravimetric analysis (TGA) was performed on a Thermal Analysis TGA 2950 instrument with a heating rate of 10 °C min−1 and an oxygen flow rate of 80 cm3 min−1.
3. Results and discussion
Fig. 2 shows the PXRD patterns of PSS before the removal of surfactant (PSS, curve a), after the removal of surfactant by ethanol extraction (MPS, curve b), after the carbonization in nitrogen at 900 °C (MCS, curve c), and after the removal of silica by NaOH solution from MCS (HMC, curve d). PSS (curve a) and MPS (curve b) exhibit typical low-angle diffraction peaks that correspond to a hexagonal mesostructure with a d100 spacing of 9.3 nm and the peak of the latter is much stronger due to removal of the P123 template. Curve b is magnified by 5× to reveal d110 and d200 spacings that correspond to the typical P123-directed hexagonal pore structure. Carbonization of PSS powders not only removed P123, but also converted the phenylene/silica network into a periodically arranged carbon/silica composite with an hexagonal pore structure (i.e. MCS). Due to structure contraction or increased skeletal density during the carbonization at 900 °C, the low-angle diffraction peak of PPS (curve c) was retained in MCS but with a decreased d100 spacing of 6.8 nm. The d100 peak intensity of HMC (curve d) decreases dramatically compared to that of MCS (curve c) due to some damaged highly-ordered pore structure caused by the dissolution of silica. | ||
| Fig. 2 Low-angle PXRD patterns of PSS (curve a), MPS (curve b), MCS (curve c), and HMC (curve d). | ||
Fig. 3 shows typical TEM images of MCS taken along (Fig. 3a) and perpendicular to (Fig. 3b) the pore channel direction. The TEM images clearly reveal that MCS has a highly-ordered hexagonal mesostructure, proving that the ordered P123-directed mesostructure is well retained during the carbonization process and removal of the P123 template. The center-to-center distance of adjacent pores in Fig. 3 is approximately 6.8 nm, which is also highly consistent with the PXRD result (see Fig. 2, curve c).
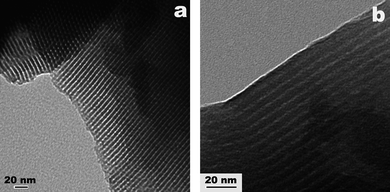 | ||
| Fig. 3 Typical TEM images of MCS obtained along (a) and perpendicular to (b) the pore channel direction. | ||
Typical TEM images of HMC were taken along (Figs. 4a and c) and perpendicular to (Fig. 4b) the pore channel direction. Figs. 4a and b confirm the existence of retained P123-directed hexagonal pore structure after the removal of silica from MCS, but the ordered pore structure is not present throughout the entire area of the sample, which results in a much weaker d100 peak (see Fig. 2, curve d) than that of MCS (see Fig. 2, curve c). The center-to-center distance of adjacent pores in Figs. 4a and b is also 6.8 nm, which is highly consistent with the corresponding d100 spacing (see Fig. 2, curve d). Besides the relatively ordered P123-directed hexagonal pore structure, Fig. 4c shows many worm-like pores in HMC with an estimated diameter of 1.8 nm, and some of the pores are connected to form bigger pores. All of these worm-like pores result from the dissolution of silica in NaOH solution, which also damages some of the highly-ordered hexagonal structure of MCS.
 | ||
| Fig. 4 Typical TEM images of HMC obtained along (a and c) and perpendicular to (b) the channel direction. | ||
Fig. 5 shows typical FE-SEM images of PSS (Fig. 5a), MCS (Fig. 5b), and HMC (Fig. 5c). Both MCS and HMC have denser skeletons than PSS due to structure contraction during carbonization . The structure contraction was discussed in the PXRD patterns (Fig. 2) which showed that the d100 spacing of the hexagonal pore structure was reduced by 2.5 nm (from 9.3 nm to 6.8 nm) after carbonization at 900 °C. Nonconductive PSS is the cause of the blurring in Fig. 1a; materials after carbonization (i.e. MCS, HMC) show much better conductivity, resulting in much sharper SEM images (Figs. 1b and c).
 | ||
| Fig. 5 Typical FE-SEM images of: a) PSS, b) MCS, and c) HMC. | ||
Fig. 6 shows N2 adsorption–desorption isotherms and Barrett–Joyner–Halenda (BJH) pore-size distributions (insets) of MPS (curve a), MCS (curve b), and HMC (curve c), and Table 1 summarizes the detailed physicochemical properties of these materials. All three samples show typical type-IV isotherms (Fig. 6) and exhibit narrow pore-size distributions (inset, Fig. 6). MPS exhibits a uniform pore size centered at 6.3 nm (inset a) while MCS and HMC have much smaller pore sizes centered at ca. 3.8 nm. The pore-size difference is due to structure contraction or increased skeletal density during carbonization , which was discussed in both Fig. 2 and Fig. 5. The hysteresis loop of MPS shows a significant N2 uptake between relative pressures of 0.45 and 0.76 while both MCS and HMC have much smaller uptakes between relative pressures of 0.4 and 0.54. By comparison and calculation of the surface areas and pore volumes of MPS and MSC (Table 1) during carbonization , micropores (less than 2 nm) existing in the framework of MPS (0.08 cm3 g−1) all disappeared due to shrinking of the framework, while all the mesopores remained with a significantly shrunken pore size (6.3 nm to 3.8 nm), decreased surface area (687 m2 g−1 to 338 m2 g−1) and pore volume (0.84 cm3 g−1 to 0.34 cm3 g−1). Dissolution of silica in MSC results in much higher surface area (338 m2 g−1 to 1397 m2 g−1) and pore volume (0.34 cm3 g−1 to 0.83 cm3 g−1). Not only micropores (less than 2 nm), but also mesopores, increased dramatically, which is reflected in their surface areas and pore volumes (Table 1). The reason for this was discussed in Fig. 4. Many micropores (i.e. 1.8 nm) were observed in HMC and some of them were connected and formed bigger pores (i.e. mesopores). N2 uptake between relative pressures of 0.4 and 0.54, which correspond to the P123-directed highly-ordered mesopores (i.e. 3.8 nm), reduced significantly after silica dissolution of MCS (Figs. 6b and c). The result is highly consistent with the PXRD results discussed in Fig .2, in which the peak intensity of d100 spacing in MCS decreased dramatically due to some collapsed highly-ordered pores.
| Sample code | Total surface area/m2 g−1 | Microporous area/m2 g−1 | Mesoporous area/m2 g−1 | Total pore volume/cm3 g−1 | Microporous volume/cm3 g−1 | Mesoporous volume/cm3 g−1 |
|---|---|---|---|---|---|---|
| MPS | 879 | 192 | 687 | 0.92 | 0.08 | 0.84 |
| MCS | 338 | 0 | 338 | 0.34 | 0 | 0.34 |
| HMC | 1397 | 586 | 811 | 0.83 | 0.27 | 0.56 |
 | ||
| Fig. 6 N2 adsorption–desorption isotherms and BJH pore size distributions (inset) of MPS (curve a), MCS (curve b), and HMC (curve c). Filled symbols correspond to adsorption and empty symbols correspond to desorption. *The pore size distribution of c was derived from the desorption curve of the isotherm. | ||
The carbon content in MCS was measured by TGA in O2, as shown in Fig. 7 (curve a). There is a weight loss of 34.0% from 450 to 650 °C (Fig. 7a), which is due to the decomposition or oxidation of carbon in O2. The observed weight loss of 34.0% has a negligible difference with the calculated value by assuming that all the phenylene groups are transformed into carbon and no surfactant is transformed into carbon during carbonization .6 HMC shows a weight loss of 97.5% from 100 °C to 380 °C (Fig. 7b), indicating that the silica was almost completely removed by NaOH solution as described in the Experimental section. The much higher decomposition temperature of MCS than that of HMC (difference >200 °C) shows that carbon stabilized by silica in MCS is much more temperature-resistant than in HMC and may show much lower weight loss than commercial carbon (e.g. Vulcan XC-72) in applications such as catalyst supports for fuel cells.
 | ||
| Fig. 7 TGA curves in an O2 atmosphere of the mesoporous carbon/silica composite (curve a) and the mesoporous carbon after removal of silica from the carbon/silica composite (curve b). | ||
4. Conclusions
In conclusion, we have developed and demonstrated an efficient approach for the synthesis of MCS and HMC viaself-assembly between a phenyl-bridged organosilane (BTEB) and P123surfactant, followed by carbonization and silica dissolution. The surfactant-directed highly-ordered mesoporous structure was retained after the carbonization of phenylene groups in the as-synthesized PSS. A unique pore-wall structure with molecularly repeating carbon/silica units was also obtained after carbonization . The secondary porous structure of HMC resulted from dissolution of silica in MCS. This simple method provides a new family of mesoporous carbon and carbon/silica composites for many potential applications, such as catalyst supports, adsorbents and hydrogen storage.Acknowledgements
The authors gratefully acknowledge the financial support for this work by NASA (Grant No. NAG-1-02070 and NCC-3-946), the Office of Naval Research, the Louisiana Board of Regents (Grant No. LEQSF(2001–04)-RD-B-09), and the National Science Foundation (Grant No. NSF-DMR-0124765, and CAREER award).References
- C. T. Kresge, M. E. Leonowicz, W. J. J. Roth, C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS.
- C. J. Brinker, Y. Lu, A. Sellinger and H. Fan, Adv. Mater., 1999, 11, 579 CrossRef CAS.
- Y. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. Gong, Y. Guo, H. Soyez, B. Dunn, M. H. Huang and J. I. Zink, Nature, 1997, 389, 364 CrossRef CAS.
- Y. Lu, H. Fan, N. Doke, D. A. Loy, R. A. Assink, D. A. LaVan and C. J. Brinker, J. Am. Chem. Soc., 2000, 122, 5258 CrossRef CAS.
- J. Pang, V. T. John, D. A. Loy, Z. Yang and Y. Lu, Adv. Mater., 2005, 17, 704 CrossRef CAS.
- W. Wang, W. Zhou and A. Sayari, Chem. Mater., 2003, 15, 4886 CrossRef CAS.
- A. M. Stein, J. S. Brian and C. Rick, Adv. Mater., 2000, 12, 1403 CrossRef CAS.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS.
- R. J. P. Corriu, A. R. C. Mehdi and C. Thieuleux, Chem. Commun., 2002, 1382 RSC.
- C. E. Fowler, S. L. Burkett and S. Mann, Chem. Commun., 1997, 1769 RSC.
- S. R. Hall, C. E. Fowler, S. Mann and B. Lebeau, Chem. Commun., 1999, 201 RSC.
- M. H. Lim, C. F. Blanford and A. Stein, J. Am. Chem. Soc., 1997, 119, 4090 CrossRef CAS.
- Q. Zhang, K. Ariga, A. Okabe and T. Aida, J. Am. Chem. Soc., 2004, 126, 988 CrossRef CAS.
- T. Asefa, M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 402, 867 CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611 CrossRef CAS.
- S. Hamoudi and S. Kaliaguine, Chem. Commun., 2002, 2118 RSC.
- W. Wang, W. Zhou and A. Sayari, Chem. Mater., 2003, 15, 4886 CrossRef CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, Nature, 2002, 416, 304 CrossRef CAS.
- Q. Yang, M. P. Kapoor and S. Inagaki, J. Am. Chem. Soc., 2002, 124, 9694 CrossRef CAS.
- M. P. Kapoor, Q. Yang and S. Inagaki, Chem. Mater., 2004, 16, 1209 CrossRef CAS.
- Q. Hu, R. Kou, J. Pang, T. L. Ward, M. Cai, Z. Yang and Y. Lu, Chem. Commun., 2007, 601 RSC.
- J. E. Hampsey, Q. Hu, L. Rice, J. Pang, Z. Wu and Y. Lu, Chem. Commun., 2005, 3606 RSC.
- J. E. Hampsey, Q. Hu, Z. Wu, L. Rice, J. Pang and Y. Lu, Carbon, 2005, 43, 2977 CrossRef CAS.
- J. Ozaki, N. Endo, W. Ohizumi, K. Igarashi, M. Nakahara, A. Oya, S. Yoshida and T. Iizuka, Carbon, 1997, 35, 1031 CrossRef CAS.
- Y. Goto and S. Inagaki, Chem. Commun., 2002, 2410 RSC.
- L. Xing, J. Huang, S. Wu, H. Wang, K. Song, H. Xu, Z. Wang and Q. Kan, Carbon, 2007, 45, 220 CrossRef CAS.
- J. Pang, X. Li, D. Wang, Z. Wu, V. John, Z. Yang and Y. Lu, Adv. Mater., 2004, 16, 884 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
