How does non-covalent Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) O interaction stabilize selenoxides at naphthalene 1,8-positions: structural and theoretical investigations†
O interaction stabilize selenoxides at naphthalene 1,8-positions: structural and theoretical investigations†
Satoko Hayashia, Waro Nakanishi*a, Atsushi Furutaa, Jozef Drabowiczb, Takahiro Sasamoric and Norihiro Tokitohc
aDepartment of Material Science and Chemistry, Faculty of Systems Engineering, Wakayama University, 930 Sakaedani, Wakayama 640-8510, Japan. E-mail: nakanisi@sys.wakayama-u.ac.jp; Fax: +81 73 457 8253; Tel: +81 73 457 8253
bCenter of Molecular and Macromolecular Studies, Polish Academy of Science, Sienkiewicza, 112, 90-363, Lodz, Poland
cInstitute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan
First published on 17th November 2008
Abstract
Bis-selenides![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), such as 8-[MeSe(X)]-1-[MeSe(Z)]C10H6 (1
(LL), such as 8-[MeSe(X)]-1-[MeSe(Z)]C10H6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)), 8-[EtSe(X)]-1-[EtSe(Z)]C10H6 (2
(LL)), 8-[EtSe(X)]-1-[EtSe(Z)]C10H6 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)), 8-[p-YC6H4Se(X)]-1-[MeSe(Z)]C10H6 (3
(LL)), 8-[p-YC6H4Se(X)]-1-[MeSe(Z)]C10H6 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)) and 8-[p-YC6H4Se(X)]-1-[p-YC6H4Se(Z)]C10H6 (4
(LL)) and 8-[p-YC6H4Se(X)]-1-[p-YC6H4Se(Z)]C10H6 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)) were oxidized with ozone at 0 °C, where (X, Z) = (lone pair, lone pair) for LL. Bis-selenoxides, 1
(LL)) were oxidized with ozone at 0 °C, where (X, Z) = (lone pair, lone pair) for LL. Bis-selenoxides, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 3
(OO), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) and 4
(OO) and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) where (X, Z) = (oxygen, oxygen), were obtained in the oxidation of 1
(OO) where (X, Z) = (oxygen, oxygen), were obtained in the oxidation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), 3
(LL), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) and 4
(LL) and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), respectively, via corresponding selenide-selenoxides, 1
(LL), respectively, via corresponding selenide-selenoxides, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 3
(LO), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 4
(LO) and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), respectively. A facile Se–C bond cleavage was observed in 2
(LO), respectively. A facile Se–C bond cleavage was observed in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL). The structures of 1
(LL). The structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) were determined by the X-ray analysis. Three Se⋯Se
(OO) were determined by the X-ray analysis. Three Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O atoms in 1
O atoms in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and four O
(LO) and four O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O atoms in 1
O atoms in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) align linearly. While the non-covalent Se⋯Se
(OO) align linearly. While the non-covalent Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O 3c–4e interaction operates to stabilize 1
O 3c–4e interaction operates to stabilize 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), the non-covalent O
(LO), the non-covalent O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O 4c–4e interaction would not stabilize 1
O 4c–4e interaction would not stabilize 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). The 3c–4e interaction must play an important role to control the stereochemistry of selenoxides. The 8-G-1-[MeSe(OH)2]C10H6 (n
(OO). The 3c–4e interaction must play an important role to control the stereochemistry of selenoxides. The 8-G-1-[MeSe(OH)2]C10H6 (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)) are the key intermediates in the racemization of 8-G-1-[MeSe(O)]C10H6 (n
(OH·OH)) are the key intermediates in the racemization of 8-G-1-[MeSe(O)]C10H6 (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O)) in solutions, where G = SeMe (1), H (5), F (6), Cl (7) and Br (8). Energies of n
(O)) in solutions, where G = SeMe (1), H (5), F (6), Cl (7) and Br (8). Energies of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH), relative to n
(OH·OH), relative to n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O), are evaluated based on the theoretical calculations. G of SeMe is demonstrated to operate most effectively to protect from racemization of selenoxides among n = 1 and 5–8, since the relative energies for G of cis- and trans-SeMe are largest.
(O), are evaluated based on the theoretical calculations. G of SeMe is demonstrated to operate most effectively to protect from racemization of selenoxides among n = 1 and 5–8, since the relative energies for G of cis- and trans-SeMe are largest.
Introduction
Selonoxides1–4 [RSe(O)R′] afford optically active enantiomers, as well as sulfoxides,5,6 if R and R′ are not the same, since Se in each selenoxide is three-coordinated containing a lone pair. However, it is usually difficult to utilize optical active selenoxides to introduce the optical activity in a target molecule,1,2,4,7 since the racemization of optical active selenoxides is usually very fast. Nevertheless, some efforts have been made to stabilize the stereochemistry of selenoxides, by taking advantage of non-covalent coordination by the neighboring groups (G) of the G⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O type.2,4,7
O type.2,4,7Naphthalene 1,8-positions supply a good system to investigate such interactions, since the non-bonded distances between heteroatoms at the positions are close to the sum of the van der Waals radii minus 1 Å.8,9 Various types of non-covalent interactions are detected in naphthalene 1,8-positions.8–11 The σ-type three center-four electron interactions (σ(3c–4e)),12–14σ(2c–4e),12π(2c–4e),12 distorted π(2c–4e),12 and Z4 4c–6e13 are typical examples. Such non-covalent interactions are demonstrated to control the fine structures of molecules.15 Recently, we investigated fine structures of 8-G-1-(arylseleninyl)naphthalene with G = H, F, Cl and Br, together with the factors to control the structures, as the first step to control the stereochemistry of selenoxides.16 The factors are called G, O and Y dependences, which originate from the np(G)⋯σ*(Se–O), np(O)⋯π(Nap) and np(O)⋯π(Ar) interactions, respectively.16
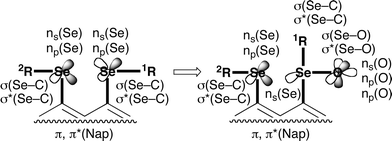
We paid much attention to G = MeSe and ArSe in 8-G-1-(arylseleninyl)naphthalenes, since many conformers are plausible around the two Se–CNap bonds, relative to the case of G = H and halogens. Scheme 1 shows the orbitals taking part in the non-covalent Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction. A bis-selenide contains double ns(Se), np(Se), σ(Se–C) and σ*(Se–C) orbitals. However, ns(O), np(O), np′(O), σ(Se–O) and σ*(Se–O) appear newly with the quit of an np(Se), when a selenide-selenoxide is formed from the bis-selenide.
O interaction. A bis-selenide contains double ns(Se), np(Se), σ(Se–C) and σ*(Se–C) orbitals. However, ns(O), np(O), np′(O), σ(Se–O) and σ*(Se–O) appear newly with the quit of an np(Se), when a selenide-selenoxide is formed from the bis-selenide.
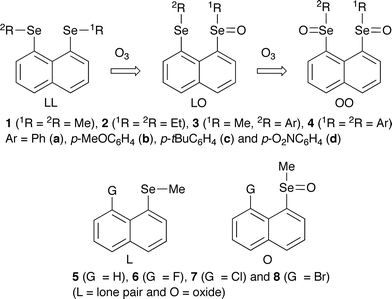
The oxidation and formation of 8-[2RSe(X)]-1-[1RSe(Z)]C10H6 (1 (1R = 2R = Me), 2 (1R = 2R = Et), 3 (1R = Me, 2R = p-YC6H4: Y = H (a), MeO (b) and NO2 (d)) and 4 (1R = 2R = p-YC6H4: Y = H (a) and tBu (c)) are investigated for LL where (X, Z) = (lone pair, lone pair), LO (lone pair, oxygen) and OO (oxygen, oxygen) (Chart 1). The reactions are easily controlled and each process is followed by the spectroscopic method. Non-bonded O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interactions are also the subject of interest.
O interactions are also the subject of interest.
The structures around the naphthyl group (Nap) in 8-G-1-RSeC10H6 are well explained by three types, type A (A), B and C.8c,d,f–h,17,18 The combined notation are used to specify the structures of 1–4 with G = RSe, where the notation, such as AA, BA or CA, shows the conformers around the two CNap–Se bonds. Scheme 2 draws the notations employed in this work, exemplified by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO).
(LO).
The structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are determined by X-ray crystallographic analysis. Quantum chemical (QC) calculations are performed on 1
(OO) are determined by X-ray crystallographic analysis. Quantum chemical (QC) calculations are performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), to elucidate the role of the Se⋯Se
(OO), to elucidate the role of the Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction in 1
O interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and the O
(LO) and the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction in 1
O interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) as the factor to control the fine structures. Orbitals of two Se atoms in 1
(OO) as the factor to control the fine structures. Orbitals of two Se atoms in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) must overlap directly with each other, which would stabilize the fine structures. QC calculations are also performed on 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O (O), OH+ (OH+) and O2H2 (OH·OH)], where OH·OH must be the key intermediate in the racemization of 1 and 5–8, in the presence of a trace of water. The relative energy [ΔE = E(n
(OO) must overlap directly with each other, which would stabilize the fine structures. QC calculations are also performed on 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O (O), OH+ (OH+) and O2H2 (OH·OH)], where OH·OH must be the key intermediate in the racemization of 1 and 5–8, in the presence of a trace of water. The relative energy [ΔE = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)) − (E(n
(OH·OH)) − (E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O)) + E(H2O)) (n = 1 and 5–8)] is evaluated: that for G = MeSe is largest among them. The larger value must correspond to a selenoxide with the stronger resistance for racemization, although n
(O)) + E(H2O)) (n = 1 and 5–8)] is evaluated: that for G = MeSe is largest among them. The larger value must correspond to a selenoxide with the stronger resistance for racemization, although n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) is not the transition state. The G⋯Se
(OH·OH) is not the transition state. The G⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interactions containing the Se⋯Se
O interactions containing the Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O
O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interactions are also analyzed with the natural orbital (NBO)19,20 and atoms-in-molecules (AIM)21,22 analyses.
O interactions are also analyzed with the natural orbital (NBO)19,20 and atoms-in-molecules (AIM)21,22 analyses.
Oxidation of 1,8-bis(selanyl)naphthalenes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) with ozone is well controlled and monitored, which gives 1,8-bis(seleninyl)naphthalenes (OO) via 8-selanyl-1-seleninylnaphthalenes (LO). Factors to control the fine structures of 1
(LL) with ozone is well controlled and monitored, which gives 1,8-bis(seleninyl)naphthalenes (OO) via 8-selanyl-1-seleninylnaphthalenes (LO). Factors to control the fine structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are clarified based on QC calculations, after determination of the structures. The Se⋯Se
(OO) are clarified based on QC calculations, after determination of the structures. The Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction is demonstrated to control the fine structure of 1
O interaction is demonstrated to control the fine structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), whereas the role of the O
(LO), whereas the role of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interaction in 1
O interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) is critically discussed. The role of G in 1 and 5–8 in the racemization process is also evaluated.
(OO) is critically discussed. The role of G in 1 and 5–8 in the racemization process is also evaluated.
 | ||
Scheme 1 Orbitals taking part in the non-bonded Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O interactions in naphthalene 1,8-positions. O interactions in naphthalene 1,8-positions. | ||
![Structures around naphthyl group in 8-G-1-[RSe(X)]C10H6, exemplified by 1 (LO).](/image/article/2009/NJ/b809763a/b809763a-s2.gif) | ||
Scheme 2 Structures around naphthyl group in 8-G-1-[RSe(X)]C10H6, exemplified by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO). (LO). | ||
 | ||
| Chart 1 Bis(selanyl)naphthalenes, 1–4, together with 5–8. | ||
Results and discussion
Survey of oxidation
Bis-selenides (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL): n = 1–4) were oxidized with ozone in the methylene dichloride solution of each bis-selenide at 0 °C. The bis-selenides (n
(LL): n = 1–4) were oxidized with ozone in the methylene dichloride solution of each bis-selenide at 0 °C. The bis-selenides (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)) gave corresponding bis-selenoxides (n
(LL)) gave corresponding bis-selenoxides (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O)) via corresponding selenide-selenoxides (n
(O)) via corresponding selenide-selenoxides (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)), except for 2
(LO)), except for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL). While 1
(LL). While 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) gave 1
(LL) gave 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), followed by the quantitative formation of 1
(LO), followed by the quantitative formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), a facile Se–C bond cleavage occurred on the oxidation of 2
(OO), a facile Se–C bond cleavage occurred on the oxidation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), resulting in the formation of naphtho-1,8-[c,d]-1,2-diselenole (9).23β-Elimination of the selenoxide may be responsible for the facile Se–C bond cleavage. In the case of 3
(LL), resulting in the formation of naphtho-1,8-[c,d]-1,2-diselenole (9).23β-Elimination of the selenoxide may be responsible for the facile Se–C bond cleavage. In the case of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), the methylselanyl Se atoms were attacked exclusively. 3
(LL), the methylselanyl Se atoms were attacked exclusively. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) were consumed to produce the corresponding 3
(LO) were consumed to produce the corresponding 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) with more ozone. 4
(OO) with more ozone. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) were also produced from the corresponding 4
(LO) were also produced from the corresponding 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) with ozone, followed by the formation of the corresponding 4
(LL) with ozone, followed by the formation of the corresponding 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), respectively. The results are summarized in Chart 1. The reactions are well followed by NMR.
(OO), respectively. The results are summarized in Chart 1. The reactions are well followed by NMR.Structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) (OO)
(OO)
Single crystals of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) were obtained via slow evaporation of methylene dichloride-hexane solutions and one of suitable crystals was subjected to X-ray crystallographic analysis for each compound.24 Only one type of structure corresponds to each of 1
(OO) were obtained via slow evaporation of methylene dichloride-hexane solutions and one of suitable crystals was subjected to X-ray crystallographic analysis for each compound.24 Only one type of structure corresponds to each of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) in the crystals. Table 1 shows the crystallographic data of 1
(OO) in the crystals. Table 1 shows the crystallographic data of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). Fig. 1 shows the structures of 1
(OO). Fig. 1 shows the structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO).25 The packing structure of 1
(OO).25 The packing structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) is shown in Fig. S1 of the Electronic Supplementary Information (ESI†). Selected interatomic distances, angles and torsional angles of the compounds 1
(OO) is shown in Fig. S1 of the Electronic Supplementary Information (ESI†). Selected interatomic distances, angles and torsional angles of the compounds 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are collected in Table 2, together with those of 1
(OO) are collected in Table 2, together with those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), which contains two types, 1
(LL), which contains two types, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)A and 1
(LL)A and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)B.26
(LL)B.26The structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are all AA for two methyl groups (Fig. 1 and Table 2).15 The planarity of the naphthyl (Nap) planes is very good. All Se–O bonds are placed in the naphthyl plane. The superior tendency of the Se–O bonds to stay on the naphthyl plane (O dependence)16 must be the driving force for the structures of 1
(OO) are all AA for two methyl groups (Fig. 1 and Table 2).15 The planarity of the naphthyl (Nap) planes is very good. All Se–O bonds are placed in the naphthyl plane. The superior tendency of the Se–O bonds to stay on the naphthyl plane (O dependence)16 must be the driving force for the structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). Three Se⋯Se–O atoms align linearly (∠SeSeO = 173.31(15)°) and the Se–O bond is almost perpendicular to another CNapSeCMe plane in 1
(OO). Three Se⋯Se–O atoms align linearly (∠SeSeO = 173.31(15)°) and the Se–O bond is almost perpendicular to another CNapSeCMe plane in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO). The non-covalent np(Se)⋯σ*(Se–O) 3c–4e interaction operates effectively to keep the Se–O bond on the naphthyl plane in 1
(LO). The non-covalent np(Se)⋯σ*(Se–O) 3c–4e interaction operates effectively to keep the Se–O bond on the naphthyl plane in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (G dependence).16 These results show that the structure of 1
(LO) (G dependence).16 These results show that the structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) is well stabilized by the O and G dependences observed in 1-naphthyl selenoxides.16
(LO) is well stabilized by the O and G dependences observed in 1-naphthyl selenoxides.16
On the other hand, there is no np(Se) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). Therefore, the G dependence of the np(Se)⋯σ*(Se–O) type cannot operate in 1
(OO). Therefore, the G dependence of the np(Se)⋯σ*(Se–O) type cannot operate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). Consequently, the driving force for the structure must come from the O dependence for both Se–O bonds. Namely, the non-covalent O–Se⋯Se–O σ(4c–4e) interaction must be carefully examined as a factor to stabilize the fine structure of 1
(OO). Consequently, the driving force for the structure must come from the O dependence for both Se–O bonds. Namely, the non-covalent O–Se⋯Se–O σ(4c–4e) interaction must be carefully examined as a factor to stabilize the fine structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), although the non-bonded Se⋯Se distances are less than the sum of van der Waals radii by ca. 0.65 Å.27 The σ(4c–4e) interaction seems not so important.
(OO), although the non-bonded Se⋯Se distances are less than the sum of van der Waals radii by ca. 0.65 Å.27 The σ(4c–4e) interaction seems not so important.
How does G of MeSe control the fine structure and the behavior? QC calculations are performed on 1 and 5–8.
 | ||
Fig. 1 Structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (a) and 1 (LO) (a) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (b) with atomic numbering scheme for selected atoms (thermal ellipsoids are shown at the 50% probability level). (OO) (b) with atomic numbering scheme for selected atoms (thermal ellipsoids are shown at the 50% probability level). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO)
(OO)
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (LO) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (OO) | |
|---|---|---|
| Empirical formula | C12H12OSe2 | C12H12O2Se2·2.5H2O |
| Formula weight | 330.14 | 391.18 |
| Temperature/K | 298(2) | 103(2) |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n (#14) | C2/c (#15) |
| a/Å | 5.8460(19) | 25.549(9) |
| b/Å | 14.473(3) | 5.8653(18) |
| c/Å | 14.1490(16) | 20.850(8) |
| β/° | 97.660(17) | 117.329(4) |
| V/Å3 | 1186.5(5) | 2775.6(16) |
| Z | 4 | 8 |
| Dc/g cm−3 | 1.848 | 1.872 |
| F(000) | 640 | 1544 |
| Reflections observed [I > 2σ(I)] | 2200 | 2435 |
| Parameters | 136 | 190 |
| R1 [I > 2σ(I)] | 0.032 | 0.021 |
| R1 [all data] | 0.082 | 0.022 |
| ωR2 [I > 2σ(I)] | 0.065 | 0.053 |
| ωR2 [all data] | 0.077 | 0.054 |
| Goodness-of-fit on F2 | 1.029 | 1.109 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), together with those of 1
(OO), together with those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)
(LL)
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)Aa (LL)Aa | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)Ba (LL)Ba | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (LO) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (OO) | |
|---|---|---|---|---|
| a Ref. 26. | ||||
| Interatomic distances | ||||
| Se1–Se2 | 3.051(4) | 3.064(4) | 3.1587(10) | 3.1512(8) |
| Se1–C1 | 1.929(4) | 1.932(3) | 1.983(5) | 1.959(2) |
| Se1–C11 | 1.944(4) | 1.953(4) | 1.954(5) | 1.940(2) |
| Se1–O1 | 1.653(4) | 1.6771(15) | ||
| Se2–C9 | 1.926(4) | 1.932(4) | 1.928(5) | 1.970(2) |
| Se2–C12 | 1.944(4) | 1.949(4) | 1.938(6) | 1.934(2) |
| Se2–O2 | 1.680(15) | |||
| Angles | ||||
| Se2–Se1–C11 | 164.47(3) | 146.46(3) | 85.93(16) | 88.26(7) |
| Se2–Se1–O1 | 173.31(15) | 167.54(5) | ||
| Se1–Se2–C12 | 150.34(3) | 159.73(3) | 85.65(18) | 89.41(7) |
| Se1–Se2–O2 | 167.51(5) | |||
| Se1–C1–C10 | 122.9(3) | 123.9(3) | 126.9(4) | 124.33(16) |
| C1–Se1–C11 | 99.29(16) | 98.41(16) | 96.0(2) | 94.73(9) |
| C1–Se1–O1 | 101.1(2) | 102.69(8) | ||
| C11–Se1–O1 | 100.7(2) | 102.85(9) | ||
| Se2–C9–C10 | 123.9(3) | 122.9(3) | 124.1(4) | 124.67(16) |
| C9–Se2–C12 | 99.27(16) | 98.50(16) | 98.1(2) | 93.38(9) |
| C9–Se2–O2 | 102.21(8) | |||
| C12–Se2–O2 | 102.29(9) | |||
| C1–C10–C9 | 126.4(3) | 127.2(3) | 127.0(4) | 128.1(2) |
| Torsional angles | ||||
| Se1–C1–C10–C5 | 173.5(2) | −176.0(2) | −177.6(4) | 179.19(15) |
| C10–C1–Se1C11 | −154.1(3) | 136.8(3) | 82.8(4) | −86.49(19) |
| C10–C1–Se1–O1 | −175.0(4) | 169.19(17) | ||
| Se2–C9–C10–C5 | 172.2(2) | −170.2(2) | 178.8(4) | 178.90(15) |
| C10–C9–Se2–C12 | −138.8(3) | 148.0(3) | 84.6(4) | −87.10(19) |
| C10–C9–Se2–O2 | 169.54(18) | |||
| O1–Se1–Se2–O2 | 140.3(3) | |||
QC calculations
QC calculations were performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) with the B3LYP/6-311+G(d) method of the Gaussian 98 program.28–30 QC calculations revealed energy profiles of the compounds.31Table 3 collects the results of the QC calculations. The NBO analysis19,20 were performed on 1
(LO) with the B3LYP/6-311+G(d) method of the Gaussian 98 program.28–30 QC calculations revealed energy profiles of the compounds.31Table 3 collects the results of the QC calculations. The NBO analysis19,20 were performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) with the B3LYP/6-311+G(d) method. The results are shown in Table 4. The AIM parameters21,22 are calculated for 1
(OO) with the B3LYP/6-311+G(d) method. The results are shown in Table 4. The AIM parameters21,22 are calculated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) with the Gaussian 03 program32 employing the 6-311+G(3df) basis sets for Se with the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. They are analyzed employing the AIM 2000 program.33Table 5 collects the results of AIM calculations.
(OO) with the Gaussian 03 program32 employing the 6-311+G(3df) basis sets for Se with the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. They are analyzed employing the AIM 2000 program.33Table 5 collects the results of AIM calculations.Indeed, the results of QC calculations essentially correspond to those in the gas phase, but the factors to control and/or stabilize the structures in gas phase must also operate in solid states and in solutions. Therefore, it must be instructive to consider those predicted by QC calculations, although we must be careful for the crystal packing effect in crystals and the solvent effect in solutions, since such effects often larger than the predicted factors.
The effect of G to stabilize 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O (O), OH+ (OH+) and O2H2 (OH·OH)] will be discussed in detail, here. The results clarified the factors for the racemization of selenoxides. n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) (n = 1 and 5–8) must be the key intermediates in the racemization of n
(OH·OH) (n = 1 and 5–8) must be the key intermediates in the racemization of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O), in the presence of (a trace of) water in solutions.
(O), in the presence of (a trace of) water in solutions.
| Form | O: A/AAb | OH+: A/AAb | OH·OH: AC |
|---|---|---|---|
a Calculated with the B3LYP/6-311+G(d) method.b A for 5–8 and AA for 1.c Evaluated based on the values of E(H2O2) = −151.5891 au, E(H2O) = −76.4438 au and E(OH−) = −75.8181 au calculated with the same method.d Relative to that of the corresponding n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O): A.e Relative to the same structure derived from 5 (G = H) being given in parenthesis.f 7.1 kJ mol−1 from the corresponding species of 1 (G = trans-MeSe; O): AA. (O): A.e Relative to the same structure derived from 5 (G = H) being given in parenthesis.f 7.1 kJ mol−1 from the corresponding species of 1 (G = trans-MeSe; O): AA. | |||
| 5 (G = H) | −2902.0303 | −2902.4017 | −2978.4686 |
| Qn(Se) | 1.309 | 1.307 | 1.324 |
| Qn(O) | −0.968 | −0.837 | −0.996, −0.993 |
| Qn(H) | 0.497 | 0.433, 0.433 | |
| +Wc | −2978.4741 | −2978.2198 | −2978.4686 |
| Δde | as 0.0 | 667.7 (as 0.0) | 14.4 (as 0.0) |
| 6 (G = F) | −3001.3000 | −3001.6744 | −3077.7371 |
| +Wc | −3077.7438 | −3077.4925 | −3077.7371 |
| Δde | as 0.0 | 659.8 (−7.9) | 17.6 (3.2) |
| 7 (G = Cl) | −3361.6478 | −3362.0250 | −3438.0833 |
| +Wc | −3438.0916 | −3437.8431 | −3438.0833 |
| Δde | as 0.0 | 652.4 (−15.2) | 21.8 (7.4) |
| 8 (G = Br) | −5475.5651 | −5475.9442 | −5552.0007 |
| +Wc | −5552.0089 | −5551.7623 | −5552.0007 |
| Δde | as 0.0 | 647.4 (−20.2) | 21.5 (7.1) |
| 1 (G = trans-MeSe) | −5342.8896 | −5343.2854 | −5419.3241 |
| +Wc | −5419.3334 | −5419.1035 | −5419.3241 |
| Δde | as 0.0 | 603.6 (−64.1) | 24.4 (10.0) |
| 1 (G = cis-MeSe) | −5342.8869 | −5343.2821 | −5419.3214 |
| +Wc | −5419.3307 | −5419.1002 | −5419.3214 |
| Δde | as 0.0f | 612.3 (−55.4) | 31.5 (17.1) |
| D; A | np(G); σ*(Se–O) | np(G): σ*(Se+–OH) |
|---|---|---|
| a The 6-311+G(d) basis sets being employed.b In kcal mol−1.c Corresponding to the ns(G)⋯σ*(Se+–OH) interaction.d 0.76 kcal mol−1 for the ns(Se)⋯σ*(Se–O) type interaction.e The 6-311+G(3df) basis sets being employed for Se with the 6-311+G(3d,2p) basis sets for C and H.f Corresponding to the ns(Se)⋯σ*(Se–O) interactions. | ||
| G = F | 1.44 | 9.15 (0.87)c |
| G = Cl | 3.29 | 13.65 (1.09)c |
| G = Br | 3.73 | 27.95 (1.19)c |
| G = cis-SeMe | 4.77d | 34.99 (1.76)c |
| G = trans-SeMe | 5.52 | 41.86 (2.69)c |
| G = trans-SeMee | 5.86 | |
| G = trans-Se(O)Mee | 1.53 (×2)f | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), calculated with the NBO methoda
(OO), calculated with the NBO methoda
| Compound | ro(Se, Se)/Å | ρb(rc)/eao−3 | Δρb(rc)/eao−5 | Hb(rc)/au |
|---|---|---|---|---|
| a The 6-311+G(3df) basis sets being employed for Se and the 6-311+G(3d,2p) basis sets for C and H. | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (LO) | 3.2521 | 0.0195 | 0.0420 | −0.0005 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (OO) | 3.2851 | 0.0160 | 0.0393 | 0.0002 |
Effect of G in 1 and 5–8
Racemization of an optically active selenoxide is believed to proceed via a selenide dihydroxide (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)).4a–dScheme 3 shows a hypothetical racemization process of optically active n
(OH·OH)).4a–dScheme 3 shows a hypothetical racemization process of optically active n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*) vian
(O*) vian![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH).
(OH·OH).Protonation of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*) occurs at O of an optically active isomer of n
(O*) occurs at O of an optically active isomer of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*: R) to give n
(O*: R) to give n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*H+: R) at the initial stage of the reaction. n
(O*H+: R) at the initial stage of the reaction. n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) will form in the reaction of n
(OH·OH) will form in the reaction of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*H+: R) with water followed by the deprotonation to yield n
(O*H+: R) with water followed by the deprotonation to yield n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH). Elimination of water from n
(OH·OH). Elimination of water from n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) results in the racemization, since of n
(OH·OH) results in the racemization, since of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) is not optically active as a whole. Similar reactions occur starting from n
(OH·OH) is not optically active as a whole. Similar reactions occur starting from n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*: S) to yield n
(O*: S) to yield n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) vian
(OH·OH) vian![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*H+: S), which also leads to racemization. Water may originate from the solvent and the racemization would proceed under the neutral conditions. The stability of n
(O*H+: S), which also leads to racemization. Water may originate from the solvent and the racemization would proceed under the neutral conditions. The stability of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) must affect on the rates of racemization for the optical active selenoxides.
(OH·OH) must affect on the rates of racemization for the optical active selenoxides.
The effect of G on the stability of 8-G-1-[MeSe(OiHj)]C10H6 [1 and 5–8: L (i = j = 0), O (i = 1, j = 0), OH+ (i = j = 1) and OH·OH (i = j = 2)] are examined based on the QC calculations. The results of QC calculations performed with the B3LYP/6-311+G(d) method are collected in Table 3. Table 3 also contains natural charges (Qn) of Se and O calculated employing the natural population analysis.20Scheme 4 shows optimized structures of the global minimum for each of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), 1
(LL), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1 (LOH+), together with the three types, AA′, BB and AC, for 1 (LOH·OH). The values for AC of n
(LO) and 1 (LOH+), together with the three types, AA′, BB and AC, for 1 (LOH·OH). The values for AC of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LOH·OH) are given in Table 3, since AC is most stable among the three for each.34
(LOH·OH) are given in Table 3, since AC is most stable among the three for each.34
 | ||
Scheme 3 Mechanism for racemization of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*) vian (O*) vian![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) (n = 1 and 5–8). (OH·OH) (n = 1 and 5–8). | ||
 | ||
| Scheme 4 Optimized structures for 1 (G = SeMe) and the derivatives. | ||
Energy differences of the reactions in Scheme 3 are examined based on the values shown in Table 3. The energy of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O (E(n
(O) + H2O (E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)) is taken as the standard for each, for convenience of comparison. How are the selenoxides stabilized by G at the 8-position? The effect of G on the stabilization of selenoxides is examined before discussion the energy profile shown in Scheme 3.
(O) + H2O)) is taken as the standard for each, for convenience of comparison. How are the selenoxides stabilized by G at the 8-position? The effect of G on the stabilization of selenoxides is examined before discussion the energy profile shown in Scheme 3.
Eqn (1) shows the energies of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2 (E(n
(L) + H2O2 (E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2)) relative to E(n
(L) + H2O2)) relative to E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O) [ΔE(n
(O) + H2O) [ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) = E(n
(LO) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2) −E(n
(L) + H2O2) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)) (see Table S1 in the ESI†). Similarly, eqn (2) and (3) exhibit ΔE(n
(O) + H2O)) (see Table S1 in the ESI†). Similarly, eqn (2) and (3) exhibit ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+)) and ΔE(n
(OH+)) and ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)),35 respectively, which are defined as [E(n
(OH·OH)),35 respectively, which are defined as [E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+) + HO−) −E(n
(OH+) + HO−) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)] and [E(n
(O) + H2O)] and [E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)) −E(n
(OH·OH)) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)], respectively.36
(O) + H2O)], respectively.36
ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)) = E(n (LO)) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2) −E(n (L) + H2O2) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O) (O) + H2O)G = H (121.8 kJ mol−1) < F (131.3) < cis-MeSe (133.9) < Cl (136.8) ≤ Br (137.6) < trans-MeSe (141.0) | (1) |
ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+)) = E(n (OH+)) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+) + HO−) −E(n (OH+) + HO−) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O) (O) + H2O)G = H (667.7 kJ mol−1) > F (659.8) > Cl (652.4) > Br (647.4) ≫cis-MeSe (605.2) > trans-MeSe (603.6) | (2) |
ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)) = E(n (OH·OH)) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)) −E(n (OH·OH)) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O) (O) + H2O)G = H (14.4 kJ mol−1) < F (17.6) < Cl (21.8) ≈ Br (21.5) < trans-MeSe (24.4) < cis-MeSe (31.5) | (3) |
The order in eqn (1) corresponds the energy lowering effect by the G⋯Se–O interactions in the formation selenoxides relative to the G⋯Se–C interactions in selenides. However, we must be careful to examine the values for G = cis-MeSe and trans-MeSe, since the structure of the corresponding selenide is commonly CC (see Table S1 in the ESI†).
Eqn (2) exhibits that the protonation on the seleninyl O atom occurs more easily in the order of G = H < F < Cl < Br ≪cis-MeSe < trans-MeSe. The results show that the protonation occurs more easily when G become better donors, especially for G = MeSe. The evaluated ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+)) values are very large in magnitudes, however, they do not mean that the process is very difficult to occur. The large magnitudes are the results of the calculations for the charge separated species of the n
(OH+)) values are very large in magnitudes, however, they do not mean that the process is very difficult to occur. The large magnitudes are the results of the calculations for the charge separated species of the n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH+) + HO− type. Only the relative values are important, since protonation will occur easily in solutions. Resulting hypervalent np(G)⋯σ*(Se–OH+) interactions stabilize further the species in the order shown in eqn (2), relative to the case of the selenoxides.
(OH+) + HO− type. Only the relative values are important, since protonation will occur easily in solutions. Resulting hypervalent np(G)⋯σ*(Se–OH+) interactions stabilize further the species in the order shown in eqn (2), relative to the case of the selenoxides.
The activation energies for the racemization of optically active selenoxides are closely related to the values shown in eqn (3), although they are the energies for the intermediates, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH). The activation energies are expected to increase in this order. The activation energy for G = cis-MeSe is predicted to be larger than that with trans-MeSe. However, cis-MeSe and trans-MeSe isomers interconvert with each other. Therefore, it may be better to evaluate the value by G = trans-MeSe under the experimental conditions: The activation energy of 1
(OH·OH). The activation energies are expected to increase in this order. The activation energy for G = cis-MeSe is predicted to be larger than that with trans-MeSe. However, cis-MeSe and trans-MeSe isomers interconvert with each other. Therefore, it may be better to evaluate the value by G = trans-MeSe under the experimental conditions: The activation energy of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) with G = MeSe is estimated to be about 10 kJ mol−1 larger than that of 5 (L) with G = H and the former is also larger than the case of G = Br by ca. 3 kJ mol−1. Fig. 2 summarizes the effect of G given in eqn (2).
(LO) with G = MeSe is estimated to be about 10 kJ mol−1 larger than that of 5 (L) with G = H and the former is also larger than the case of G = Br by ca. 3 kJ mol−1. Fig. 2 summarizes the effect of G given in eqn (2).
 | ||
Fig. 2 Energies of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH), relative to n (OH·OH), relative to n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) for n = 1 and 5–8. (O) for n = 1 and 5–8. | ||
G at the 8-position will protect sterically from the racemization of an optical active n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*). G must stabilize the optical active n
(O*). G must stabilize the optical active n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*) and the protonated n
(O*) and the protonated n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*H+) whereas G would destabilize n
(O*H+) whereas G would destabilize n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH). The steric congestion at the backside of the Se+–OH bond in n
(OH·OH). The steric congestion at the backside of the Se+–OH bond in n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O*H+) by G will block the space for H2O to attack to produce n
(O*H+) by G will block the space for H2O to attack to produce n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) (Scheme 4). We must be careful, since G could also stabilize n
(OH·OH) (Scheme 4). We must be careful, since G could also stabilize n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) in some cases. The calculated values might correspond to the total effects of the electronic and steric effects. Energy profiles for the racemization evaluated by above calculations must contain main factors. The energies for the transition states must be close to those of the intermediates, n
(OH·OH) in some cases. The calculated values might correspond to the total effects of the electronic and steric effects. Energy profiles for the racemization evaluated by above calculations must contain main factors. The energies for the transition states must be close to those of the intermediates, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH).
(OH·OH).
NBO analysis for n(G)⋯σ*(Se–O) interactions
Table 4 summarizes the second order perturbation energies (E(2)) for the charge transfer (CT) interactions of the n(G)⋯σ*(Se–O) type in 8-G-1-[MeSe(O)]C10H6 and 8-G-1-[MeSe+(OH)]C10H6 evaluated with the NBO method.37 The B3LYP/6-311+G(d) method is employed for the calculations. The E(2) values becomes larger in an order shown in eqn (4).| E(2): G = F (1.44) < Cl (3.29) < Br (3.73) < cis-MeSe (4.77) < trans-MeSe (5.52) | (4) |
The E(2) values are also evaluated for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), employing the 6-311+G(3df) basis sets for Se and the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level.38Table 4 also contains the values. The np(G)⋯σ*(Se–O) interaction are evaluated to be 5.9 kcal mol−1 for 1
(OO), employing the 6-311+G(3df) basis sets for Se and the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level.38Table 4 also contains the values. The np(G)⋯σ*(Se–O) interaction are evaluated to be 5.9 kcal mol−1 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)39 and as 1.5 (× 2) kcal mol−1 for the ns(G)⋯σ*(Se–O) interactions in 1
(LO)39 and as 1.5 (× 2) kcal mol−1 for the ns(G)⋯σ*(Se–O) interactions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). The larger value for 1
(OO). The larger value for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) relative to 1
(LO) relative to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) implies the more effective interaction of the np(G)⋯σ*(Se–O) type in 1
(OO) implies the more effective interaction of the np(G)⋯σ*(Se–O) type in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO). The contribution of the 4c–4e interaction of the O–Se⋯Se–O type was not detected by the NBO analysis. Fig. 3 summarizes the interactions.
(LO). The contribution of the 4c–4e interaction of the O–Se⋯Se–O type was not detected by the NBO analysis. Fig. 3 summarizes the interactions.
 | ||
Fig. 3 The np(G)⋯σ*(Se–O) interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and the ns(G)⋯σ*(Se–O) interactions in 1 (LO) and the ns(G)⋯σ*(Se–O) interactions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) evaluated by the NBO method. (OO) evaluated by the NBO method. | ||
The nature of the np(G)⋯σ*(Se–O) interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and the ns(G)⋯σ*(Se–O) interactions in 1
(LO) and the ns(G)⋯σ*(Se–O) interactions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are evaluated based on the AIM analysis, next.
(OO) are evaluated based on the AIM analysis, next.
AIM analysis of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) (OO)
(OO)
The AIM analysis are carried out on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). The 6-311+G(3df) basis sets are employed for Se and the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. Table 5 collects the AIM parameters of 1
(OO). The 6-311+G(3df) basis sets are employed for Se and the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. Table 5 collects the AIM parameters of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) for the bond critical points (BCPs: rc) on the interaction lines between non-bonded Se atoms.
(OO) for the bond critical points (BCPs: rc) on the interaction lines between non-bonded Se atoms.The low values of electron densities at BCPs (ρb(rc)) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (0.016–0.020 eao−3) show that the interactions are ionic in nature. Laplacian values of ρb(rc) (Δρb(rc)) are both positive, whereas the total electron energy densities at BCPs (Hb(rc)) for 1
(OO) (0.016–0.020 eao−3) show that the interactions are ionic in nature. Laplacian values of ρb(rc) (Δρb(rc)) are both positive, whereas the total electron energy densities at BCPs (Hb(rc)) for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) is negative but it is positive for 1
(LO) is negative but it is positive for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). The results strongly suggest that the np(G)⋯σ*(Se–O) interaction in 1
(OO). The results strongly suggest that the np(G)⋯σ*(Se–O) interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) is the CT interaction in nature similarly to the case of R2Se⋯Br2 (MC) but the ns(G)⋯σ*(Se–O) interactions in 1
(LO) is the CT interaction in nature similarly to the case of R2Se⋯Br2 (MC) but the ns(G)⋯σ*(Se–O) interactions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) seems weaker than such CT interactions.40
(OO) seems weaker than such CT interactions.40
Fig. 4 shows the counter map of ρb(rc) in the SeSeC9 plane for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), together with BCPs (
(LO), together with BCPs ( ), ring critical points (
), ring critical points ( ), bond paths and the interaction lines. BCP are detected on the Se⋯Se and O⋯2H interaction lines. The BCP on the Se⋯Se interaction line well visualize the np(Se)⋯σ*(Se–O) interaction in 1
), bond paths and the interaction lines. BCP are detected on the Se⋯Se and O⋯2H interaction lines. The BCP on the Se⋯Se interaction line well visualize the np(Se)⋯σ*(Se–O) interaction in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO). While BCP is also detected on the O⋯2H interaction line, the interaction is very small. A similar counter map is also drawn for 1
(LO). While BCP is also detected on the O⋯2H interaction line, the interaction is very small. A similar counter map is also drawn for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), which is shown in Fig. S2 of the ESI.†
(OO), which is shown in Fig. S2 of the ESI.†
![Contour map of ρb(rc) for 1 (LO) in the SeSeC9 plane, together with BCPs (), ring critical points () and bond paths. The contours [eao−3] are at 2l (l = ±8, ±7,…0) and 0.0047 (heavy line). Two Me groups are located upside and downside of the SeSeC9 plane. The C2, C3, C6 and C7 atoms with the C–H bonds deviate substantially from the plane.](/image/article/2009/NJ/b809763a/b809763a-f4.gif) | ||
Fig. 4 Contour map of ρb(rc) for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) in the SeSeC9 plane, together with BCPs ( (LO) in the SeSeC9 plane, together with BCPs ( ), ring critical points ( ), ring critical points ( ) and bond paths. The contours [eao−3] are at 2l (l = ±8, ±7,…0) and 0.0047 (heavy line). Two Me groups are located upside and downside of the SeSeC9 plane. The C2, C3, C6 and C7 atoms with the C–H bonds deviate substantially from the plane. ) and bond paths. The contours [eao−3] are at 2l (l = ±8, ±7,…0) and 0.0047 (heavy line). Two Me groups are located upside and downside of the SeSeC9 plane. The C2, C3, C6 and C7 atoms with the C–H bonds deviate substantially from the plane. | ||
Conclusion
X-Ray crystallographic analysis of 8-methylselanyl-1-(methylseleninyl)naphthalene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)) and 1,8-bis(methylseleninyl)naphthalene 1
(LO)) and 1,8-bis(methylseleninyl)naphthalene 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) revealed that the three Se⋯Se
(OO) revealed that the three Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O atoms in 1
O atoms in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and the four O
(LO) and the four O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se⋯Se
Se⋯Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O atoms in 1
O atoms in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) align linearly. All Se–O bonds are placed in the naphthyl plane. The superior tendency for the Se–O bonds to stay on the naphthyl plane (O dependence) must be the driving force for the fine structures of 1
(OO) align linearly. All Se–O bonds are placed in the naphthyl plane. The superior tendency for the Se–O bonds to stay on the naphthyl plane (O dependence) must be the driving force for the fine structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO). The noncovalent np(Se)⋯σ(Se–O) 3c–4e interactions (G dependence) operate effectively to stabilize the structure of 1
(OO). The noncovalent np(Se)⋯σ(Se–O) 3c–4e interactions (G dependence) operate effectively to stabilize the structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO). On the other hand, the driving force for the structure of 1
(LO). On the other hand, the driving force for the structure of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) must mainly come from the O dependence for each Se–O bond in 1
(OO) must mainly come from the O dependence for each Se–O bond in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), since the G dependence cannot operate without np(Se).
(OO), since the G dependence cannot operate without np(Se).QC calculations clarify the factors that protect from racemization of selenoxides. The energies of 8-G-1-[MeSe(OH)2]C10H6 from (8-G-1-[MeSe(O)]C10H6 + H2O) are shown to be in an order of G = H (14.4 kJ mol−1) < F (17.6) < Cl (21.8) ≈ Br (21.5) < trans-SeMe (24.4) < cis-SeMe (31.5). The activation energies for the racemization should increase in this order, since 8-G-1-[MeSe(OH)2]C10H6 must be the key intermediates. The activation energy of 1 (LO: G = MeSe) is evaluated to be larger than that of 5 (L: G = H) and 8 (L: G = Br) by 10 and 3 kJ mol−1, respectively. The results will help to design the optically stable selenoxides. The NBO and AIM analyses support the discussion and visualize the interactions.
Investigations on the chiral 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), prepared in the oxidation of 3a
(LO), prepared in the oxidation of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) with chiral reagents, are in progress. Details will be reported elsewhere.
(LL) with chiral reagents, are in progress. Details will be reported elsewhere.
Experimental
General considerations
Manipulations were performed under an argon atmosphere with standard vacuum-line techniques. Glassware was dried at 130 °C overnight. Solvents and reagents were purified by standard procedures if necessary. Melting points were determined on a Yanaco MP-S3 melting point apparatus and uncorrected. NMR spectra were recorded at room temperature on a JEOL AL-300 spectrometer (1H, 300 MHz; 13C, 75 MHz) and on a JEOL Lambda-400 spectrometer (1H, 400 MHz; 77Se, 76 MHz). The 1H, 13C and 77Se NMR spectra were recorded in CDCl3. Chemical shifts are given in ppm relative to Me4Si for the 1H and 13C NMR spectra and relative to reference compound MeSeMe for the 77Se NMR spectra. Column chromatography was performed by using silica gel (Fujishilysia PSQ-100B) and basic alumina (E. Merck) and analytical thin layer chromatography was performed on precoated silica gel plates (60F-254) with the systems (v/v) indicated.Syntheses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LL)). To a solution of the dianion of naphtho[1,8-c,d]-1,2-diselenole, which was prepared by reduction of the diselenole 923 (1.03 g, 3.64 mmol) with NaBH4 in an aqueous THF solution, was added methyl iodide (1.29 g, 9.06 mmol) at room temperature. After a usual workup, the crude was purified by column chromatography (flash column, SiO2, hexane). Recrystallization of the chromatographed product from hexane gave 1
(LL)). To a solution of the dianion of naphtho[1,8-c,d]-1,2-diselenole, which was prepared by reduction of the diselenole 923 (1.03 g, 3.64 mmol) with NaBH4 in an aqueous THF solution, was added methyl iodide (1.29 g, 9.06 mmol) at room temperature. After a usual workup, the crude was purified by column chromatography (flash column, SiO2, hexane). Recrystallization of the chromatographed product from hexane gave 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) as colorless prisms in 98% yield, mp 85.0–85.5 °C, 1H NMR (300 MHz, CDCl3, δ, ppm, TMS): 2.33 (s, 6H), 7.32 (t, 2H, J = 7.7 Hz), 7.70 (dd, 2H, J = 1.2 and 8.2 Hz), 7.73 (dd, 2H, J = 1.2 and 7.5 Hz); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 13.3, 125.7, 128.3, 131.9, 132.3, 135.3, 135.6; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 231.4. Anal. Calc. for 1
(LL) as colorless prisms in 98% yield, mp 85.0–85.5 °C, 1H NMR (300 MHz, CDCl3, δ, ppm, TMS): 2.33 (s, 6H), 7.32 (t, 2H, J = 7.7 Hz), 7.70 (dd, 2H, J = 1.2 and 8.2 Hz), 7.73 (dd, 2H, J = 1.2 and 7.5 Hz); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 13.3, 125.7, 128.3, 131.9, 132.3, 135.3, 135.6; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 231.4. Anal. Calc. for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (C12H12Se2): C, 45.88; H, 3.85%. Found: C, 45.73; H, 3.77%.
(LL) (C12H12Se2): C, 45.88; H, 3.85%. Found: C, 45.73; H, 3.77%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). 1
(LO)). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (0.98 mg, 3.12 mmol) was dissolved in 20 mL of CH2Cl2 and the solution was bubbling with the ozone for 5 min. TLC was checked for the completion of the reaction (rf = 0.07 (chloroform)). Then the solution was evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, Al2O3, CH2Cl2). 1
(LL) (0.98 mg, 3.12 mmol) was dissolved in 20 mL of CH2Cl2 and the solution was bubbling with the ozone for 5 min. TLC was checked for the completion of the reaction (rf = 0.07 (chloroform)). Then the solution was evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, Al2O3, CH2Cl2). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 85% yield as colorless powder, mp 129.8–130.1 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.29 (s, 3H), 2.78 (s, 3H), 7.48 (t, J 7.6 Hz, 2H), 7.76 (t, J 7.7 Hz, 2H), 7.98–8.05 (m, 2H), 8.10 (dd, J 1.1 and 7.2 Hz, 1H), 8.88 (dd, J 1.2 and 7.4 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 13.87, 41.12, 125.73, 126.28, 126.35, 126.57, 131.01, 132.44, 133.06, 136.13, 138.93, 141.34; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 210.8, 833.0. Anal. Calc. for 1
(LO) gave 85% yield as colorless powder, mp 129.8–130.1 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.29 (s, 3H), 2.78 (s, 3H), 7.48 (t, J 7.6 Hz, 2H), 7.76 (t, J 7.7 Hz, 2H), 7.98–8.05 (m, 2H), 8.10 (dd, J 1.1 and 7.2 Hz, 1H), 8.88 (dd, J 1.2 and 7.4 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 13.87, 41.12, 125.73, 126.28, 126.35, 126.57, 131.01, 132.44, 133.06, 136.13, 138.93, 141.34; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 210.8, 833.0. Anal. Calc. for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C12H12OSe2): C, 43.66; H, 3.66%. Found: C, 43.61; H, 3.60%.
(LO) (C12H12OSe2): C, 43.66; H, 3.66%. Found: C, 43.61; H, 3.60%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). 1
(OO)). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (0.58 g, 0.30 mmol) was dissolved in 20 mL of CH2Cl2 and the solution was bubbling with the ozone for 15 min. TLC was checked for the completion of the reaction (rf = 0.00 (chloroform)). Then the solution was evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, Al2O3, CH2Cl2). 1
(LL) (0.58 g, 0.30 mmol) was dissolved in 20 mL of CH2Cl2 and the solution was bubbling with the ozone for 15 min. TLC was checked for the completion of the reaction (rf = 0.00 (chloroform)). Then the solution was evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, Al2O3, CH2Cl2). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 59% yield as colorless powder, mp 154.8–155.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.71 (s, 6H), 7.84 (t, J 7.7 Hz, 2H), 8.18 (dd, J 1.2 and 6.9 Hz, 2H), 8.71 (dd, J 1.4 and 6.9 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.3. Anal. Calc. for 1
(OO) gave 59% yield as colorless powder, mp 154.8–155.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.71 (s, 6H), 7.84 (t, J 7.7 Hz, 2H), 8.18 (dd, J 1.2 and 6.9 Hz, 2H), 8.71 (dd, J 1.4 and 6.9 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.3. Anal. Calc. for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C12H12O2Se2): C, 41.64; H, 3.49%. Found: C, 41.55; H, 3.45%. Anal. Calc. for 1
(OO) (C12H12O2Se2): C, 41.64; H, 3.49%. Found: C, 41.55; H, 3.45%. Anal. Calc. for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO)·2.5H2O (C24H24O4Se4·5H2O): C, 36.84; H, 4.38%. Found: C, 36.87; H, 4.41%.
(OO)·2.5H2O (C24H24O4Se4·5H2O): C, 36.84; H, 4.38%. Found: C, 36.87; H, 4.41%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LL)). Following the similar method to that used for 1
(LL)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), 2
(LL), 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) gave 80% yield as colorless powder, mp 52.3–52.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.35 (t, J 7.4 Hz, 6H), 2.89 (q, J 7.5 Hz, 4H), 7.32 (t, J 7.6 Hz, 2H), 7.70 (dd, J 1.2 and 8.1 Hz, 2H), 7.76 (dd, J 1.1 and 7.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 341.7. Anal. Calc. for 2
(LL) gave 80% yield as colorless powder, mp 52.3–52.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.35 (t, J 7.4 Hz, 6H), 2.89 (q, J 7.5 Hz, 4H), 7.32 (t, J 7.6 Hz, 2H), 7.70 (dd, J 1.2 and 8.1 Hz, 2H), 7.76 (dd, J 1.1 and 7.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 341.7. Anal. Calc. for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (C14H16Se2): C, 49.14; H, 4.71%. Found: C, 49.23; H, 4.72%.
(LL) (C14H16Se2): C, 49.14; H, 4.71%. Found: C, 49.23; H, 4.72%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). Following the similar method to that used for 1
(LO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 3a
(LO), 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 80% yield as colorless needles, mp 129.8–130.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.72 (s, 3H), 6.98–7.02 (m, 2H), 7.11–7.16 (m, 3H), 7.56 (t, J 7.6 Hz, 1H), 7.76 (t, J 7.7 Hz, 1H), 8.05 (dd, J 1.2 and 8.0 Hz, 1H), 8.10 (dd, J 1.3 and 8.1 Hz, 1H), 8.15 (dd, J 1.3 and 7.2 Hz, 1H), 8.82 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS) 40.56, 123.19, 126.51, 126.57, 126.75, 126.88, 128.42 (2J(Se,C) 5.9 Hz), 129.63, 131.95, 132.42, 133.03, 133.50, 136.29, 140.89, 141.37; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 398.2, 831.4. Anal. Calc. for 3a
(LO) gave 80% yield as colorless needles, mp 129.8–130.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.72 (s, 3H), 6.98–7.02 (m, 2H), 7.11–7.16 (m, 3H), 7.56 (t, J 7.6 Hz, 1H), 7.76 (t, J 7.7 Hz, 1H), 8.05 (dd, J 1.2 and 8.0 Hz, 1H), 8.10 (dd, J 1.3 and 8.1 Hz, 1H), 8.15 (dd, J 1.3 and 7.2 Hz, 1H), 8.82 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS) 40.56, 123.19, 126.51, 126.57, 126.75, 126.88, 128.42 (2J(Se,C) 5.9 Hz), 129.63, 131.95, 132.42, 133.03, 133.50, 136.29, 140.89, 141.37; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 398.2, 831.4. Anal. Calc. for 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C17H14OSe2): C, 52.06; H, 3.60%. Found: C, 52.11; H, 3.66%.
(LO) (C17H14OSe2): C, 52.06; H, 3.60%. Found: C, 52.11; H, 3.66%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). Following the similar method to that used for 1
(OO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 3a
(OO), 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 63% yield as colorless needles, mp 148.0–148.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.74 (s, 3H), 7.33–7.50 (m, 3H), 7.51–7.58 (m, 2H), 7.78 (t, J 7.7 Hz, 1H), 7.81 (t, J 7.7 Hz, 1H), 8.13 (dd, J 1.1 and 8.1 Hz, 1H), 8.14 (dd, J 1.1 and 8.1 Hz, 1H), 8.63 (dd, J 1.4 and 7.4 Hz, 1H), 8.72 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 38.25, 126.72, 126.80, 126.85, 127.01, 127.64, 127.92, 130.02, 131.70, 133.33, 133.71, 135.55, 138.88, 139.22, 141.66; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 820.0, 832.5. Anal. Calc. for 3a
(OO) gave 63% yield as colorless needles, mp 148.0–148.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.74 (s, 3H), 7.33–7.50 (m, 3H), 7.51–7.58 (m, 2H), 7.78 (t, J 7.7 Hz, 1H), 7.81 (t, J 7.7 Hz, 1H), 8.13 (dd, J 1.1 and 8.1 Hz, 1H), 8.14 (dd, J 1.1 and 8.1 Hz, 1H), 8.63 (dd, J 1.4 and 7.4 Hz, 1H), 8.72 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 38.25, 126.72, 126.80, 126.85, 127.01, 127.64, 127.92, 130.02, 131.70, 133.33, 133.71, 135.55, 138.88, 139.22, 141.66; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 820.0, 832.5. Anal. Calc. for 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C17H14O2Se2): C, 50.02; H, 3.46%. Found: C, 50.07; H, 3.57%.
(OO) (C17H14O2Se2): C, 50.02; H, 3.46%. Found: C, 50.07; H, 3.57%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). Following the similar method to that used for 1
(LO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 3b
(LO), 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 88% yield as colorless needles, mp 129.6–130.4 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.70 (s, 3H), 3.82 (s, 3H), 6.70 (d, J 8.8 Hz, 2H), 7.01 (d, J 8.8 Hz, 2H), 7.51 (t, J 7.2 Hz, 1H), 7.74 (t, J 7.2 Hz, 1H), 7.88 (dd, J 1.1 and 6.8 Hz, 1H), 8.01 (dd, J 1.1 and 6.8 Hz, 1H), 8.03 (dd, J 1.1 and 6.8 Hz, 1H), 8.10 (dd, J 1.1 and 6.8 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 385.9, 833.9. Anal. Calc. for 3b
(LO) gave 88% yield as colorless needles, mp 129.6–130.4 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.70 (s, 3H), 3.82 (s, 3H), 6.70 (d, J 8.8 Hz, 2H), 7.01 (d, J 8.8 Hz, 2H), 7.51 (t, J 7.2 Hz, 1H), 7.74 (t, J 7.2 Hz, 1H), 7.88 (dd, J 1.1 and 6.8 Hz, 1H), 8.01 (dd, J 1.1 and 6.8 Hz, 1H), 8.03 (dd, J 1.1 and 6.8 Hz, 1H), 8.10 (dd, J 1.1 and 6.8 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 385.9, 833.9. Anal. Calc. for 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C18H16O2Se2): C, 51.20; H, 3.82%. Found: C, 50.98; H, 3.83%.
(LO) (C18H16O2Se2): C, 51.20; H, 3.82%. Found: C, 50.98; H, 3.83%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). Following the similar method to that used for 1
(OO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 3b
(OO), 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 43% yield as colorless powder, mp 144.5–145.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.75 (s, 3H), 3.76 (s, 3H), 6.87 (d, J 8.9 Hz, 2H), 7.45 (d, J 8.9 Hz, 2H), 7.83 (t, J 7.7 Hz, 2H), 8.15 (dd, J 1.0, 8.2 Hz, 1H), 8.17 (dd, J 1.0, 8.2 Hz, 1H), 8.69 (dd, J 1.3, 9.1 Hz, 1H), 8.71 (dd, J 1.2, 9.1 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.6, 846.4. Anal. Calc. for 3b
(OO) gave 43% yield as colorless powder, mp 144.5–145.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.75 (s, 3H), 3.76 (s, 3H), 6.87 (d, J 8.9 Hz, 2H), 7.45 (d, J 8.9 Hz, 2H), 7.83 (t, J 7.7 Hz, 2H), 8.15 (dd, J 1.0, 8.2 Hz, 1H), 8.17 (dd, J 1.0, 8.2 Hz, 1H), 8.69 (dd, J 1.3, 9.1 Hz, 1H), 8.71 (dd, J 1.2, 9.1 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.6, 846.4. Anal. Calc. for 3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C18H16O3Se2): C, 49.33; H, 3.68%. Found: C, 49.30; H, 3.73%.
(OO) (C18H16O3Se2): C, 49.33; H, 3.68%. Found: C, 49.30; H, 3.73%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). Following the similar method to that used for 1
(LO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 3d
(LO), 3d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 61% yield as colorless powder, mp 141.5–142.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.67 (s, 3H), 7.07 (dt, J 2.1 and 9.0 Hz, 2H), 7.64 (t, J 7.5 Hz, 1H), 7.83 (t, J 7.5 Hz, 1H), 7.99 (dt, J 2.4 and 9.0 Hz, 2H), 8.11 (dd, J 1.2 and 6.9 Hz, 2H), 8.18 (dd, J 1.2 and 4.2 Hz, 1H), 8.21 (dd, J 1.5 and 4.8 Hz, 1H), 8.84 (dd, J 1.2 and 6.0 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 426.4, 835.6. Anal. Calc. for 3d
(LO) gave 61% yield as colorless powder, mp 141.5–142.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.67 (s, 3H), 7.07 (dt, J 2.1 and 9.0 Hz, 2H), 7.64 (t, J 7.5 Hz, 1H), 7.83 (t, J 7.5 Hz, 1H), 7.99 (dt, J 2.4 and 9.0 Hz, 2H), 8.11 (dd, J 1.2 and 6.9 Hz, 2H), 8.18 (dd, J 1.2 and 4.2 Hz, 1H), 8.21 (dd, J 1.5 and 4.8 Hz, 1H), 8.84 (dd, J 1.2 and 6.0 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 426.4, 835.6. Anal. Calc. for 3d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C17H13NO3Se2): C, 46.70; H, 3.00; N, 3.20%. Found: C, 46.75; H, 3.03; N, 3.22%.
(LO) (C17H13NO3Se2): C, 46.70; H, 3.00; N, 3.20%. Found: C, 46.75; H, 3.03; N, 3.22%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). Following the similar method to that used for 1
(OO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 3d
(OO), 3d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 82% yield as colorless powder, mp 151.2–152.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.83 (s, 3H), 7.72–7.92 (m, 4H), 8.12–8.27 (m, 4H), 8.57 (dd, J 1.1 and 6.2 Hz, 1H), 8.74 (dd, J 1.3 and 6.1 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.4, 849.0. Anal. Calc. for 3d
(OO) gave 82% yield as colorless powder, mp 151.2–152.0 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.83 (s, 3H), 7.72–7.92 (m, 4H), 8.12–8.27 (m, 4H), 8.57 (dd, J 1.1 and 6.2 Hz, 1H), 8.74 (dd, J 1.3 and 6.1 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 821.4, 849.0. Anal. Calc. for 3d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C17H13NO4Se2): C, 45.05; H, 2.89; N, 3.09%. Found: C, 45.12; H, 2.83; N, 3.12%.
(OO) (C17H13NO4Se2): C, 45.05; H, 2.89; N, 3.09%. Found: C, 45.12; H, 2.83; N, 3.12%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LL)). Under an argon atmosphere, 1,8-diiodonaphthalene (4.33 g, 11.40 mmol) was dissolved in 100 mL of dry THF and the solution was added to nBuLi (15.0 mL, 23.94 mmol, 1.6 N) at −78 °C. After 20 min, a THF solution of phenylselenobromide (22.80 mmol) was added to the above solution at −78 °C. Then the reaction mixture was stirring for 2 h and warmed up room temperature. Then, 20 mL of 5% acetone hydrochloric acid and 100 mL of benzene were added. The organic layer was separated, washed with brine, 10% aqueous solution of sodium hydroxide, saturated aqueous solution of sodium bicarbonate and brine. Then the solution was dried over sodium sulfate, evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, SiO2, hexane). 4a
(LL)). Under an argon atmosphere, 1,8-diiodonaphthalene (4.33 g, 11.40 mmol) was dissolved in 100 mL of dry THF and the solution was added to nBuLi (15.0 mL, 23.94 mmol, 1.6 N) at −78 °C. After 20 min, a THF solution of phenylselenobromide (22.80 mmol) was added to the above solution at −78 °C. Then the reaction mixture was stirring for 2 h and warmed up room temperature. Then, 20 mL of 5% acetone hydrochloric acid and 100 mL of benzene were added. The organic layer was separated, washed with brine, 10% aqueous solution of sodium hydroxide, saturated aqueous solution of sodium bicarbonate and brine. Then the solution was dried over sodium sulfate, evaporated and dried in vacuo. The crude product was purified by column chromatography (flash column, SiO2, hexane). 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) gave 89% yield as yellow prisms, mp 64.0–64.8 °C; 1H NMR (300 MHz, CDCl3, δ, ppm, TMS): 7.22–7.28 (m, 8H), 7.39–7.45 (m, 4H), 7.64 (dd, J 1.1 and 7.3 Hz, 2H), 7.74 (dd, J 1.1 and 8.3 Hz, 2H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 126.0, 127.4, 129.2, 129.4, 131.4, 133.4, 135.18, 135.19, 135.5, 135.9; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 435.4. Anal. Calc. for 4a
(LL) gave 89% yield as yellow prisms, mp 64.0–64.8 °C; 1H NMR (300 MHz, CDCl3, δ, ppm, TMS): 7.22–7.28 (m, 8H), 7.39–7.45 (m, 4H), 7.64 (dd, J 1.1 and 7.3 Hz, 2H), 7.74 (dd, J 1.1 and 8.3 Hz, 2H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 126.0, 127.4, 129.2, 129.4, 131.4, 133.4, 135.18, 135.19, 135.5, 135.9; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 435.4. Anal. Calc. for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (C22H16Se2): C, 60.29; H, 3.68%. Found: C, 60.21; H, 3.75%.
(LL) (C22H16Se2): C, 60.29; H, 3.68%. Found: C, 60.21; H, 3.75%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). Following the similar method to that used for 1
(LO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 4a
(LO), 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 65% yield as colorless prisms, mp 155.5–156.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 6.90–6.95 (m, 4H), 7.10–7.13 (m, 6H), 7.22–7.26 (m, 6H), 7.48–7.53 (m, 4H), 7.52 (t, J 8.2 Hz, 1H), 7.83 (d, J 7.7 Hz, 1H), 8.07 (dd, J 1.3 and 7.2 Hz, 1H), 8.08 (dd, J 1.3 and 8.2 Hz, 1H), 9.02 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 123.90, 126.45, 126.51, 126.79, 127.86, 127.91, 128.70, 129.17, 129.49, 130.11, 131.73, 132.76, 133.27, 133.78, 136.31, 140.17, 140.71, 146.21; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 400.1, 863.7. Anal. Calc. for 4a
(LO) gave 65% yield as colorless prisms, mp 155.5–156.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 6.90–6.95 (m, 4H), 7.10–7.13 (m, 6H), 7.22–7.26 (m, 6H), 7.48–7.53 (m, 4H), 7.52 (t, J 8.2 Hz, 1H), 7.83 (d, J 7.7 Hz, 1H), 8.07 (dd, J 1.3 and 7.2 Hz, 1H), 8.08 (dd, J 1.3 and 8.2 Hz, 1H), 9.02 (dd, J 1.3 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 123.90, 126.45, 126.51, 126.79, 127.86, 127.91, 128.70, 129.17, 129.49, 130.11, 131.73, 132.76, 133.27, 133.78, 136.31, 140.17, 140.71, 146.21; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 400.1, 863.7. Anal. Calc. for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C22H16OSe2): C, 58.17; H, 3.55%. Found: C, 58.11; H, 3.65%.
(LO) (C22H16OSe2): C, 58.17; H, 3.55%. Found: C, 58.11; H, 3.65%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). Following the similar method to that used for 1
(OO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 4a
(OO), 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 78% yield as colorless prisms, mp. 187.5–188.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 7.21–7.30 (m, 8H), 7.37 (tt, J 1.5 and 6.8 Hz, 2H), 7.76 (t, J 7.7 Hz, 2H), 8.15 (dd, J 0.9 and 7.5 Hz, 2H), 8.47 (dd, J 1.1 and 6.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 877.1. Anal. Calc. for 4a
(OO) gave 78% yield as colorless prisms, mp. 187.5–188.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 7.21–7.30 (m, 8H), 7.37 (tt, J 1.5 and 6.8 Hz, 2H), 7.76 (t, J 7.7 Hz, 2H), 8.15 (dd, J 0.9 and 7.5 Hz, 2H), 8.47 (dd, J 1.1 and 6.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 877.1. Anal. Calc. for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C22H16O2Se2): C, 56.19; H, 3.43%. Found: C, 56.22; H, 3.53%.
(OO) (C22H16O2Se2): C, 56.19; H, 3.43%. Found: C, 56.22; H, 3.53%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LL)). Following the similar method to that used for 4a
(LL)). Following the similar method to that used for 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), 4c
(LL), 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) gave 87% yield as yellow prisms, mp 97.8–98.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.30 (s 18H), 7.24 (t, J 7.7 Hz, 2H), 7.27 (d, J 8.6 Hz, 4H), 7.38 (d, J 8.6 Hz, 4H), 7.65 (dd, J 1.3 and 6.1 Hz, 2H), 7.73 (dd, J 1.3 and 7.0 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 424.6. Anal. Calc. for 4c
(LL) gave 87% yield as yellow prisms, mp 97.8–98.3 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.30 (s 18H), 7.24 (t, J 7.7 Hz, 2H), 7.27 (d, J 8.6 Hz, 4H), 7.38 (d, J 8.6 Hz, 4H), 7.65 (dd, J 1.3 and 6.1 Hz, 2H), 7.73 (dd, J 1.3 and 7.0 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 424.6. Anal. Calc. for 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL) (C30H32Se2): C, 65.45; H, 5.86%. Found: C, 65.41; H, 5.88%.
(LL) (C30H32Se2): C, 65.45; H, 5.86%. Found: C, 65.41; H, 5.88%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (LO)). Following the similar method to that used for 1
(LO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 4c
(LO), 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) gave 86% yield as colorless powder, mp 179.5–180.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.22 (s, 9H), 1.27 (s 9H), 6.91 (d, J 8.3 Hz, 2H), 7.15 (d, J 8.1 Hz, 2H), 7.28 (d, J 8.8 Hz, 2H), 7.43 (d, J 8.6 Hz, 2H), 7.51 (t, J 7.9 Hz, 1H), 7.82 (t, J 7.7 Hz, 1H), 8.07 (d, J 8.3 Hz, 3H), 9.01 (dd, J 1.3 and 7.5 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 393.2, 861.2. Anal. Calc. for 4c
(LO) gave 86% yield as colorless powder, mp 179.5–180.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.22 (s, 9H), 1.27 (s 9H), 6.91 (d, J 8.3 Hz, 2H), 7.15 (d, J 8.1 Hz, 2H), 7.28 (d, J 8.8 Hz, 2H), 7.43 (d, J 8.6 Hz, 2H), 7.51 (t, J 7.9 Hz, 1H), 7.82 (t, J 7.7 Hz, 1H), 8.07 (d, J 8.3 Hz, 3H), 9.01 (dd, J 1.3 and 7.5 Hz, 1H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 393.2, 861.2. Anal. Calc. for 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) (C30H32OSe2): C, 63.61; H, 5.69%. Found: C, 63.55; H, 5.58%.
(LO) (C30H32OSe2): C, 63.61; H, 5.69%. Found: C, 63.55; H, 5.58%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (OO)). Following the similar method to that used for 1
(OO)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), 4c
(OO), 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) gave 87% yield as colorless powder, mp 172.5–173.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.63 (s, 18H), 7.39 (d, J 8.6 Hz, 4H), 7.47 (d, J 8.3 Hz, 4H), 7.83 (d, J 7.6 Hz, 2H), 8.16 (dd, J 0.8 and 7.3 Hz, 2H), 8.73 (dd, J 1.0 and 6.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 843.7. Anal. Calc. for 4c
(OO) gave 87% yield as colorless powder, mp 172.5–173.2 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 1.63 (s, 18H), 7.39 (d, J 8.6 Hz, 4H), 7.47 (d, J 8.3 Hz, 4H), 7.83 (d, J 7.6 Hz, 2H), 8.16 (dd, J 0.8 and 7.3 Hz, 2H), 8.73 (dd, J 1.0 and 6.2 Hz, 2H); 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 843.7. Anal. Calc. for 4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (C30H32O2Se2): C, 61.86; H, 5.54%. Found: C, 61.93; H, 5.58%.
(OO) (C30H32O2Se2): C, 61.86; H, 5.54%. Found: C, 61.93; H, 5.58%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL), 5 (L) gave 99% yield as pale yellow oil; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.37 (s, 2JSe,H 11.7 Hz, 3H), 7.35 (dd, J 7.3 and 8.1 Hz, 1H), 7.47 (ddd, J 1.6, 6.9 and 8.2 Hz, 1H), 7.53 (ddd, J 1.6, 6.9 and 8.3 Hz, 1H), 7.66 (dd, J 1.1 and 7.3 Hz, 1H), 7.71 (d, J 8.2 Hz, 1H), 7.80 (dd, J 1.7 and 7.9 Hz, 1H), 8.24 (ddd, J 0.7, 1.6 and 8.1 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 36.54, 122.08 (J 14.9 Hz), 124.03 (J 6.2 Hz), 126.11, 126.79, 127.56, 129.35, 130.27, 131.40, 133.88, 138.68; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 158.6. Anal. Calc. for 5 (L) (C11H10Se): C, 59.74; H, 4.56%. Found: C, 59.90; H, 4.49%.
(LL), 5 (L) gave 99% yield as pale yellow oil; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.37 (s, 2JSe,H 11.7 Hz, 3H), 7.35 (dd, J 7.3 and 8.1 Hz, 1H), 7.47 (ddd, J 1.6, 6.9 and 8.2 Hz, 1H), 7.53 (ddd, J 1.6, 6.9 and 8.3 Hz, 1H), 7.66 (dd, J 1.1 and 7.3 Hz, 1H), 7.71 (d, J 8.2 Hz, 1H), 7.80 (dd, J 1.7 and 7.9 Hz, 1H), 8.24 (ddd, J 0.7, 1.6 and 8.1 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 36.54, 122.08 (J 14.9 Hz), 124.03 (J 6.2 Hz), 126.11, 126.79, 127.56, 129.35, 130.27, 131.40, 133.88, 138.68; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 158.6. Anal. Calc. for 5 (L) (C11H10Se): C, 59.74; H, 4.56%. Found: C, 59.90; H, 4.49%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) (O)). Following the similar method to that used for 1
(O)). Following the similar method to that used for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO), 5
(LO), 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) gave 67% yield as colorless needles, mp 97.2–97.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.71 (s, 2JSe,H 12.3 Hz, 3H), 7.56–7.65 (m, 2H), 7.70 (dd, J 7.4 and 8.2 Hz, 1H), 7.81–7.87 (m, 1H), 7.95–8.02 (m, 2H), 8.29 (dd, J 1.1 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 7.52, 125.83, 126.14, 126.39, 126.63, 127.16, 128.58, 128.67, 131.10, 133.29, 133.77; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 809.3. Anal. Calc. for 5
(O) gave 67% yield as colorless needles, mp 97.2–97.8 °C; 1H NMR (400 MHz, CDCl3, δ, ppm, TMS): 2.71 (s, 2JSe,H 12.3 Hz, 3H), 7.56–7.65 (m, 2H), 7.70 (dd, J 7.4 and 8.2 Hz, 1H), 7.81–7.87 (m, 1H), 7.95–8.02 (m, 2H), 8.29 (dd, J 1.1 and 7.3 Hz, 1H); 13C NMR (75 MHz, CDCl3, δ, ppm, TMS): 7.52, 125.83, 126.14, 126.39, 126.63, 127.16, 128.58, 128.67, 131.10, 133.29, 133.77; 77Se NMR (76 MHz, CDCl3, δ, ppm, Me2Se): 809.3. Anal. Calc. for 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) (C11H10OSe): C, 55.71; H, 4.25%. Found: C, 55.88; H, 4.18%.
(O) (C11H10OSe): C, 55.71; H, 4.25%. Found: C, 55.88; H, 4.18%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) were grown by slow evaporation of methylene dichloride-hexane solutions at room temperature. A crystal of 1
(OO) were grown by slow evaporation of methylene dichloride-hexane solutions at room temperature. A crystal of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) was measured on a Rigaku AFC5R diffractometer with graphite monochromated Mo-Kα radiation source (λ = 0.71069 Å) and a rotating anode generator at 298(2) K. That of 1
(LO) was measured on a Rigaku AFC5R diffractometer with graphite monochromated Mo-Kα radiation source (λ = 0.71069 Å) and a rotating anode generator at 298(2) K. That of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) was measured on a Rigaku/MSC Mercury CCD diffractometer equipped with a graphite-monochromated Mo-Kα radiation source (λ = 0.71070 Å) at 103(2) K. The structures of 1
(OO) was measured on a Rigaku/MSC Mercury CCD diffractometer equipped with a graphite-monochromated Mo-Kα radiation source (λ = 0.71070 Å) at 103(2) K. The structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) were solved by direct method (SHELXS-97)41 and refined by full-matrix least-square method on F2 for all reflections (SHELXL-97).42 All the non-hydrogen atoms were refined anisotropically.
(OO) were solved by direct method (SHELXS-97)41 and refined by full-matrix least-square method on F2 for all reflections (SHELXL-97).42 All the non-hydrogen atoms were refined anisotropically.QC calculations
QC calculations are performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), as the models of n
(OO), as the models of n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and n
(LO) and n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) (n = 1, 3 and 4), respectively, employing the 6-311+G(d) basis sets of the Gaussian 98 program.27 Calculations are performed at the density functional theory (DFT) level of the Becke three parameter hybrid functionals combined with the Lee-Yang-Parr correlation functional (B3LYP).28,29 QC calculations are also performed on 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O (O), OH+ (OH+) and O2H2 (OH·OH)], employing the B3LYP/6-311+G(d) method. The NBO19,20 analysis were performed with the B3LYP/6-311+G(d) method. The AIM21,22 analysis are performed on 1
(OO) (n = 1, 3 and 4), respectively, employing the 6-311+G(d) basis sets of the Gaussian 98 program.27 Calculations are performed at the density functional theory (DFT) level of the Becke three parameter hybrid functionals combined with the Lee-Yang-Parr correlation functional (B3LYP).28,29 QC calculations are also performed on 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O (O), OH+ (OH+) and O2H2 (OH·OH)], employing the B3LYP/6-311+G(d) method. The NBO19,20 analysis were performed with the B3LYP/6-311+G(d) method. The AIM21,22 analysis are performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) with the Gaussian 03 program employing the 6-311+G(3df) basis sets for Se with the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. They are analyzed employing the AIM 2000 program.21,22 NBO analysis are also performed on 1
(OO) with the Gaussian 03 program employing the 6-311+G(3df) basis sets for Se with the 6-311+G(3d,2p) basis sets for C and H at the B3LYP level. They are analyzed employing the AIM 2000 program.21,22 NBO analysis are also performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) with the same method for the AIM analysis. Optimized structures and the molecular orbitals are drawn using MolStudio R3.2 (Rev 1.0).43
(OO) with the same method for the AIM analysis. Optimized structures and the molecular orbitals are drawn using MolStudio R3.2 (Rev 1.0).43Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (Nos. 16550038, 19550041 and 20550042) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.References
- (a) The Chemistry of Organic Selenium and Tellurium Compounds, eds. S. Patai and Z. Rappoport, John-Wiley and Sons, New York, 1986, vol. 1 Search PubMed; (b) The Chemistry of Organic Selenium and Tellurium Compounds, ed. S. Patai, John-Wiley and Sons, New York, 1986, vol. 2 Search PubMed; (c) Organoselenium Chemistry, A Practical Approach, ed. T. G. Back, Oxford University Press, Oxford, 1999. See also refs cited therein Search PubMed.
- T. Shimizu and N. Kamigata, Synthesis and Stereochemistry of Optically Active Chalcogen Compounds, in Handbook of Chalcogen Chemistry New Perspectives in Sulfur Selenium and Tellurium, ed. F. A. Devillanova, Royal Society of Chemistry, Cambridge, 2006, ch. 10.1 Search PubMed.
- (a) P. Nagy, A. Csampai, D. Szabo, J. Varga, V. Harmat, F. Ruff and A. Kucsman, J. Chem. Soc., Perkin Trans. 2, 2001, 339–349 RSC; (b) C. Reichardt, H.-P. Erfurt, K. Harms and G. Schafer, Eur. J. Org. Chem., 2002, 439–452 CrossRef CAS.
- (a) T. Shimizu, M. Enomoto, H. Taka and N. Kamigata, J. Org. Chem., 1999, 64, 8242–8247 CrossRef CAS; (b) H. Taka, A. Matsumoto, T. Shimizu and N. Kamigata, Chem. Lett., 2000, 726–727 CrossRef CAS; (c) H. Taka, Y. Yamazaki, T. Shimizu and N. Kamigata, J. Org. Chem., 2000, 65, 2127–2133 CrossRef; (d) H. Taka, A. Matsumoto, T. Shimizu and N. Kamigata, Heteroat. Chem., 2001, 12, 227–237 CrossRef CAS; (e) T. Soma, T. Shimizu, K. Hirabayashi and N. Kamigata, Heteroat. Chem., 2007, 18, 301–311 CrossRef CAS; (f) T. Soma, N. Kamigata, K. Hirabayashi and T. Shimizu, Bull. Chem. Soc. Jpn., 2007, 80, 2389–2394 CrossRef CAS . For the N⋯Si interactions at naphthalene 1,8-positions, see: P. M. Dominiak, G. P. Schiemenz and K. Woz’niak, Pol. J. Chem., 2007, 81, 663–681 Search PubMed.
- Organic Sulfur Chemistry: Theoretical and Experimental Advances, eds. F. Bernardi, I. G. Csizmadia and A. Mangini, Elsevier, Amsterdam, 1985 Search PubMed.
- (a) S. Nakamura, H. Yasuda and T. Toru, Tetrahedron: Asymmetry, 2002, 13, 1509–1518 CrossRef CAS; (b) S. Nakamura, H. Yasuda, Y. Watanabe and T. Toru, J. Org. Chem., 2000, 65, 8640–8650 CrossRef CAS; (c) S. Nakamura, H. Yasuda, Y. Watanabe and T. Toru, Tetrahedron Lett., 2000, 41, 4157–4160 CrossRef CAS.
- (a) J. Drabowicz, Hypervalent Sulfuranes as Transient and Isolable Structures: Occurrence, Synthesis, and Reactivity in Chemistry of Hypervalent Compounds, ed. K.-y. Akiba, Wiley-VCH, New York, 1999, ch. 7 Search PubMed; (b) J. Drabowicz, Heteroat. Chem., 2002, 5, 437–442 CrossRef.
- (a) W. Nakanishi, Chem. Lett., 1993, 2121–2122 CAS; (b) W. Nakanishi, S. Hayashi and S. Toyota, Chem. Commun., 1996, 371–372 RSC; (c) W. Nakanishi, S. Hayashi and H. Yamaguchi, Chem. Lett., 1996, 947–948 CAS; (d) W. Nakanishi, S. Hayashi, A. Sakaue, G. Ono and Y. Kawada, J. Am. Chem. Soc., 1998, 120, 3635–3640 CrossRef CAS; (e) W. Nakanishi, S. Hayashi and S. Toyota, J. Org. Chem., 1998, 63, 8790–8800 CrossRef CAS; (f) S. Hayashi and W. Nakanishi, J. Org. Chem., 1999, 64, 6688–6696 CrossRef CAS; (g) W. Nakanishi, S. Hayashi and T. Uehara, J. Phys. Chem. A, 1999, 103, 9906–9912 CrossRef CAS; (h) W. Nakanishi and S. Hayashi, J. Org. Chem., 2002, 67, 38–48 CrossRef CAS.
- (a) G. Gafner and F. H. Herbstein, Acta. Crystallogr., 1962, 15, 1081–1092 CrossRef CAS; (b) M. A. Davydova and Yu. T. Struchkov, Zh. Strukt. Khim., 1962, 3, 184 Search PubMed; (c) M. A. Davydova and Yu. T. Struchkov, Zh. Strukt. Khim., 1968, 9, 1968 Search PubMed; (d) H. Bock, M. Sievert and Z. Havlas, Chem. Eur. J., 1998, 4, 677–685 CrossRef CAS; (e) R. D. Jackson, S. James, A. G. Orpen and P. G. Pringle, J. Organomet. Chem., 1993, 458, C3–C4 CrossRef CAS; G. Mugesh and H. B. Singh, Acc. Chem. Rev., 2002, 35, 226–236 Search PubMed.
- (a) R. S. Glass, S. W. Andruski, J. L. Broeker, H. Firouzabadi, L. K. Steffen and G. S. Wilson, J. Am. Chem. Soc., 1989, 111, 4036–4045 CrossRef CAS; (b) R. S. Glass, L. Adamowicz and J. L. Broeker, J. Am. Chem. Soc., 1991, 113, 1065–1072 CrossRef CAS; (c) F. B. Mallory, C. W. Mallory, K. B. Butler, M. B. Lewis, A. Q. Xia, E. D. Luzik, Jr, L. E. Fredenburgh, M. M. Ramanjulu, Q. N. Van, M. M. Francl, D. A. Freed, C. C. Wray, C. Hann, M. Nerz-Stormes, P. J. Carroll and L. E. Chirlian, J. Am. Chem. Soc., 2000, 122, 4108–4116 CrossRef CAS.
- (a) A. Singh and B. Ganguly, J. Phys. Chem. A, 2007, 111, 6468–6471 CrossRef CAS; (b) N. Chanda and P. R. Sharp, Organometallics, 2007, 26, 3368–3373 CrossRef CAS; (c) R. Panisch, M. Bolte and T. Muller, Organometallics, 2007, 26, 3524–3529 CrossRef CAS; (d) A. F. Pozharskii, A. V. Degtyarev, O. V. Ryabtsova, V. A. Ozeryanskii, M. E. Kletskii, Z. A. Starikova, L. Sobczyk and A. Filarowski, J. Org. Chem., 2007, 72, 3006–3019 CrossRef CAS.
- (a) G. C. Pimentel, J. Chem. Phys., 1951, 19, 446–448 CrossRef CAS; J. I. Musher, Angew. Chem., Int. Ed. Engl., 1969, 8, 54–68 CrossRef CAS; R. J. Hatch and R. E. Rundle, J. Am. Chem. Soc., 1951, 73, 4321–4324 CrossRef CAS; (b) R. E. Rundle, J. Am. Chem. Soc., 1963, 85, 112–113 CrossRef CAS.
- (a) R. A. Hayes and J. C. Martin, Sulfurane Chemistry, in Organic Sulfur Chemistry: Theoretical and Experimental Advances, eds. F. Bernardi, I. G. Csizmadia and A. Mangini, Elsevier Scientific, Amsterdam, 1985, ch. 8 Search PubMed; (b) J. Bergman, L. Engman and J. Siden, Tetra- and Higher-Valent (Hypervalent) Derivatives of Selenium and Tellurium, in The Chemistry of Organic Selenium and Tellurium Compounds, eds. S. Patai and Z. Rappoport, John Wiley & Sons, New York, 1986, vol. 1, ch. 14 Search PubMed; (c) Chemistry of Hypervalent Compounds, ed. K.-y. Akiba, Wiley-VCH, New York, 1999 Search PubMed; (d) W. Nakanishi, Hypervalent Chalcogen Compounds, in Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, ed. F. A. Devillanova, Royal Society of Chemistry, London, 2006, ch. 10.3 Search PubMed.
- For the 3c–4e treatment of the CT complexes, see: W. Nakanishi, S. Hayashi and H. Kihara, J. Org. Chem., 1999, 64, 2630–2637 Search PubMed.
- For the weak interactions, see, (a) Molecular Interactions. From van der Waals to Strongly Bound Complexes, ed. S. Scheiner, Wiley, New York, 1997 Search PubMed; (b) K. D. Asmus, Acc. Chem. Res., 1979, 12, 436–442 CrossRef CAS; (c) W. K. Musker, Acc. Chem. Res., 1980, 13, 200–206 CrossRef CAS.
- S. Hayashi, H. Wada, T. Ueno and W. Nakanishi, J. Org. Chem., 2006, 71, 5574–5585 CrossRef CAS.
- W. Nakanishi, S. Hayashi and T. Uehara, Eur. J. Org. Chem., 2001, 3933–3943 CrossRef CAS.
- The structure is A if the Se–CAr bond is placed almost perpendicular to the naphthyl plane, it is B when the bond is located on the plane and C is the intermediate between A and B.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS; J. E. Carpenter and F. Weinhold, J. Mol. Struct. (THEOCHEM), 1988, 169, 41–62 CrossRef.
- E. D. Glendening, A. E. Reed, J. E. Carpenter and F. Weinhold, NBO Ver. 3.1.
- Atoms in Molecules. A Quantum Theory, ed. R. F. W. Bader, Oxford University Press, Oxford, 1990 Search PubMed; The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design, eds. C. F. Matta and R. J. Boyd, Wiley-VCH, Weinheim, 2007, ch. 1 Search PubMed.
- (a) R. F. W. Bader, T. S. Slee, D. Cremer and E. Kraka, J. Am. Chem. Soc., 1983, 105, 5061–5068 CrossRef CAS; (b) R. F. W. Bader, Chem. Rev., 1991, 91, 893–926 CrossRef CAS; (c) R. F. W. Bader, J. Phys. Chem. A, 1998, 102, 7314–7323 CrossRef CAS; (d) F. Biegler-König, R. F. W. Bader and T. H. Tang, J. Comput. Chem., 1982, 3, 317–328 CrossRef; (e) R. F. W. Bader, Acc. Chem. Res., 1985, 18, 9–15 CrossRef CAS; (f) T. H. Tang, R. F. W. Bader and P. MacDougall, Inorg. Chem., 1985, 24, 2047–2053 CrossRef CAS; (g) F. Biegler-König, J. Schönbohm and D. Bayles, J. Comput. Chem., 2001, 22, 545–559 CrossRef; (h) F. Biegler-König and J. Schönbohm, J. Comput. Chem., 2002, 23, 1489–1494 CrossRef CAS . See also: W. Nakanishi, T. Nakamoto, S. Hayashi, T. Sasamori and N. Tokitoh, Chem. Eur. J., 2007, 13, 255–268 Search PubMed.
- J. Meinwald, D. Dauplaise and J. Clardy, J. Am. Chem. Soc., 1977, 99, 7743–7744 CrossRef CAS.
- The structures of 3b
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 3a
(LO) and 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are also determined by the X-ray crystallographic analysis. The results are essentially the same as those of 1
(OO) are also determined by the X-ray crystallographic analysis. The results are essentially the same as those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) and 1
(LO) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), respectively, which will be reported elsewhere.
(OO), respectively, which will be reported elsewhere. - Water molecules in 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) are omitted for clarity.
(OO) are omitted for clarity. - S. Hayashi and W. Nakanishi, Bull. Chem. Soc. Jpn., 2008, 12 Search PubMed , in press (CCDC 640537 for 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LL)).
(LL)). - A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, P. Salvador, J. J. Dannenberg, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle and J. A. Pople, GAUSSIAN 98 (Revision A.11), Gaussian, Inc., Pittsburgh, PA, 2001 Search PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785–789 CrossRef CAS; B. Miehlich, A. Savin, H. Stoll and H. Preuss, Chem. Phys. Lett., 1989, 157, 200–2006 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS; A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- S. Hayashi and W. Nakanishi, J. Mol. Struct. (THEOCHEM), 2007, 811, 293–301 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03 (Revision B.05), Gaussian, Inc., Pittsburgh, PA, 2003 Search PubMed.
- The AIM2000 program (Version 2.0) is employed to analyze and visualize atoms in molecules: F. J. Biegler-König, Comput. Chem., 2000, 21, 1040–1048 Search PubMed ; see also ref. 22g.
- Data for A and B, together with LL, are given in the ESI†.
- The type C of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH) is discussed which is predicted to be most stable
among the three44.
(OH·OH) is discussed which is predicted to be most stable
among the three44. - Eqn (R1) shows the energies of n
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2 (E(n
(L) + H2O2 (E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2)) relative to E(n
(L) + H2O2)) relative to E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O) [ΔE(n
(O) + H2O) [ΔE(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)) = E(n
(LO)) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2) −E(n
(L) + H2O2) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)], although E(n
(O) + H2O)], although E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L)) are not given in Table 3.45
(L)) are not given in Table 3.45
.ΔE(n ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)) = E(n
(LO)) = E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (L) + H2O2) −E(n
(L) + H2O2) −E(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O) + H2O)
(O) + H2O) G = H (121.8 kJ mol−1) < F (131.3) < cis-MeSe (133.9) < Cl (136.8) ≤ Br (137.6) < trans-MeSe (141.0)
(R1) - NOB analysis were also performed on the AC conformer of 8-G-1-[MeSe(O2H2)]C10H6. However, the corresponding CT interactions were not detected.
- The nonbonded Se⋯Se distance in 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO) is predicted to be shorter than that of 1
(LO) is predicted to be shorter than that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO) by ca. 0.03 Å, while the observed values are almost equal (see Table 5). The crystal packing effect might contribute to the results.
(OO) by ca. 0.03 Å, while the observed values are almost equal (see Table 5). The crystal packing effect might contribute to the results. - The value is very close to that evaluated with the B3LYP/6-311+G(d) method.
- W. Nakanishi, S. Hayashi and K. Narahara, unpublished results.
- G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, Universität Göttingen, Germany, 1997.
- G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
- MolStudio R3.2 (Rev 1.0), NEC Corporation, 1997–2003.
- Three structures (type A, type B and type C) were optimized for each of n
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH). The type C is the global minimum, which is slightly stable than type B and much stable than type A, although the steric repulsion between OH and G seems largest.
(OH·OH). The type C is the global minimum, which is slightly stable than type B and much stable than type A, although the steric repulsion between OH and G seems largest. - Eqn (R1)36 shows that selenoxides are stabilized in this order through the non-bonded n(G)⋯σ*(Se–O) 3c–4e interactions, together with the O dependence.16 While G = trans-MeSe is demonstrated to be most effective to stabilize in the selenoxide relative to the corresponding bis-selenide, the effect of G = cis-MeSe places between F and Cl, where the CC form is postulated for the bis-selenide.
Footnote |
† Electronic supplementary information (ESI) available: Energies and relative energies for 8-G-1-[MeSe(X)]C10H6 [G = MeSe (1), H (5), F (6), Cl (7) and Br (8) with X = lone pair (L), O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O), OH+ (OH+) and O2H2 (O), OH+ (OH+) and O2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)], the packing structures of 1 (OH·OH)], the packing structures of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), counter map for 1 (OO), counter map for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO), Cartesian coordinates for optimized structures of 1 and 5–8 with X = lone pair (L), O (OO), Cartesian coordinates for optimized structures of 1 and 5–8 with X = lone pair (L), O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (O), OH+ (OH+) and O2H2 (O), OH+ (OH+) and O2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OH·OH)]. CCDC reference numbers 688690 (1 (OH·OH)]. CCDC reference numbers 688690 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (LO)) and 688691 (1 (LO)) and 688691 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (OO)). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b809763a (OO)). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b809763a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2009 |
