Pseudo-halide derivatives of titanoceneY: synthesis and cytotoxicity studies†
James
Claffey
,
Anthony
Deally
,
Brendan
Gleeson
,
Megan
Hogan
,
Luis Miguel Menéndez
Méndez
,
Helge
Müller-Bunz
,
Siddappa
Patil
,
Denise
Wallis
and
Matthias
Tacke
*
Conway Institute of Biomolecular and Biomedical Research, The UCD School of Chemistry and Chemical Biology, Centre for Synthesis and Chemical Biology (CSCB), University College Dublin, Belfield, Dublin 4, Ireland. E-mail: matthias.tacke@ucd.ie
First published on 2nd September 2009
Abstract
The well-known anticancer drug candidate bis-[(p-methoxybenzyl)cyclopentadienyl] titanium(IV) dichloride (TitanoceneY) was reacted with sodium azide or potassium cyanate, thiocyanate or selenocyanate in order to give pseudo-halide analogues 2a–d of TitanoceneY. 2b and 2c were characterised by single crystal X-ray diffraction, which confirmed the expected nitrogen binding of the cyanate and thiocyanate to the titanium centre. All four titanocenes had their cytotoxicity investigated through preliminary in vitro testing on the LLC-PK (pig kidney epithelial) cell line in an MTT based assay in order to determine their IC50 values. Titanocenes2a–d were found to have IC50 values of 24 (±8) μM, 101 (±14) μM, 54 (±21) μM and 27 (±4) μM respectively. All four titanocene derivatives show significant cytotoxicity improvement when compared to unsubstituted titanocene dichloride and 2a and 2d showed similiar cytotoxic behaviour to TitanoceneYin vitro.
1. Introduction
The serendipitous discovery of the anticancer activity of cis-diaminedichloroplatinum(II) (cis-platin) in 1967 by Rosenberg and co-workers1 was to lead to the first use of a transition metal complex as a chemotherapeutic reagent. Following the success of cis-platin and its second generation analogue carboplatin in the clinic, the search to find other transition metal based complexes that show promising anticancer activity began. Transition metal complexes of gold, iron, ruthenium, tin, vanadium, molybdenum and titanium have shown some promising antitumour activity during in vitro and in vivo testing. Titanium and ruthenium are, as of yet, the only transition metals to enter the clinic apart from platinum. Budotitane ([cis-diethoxybis(1-phenylbutane-1,3-dionato)titanium(IV)]) (Fig. 1) reached Phase I clinical trials2 following a promising early preclinical evaluation but did not progress any further despite the development of a Cremophor EL® based formulation for it. Titanocene dichloride is the only metallocene dichloride so far which has reached clinical trials. Cp2TiCl2 shows medium anti-proliferative activity in vitro and promising results in vivo3,4 but its efficacy in Phase II clinical trials in patients with metastatic renal cell carcinoma5 or metastatic breast cancer6 was too low to be pursued.Titanocene dichlorides as anticancer reagents got a renewed interest when McGowan et al. synthesised ring-substituted cationic titanocene dichloride derivatives, which are water-soluble and show significant activity against ovarian cancer.7
Through the use of Super Hydride, 6-anisyl fulvene can undergo selective hydridolithiation to yield an isolable lithium cyclopentadienide. Following a transmetallation reaction of this lithium cyclopentadienide with titanium tetrachloride the promising anticancer compound bis-[(p-methoxybenzyl)cyclopentadienyl] titanium(IV) dichloride (TitanoceneY)8 can be isolated.
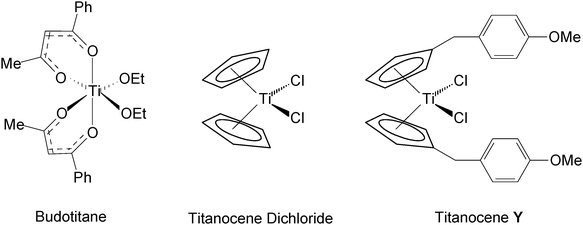 | ||
| Fig. 1 Structures of Budotitane, Titanocene dichloride and TitanoceneY. | ||
Titanocene Y has an IC50 value of 21 μM when tested on the long life epithelial pig kidney cell line LLC-PK. TitanoceneY has had its anti-proliferative activity studied in 36 human tumor cell lines and also against explanted human tumors.9 These in vitro and ex vivo experiments showed that renal cell cancer is the prime target for this compound, but it also has activity against ovary, prostate, cervix, lung, colon, and breast cancer. Titanocenes have also been shown to give a positive immune response by up-regulating the number of natural killer (NK) cells in mice.9 Animal studies reported the successful treatment of mice bearing xenografted Caki-1, MCF-79 and A43110 tumors with TitanoceneY where reduction of tumor size was seen.
For titanocene dichloride, variation of the chloride ligand using halide, dicarboxylate and pseudo-halides has been extensively investigated. From this work it appears that the nature of the ligand does not appear to have a significant affect on the cytotoxic properties of the unsubstituted titanocene.11–13 The use of Ti(C5H5)2(NCS)2 on Ehrlich’s ascites tumours in mice has also been reported14 with significant anti-tumour effect observed. The use of thiocyanate and, in particular, selenocyanate ligands on vanadocenes has been shown to assist in their cytotoxicity against human testicular cancer cell lines Tera-2 and Ntera-2.15
Recently an oxalate derivative of TitanoceneY (Oxali-TitanoceneY) has been reported as having an IC50 of 1.6 μM on the LLC-PK cell line .16 This was also shown to have good anti-angiogenic properties in HUVEC anti-angiogenesis tests and, in a mouse model, was shown to be cytostatic on xenografted Caki-1.17
Within this paper we present the synthesis of four pseudo-halide derivatives of TitanoceneY and the results of preliminary in vitro biological testing.
2. Experimental
2.1 General conditions
Potassium selenocyanate, potassium thiocyanate and potassium cyanate were obtained commercially from Aldrich Chemical Co. Sodium azide was obtained from Fluka Chemical Co. THF was dried over Na–benzophenone and it was freshly distilled and collected under an atmosphere of nitrogen prior to use. Manipulations of air and moisture sensitive compounds were done using standard Schlenk techniques, under a nitrogen atmosphere. NMR spectra were measured on a Varian 400 MHz spectrometer. Chemical shifts are reported in ppm and are referenced to TMS. IR spectra were recorded on a Perkin Elmer Paragon 1000 FT-IR Spectrometer employing a KBr disk. UV/Vis spectra were recorded on a Unicam UV4 Spectrometer, while CHN analysis was done with an Exeter Analytical CE-440 Elemental Analyser, while Cl was determined in mercurimetric titrations. X-Ray diffraction data for the compounds 2b and 2c were collected using a Bruker SMART APEX CCD area detectordiffractometer. A full sphere of reciprocal space was scanned by phi-omega scans. Pseudo-empirical absorption correction based on redundant reflections was performed by the program SADABS.18 The structures were solved by direct methods using SHELXS-9719 and refined by full matrix least-squares on F2 for all data using SHELXL-97.19 All hydrogen atoms were located in the difference Fourier map and allowed to refine freely. In 2b the C–H bond lengths were restrained to be their default values (0.95 Å for aromatic C–H, 0.98 Å for methyl groups and 0.99 Å for CH2 groups) using DFIX. Anisotropic thermal displacement parameters were used for all non-hydrogen atoms. Further details about the data collection are listed in Table 1, as well as reliability factors. Suitable crystals of 2b and 2c for X-ray diffraction were grown in saturated trichloromethane solutions with slow infusion of pentane.| Identification code | 2b | 2c |
|---|---|---|
| a The C–H bond lengths were restrained to be their idealised values (0.95 Å for aromatic, 0.98 Å for methyl and 0.99 Å for methylene hydrogens). | ||
| Empirical formula | C28 H26 N2 O4 Ti | C28 H26 N2 O2 S2 Ti |
| Formula weight | 502.41 | 534.53 |
| Temperature | 100(2) K | 170(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2 (#5) | C2/c (#15) |
| Unit cell dimensions | a = 25.394(2) Å | a = 14.6085(10) Å |
| α = 90°. | α = 90°. | |
| b = 7.3361(7) Å | b = 7.9381(6) Å | |
| β = 98.110(2)°. | β = 98.048(1)°. | |
| c = 6.3130(6) Å | c = 21.7937(15) Å | |
| γ = 90°. | γ = 90°. | |
| Volume | 1164.32(19) Å3 | 2502.4(3) Å3 |
| Z | 2 | 4 |
| Density (calculated) | 1.433 Mg/m3 | 1.419 Mg/m3 |
| Absorption coefficient | 0.407 mm−1 | 0.538 mm−1 |
| F(000) | 524 | 1112 |
| Crystal size | 0.60 × 0.20 × 0.03 mm3 | 0.60 × 0.50 × 0.10 mm3 |
| Theta range for data collection | 1.62 to 30.51°. | 1.89 to 28.00°. |
| Index ranges | −34< = h < = 34, | −19< = h < = 18, |
| −10< = k < = 10, | −10< = k < = 10, | |
| −8< = l < = 9 | −28< = l < = 28 | |
| Reflections collected | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 207 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 083 |
| Independent reflections | 3346 [R(int) = 0.0294] | 3009 [R(int) = 0.0219] |
| Completeness to thetamax | 96.7% | 99.9% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.9879 and 0.8670 | 0.9482 and 0.7687 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3346/14/211a | 3009/0/211 |
| Goodness-of-fit on F2 | 1.083 | 1.049 |
| Final R indices [I>2sigma(I)] | R1 = 0.0363, wR2 = 0.0832 | R1 = 0.0351, wR2 = 0.0883 |
| R indices (all data) | R1 = 0.0376, wR2 = 0.0838 | R1 = 0.0393, wR2 = 0.0914 |
| Largest diff. peak and hole | 0.442 and −0.228 e Å−3 | 0.383 and −0.149 e Å−3 |
| Absolute structure parameter | 0.048(19) | — |
2.2 Synthesis
Titanocene Y was synthesised according to the known literature method.71H NMR (CDCl3, 400 MHz,): δ 3.65 [s, 4H, C5H4–CH2], 3.78 [s, 6H, C6H4–(OCH3)], 6.14 [m, 8H, C5H4], 6.84 [dd, 4H, J 8.6 Hz, 2.5 Hz, C6H4–(OCH3)], 7.10 ppm [dd, 4H, J 8.6 Hz, 2.5 Hz, C6H4–(OCH3)].
13C NMR (CDCl3, 100 MHz): δ 33.9, 54.2, 112.8, 112.9, 113.0,113.5, 115.2, 128.6, 128.7, 130.3, 133.0, 157.0.
IR (KBr, cm−1): ν 2962, 2923, 2083, 2060, 1610, 1512, 1385, 1262, 1096, 1026, 803.
UV/Vis (CHCl3, nm): 246 (ε 5470), 278 (ε 4990), λmax 332 (ε 1790).
Elemental analysis calculated (%) for TiC26O2H26N6: C, 62.2%; H, 5.2%; Cl, 0.0%; Found: C, 61.6%; H, 4.9%; Cl, 0.2%.
1H NMR (CDCl3, 400 MHz,): δ 3.78 [s, 6H, C6H4–(OCH3)], 3.84 [s, 4H, C5H4–CH2], 6.19 [m, 8H, C5H4], 6.84 [d, 4H, J 8.7 Hz, C6H4–(OCH3)], 7.08 ppm [d, 4H, J 8.4 Hz, C6H4–(OCH3)].
13C NMR (CDCl3, 100 MHz): δ 34.5, 54.3, 112.7, 112.9, 113.1, 113.5, 118.4, 128.8, 129.8, 136.9, 157.4.
IR (KBr, cm−1): ν 2962, 2927,2361, 2343, 2229, 2207, 1610, 1512, 1260, 1176, 1097, 1031, 803.
UV/Vis (CHCl3, nm): 261 (ε 7130), 277 (ε 6900), 298 (ε 4500), 343 (ε 2100), λmax 382 (ε 1310).
Elemental analysis calculated (%) for TiC28O4H26N2: C, 66.9%; H, 5.2%; Cl, 0.0%; Found: C, 66.0%; H, 5.3%; Cl, 0.0%.
1H NMR (CDCl3, 400 MHz, ): δ 3.79 [s, 6H, C6H4–(OCH3)], 3.89 [s, 4H, C5H4–CH2], 6.25 [s, 8H, C5H4], 6.86 [d, 4H, J 8.2 Hz, C6H4–(OCH3)], 7.11 ppm [d, 4H, J 7.6 Hz, C6H4–(OCH3)].
13C NMR (CDCl3, 100 MHz): δ 35.3, 55.3, 114.2, 115.3, 119.9, 130.0, 130.4, 139.1, 158.5.
IR (KBr, cm−1): ν 2929, 2051, 1700, 1653, 1558, 1506, 1246, 1032, 817, 668.
UV/Vis (CHCl3, nm): 247 (ε 5850), 278 (ε 5540),350 (ε 3030), λmax 446 (ε 2340).
Elemental analysis calculated (%) for TiC28O2H26N2 S2: C, 62.9%; H, 4.9%; Cl, 0.0%; Found: C, 62.7%; H, 4.4%; Cl, 0.4%.
1H NMR (CDCl3, 400 MHz, ): δ 3.79 [s, 6H, C6H4–(OCH3)], 3.91 [s, 4H, C5H4–CH2], 6.28 [s, 8H, C5H4], 6.86 [d, 4H, J 8.2 Hz, C6H4–(OCH3)], 7.12 ppm [d, 4H, J 8.2 Hz, C6H4–(OCH3)].
13C NMR (CDCl3, 100 MHz): δ 34.4, 54.3, 113.2, 114.8, 119.2, 129.0, 129.2, 138.6, 152.2, 157.6.
IR (KBr, cm−1): ν 2961, 2071, 2005, 1610, 1511, 1261, 1092, 1029, 804.
UV/Vis (CHCl3, nm): 243 (ε 4000), 275 (ε 3810), 413 (ε 640), λmax 494 (ε 590).
Elemental analysis calculated (%) for TiC28O2H26N2Se2: C, 53.5%; H, 4.2%; Cl, 0.0%; Found: C, 52.8%; H, 4.7%; Cl, 0.0%.
2.3 Cytotoxicity studies
Preliminary in vitro cell tests were performed on the cell line LLC-PK (long-lasting cells-pig kidney) in order to compare the cytotoxicity of the compounds presented in this paper. This cell line was chosen based on their regular and long-lasting growth behaviour, which is similar to that shown in kidney carcinoma cells. It was obtained from the ATCC (American Tissue Cell Culture Collection) and maintained in Dulbecco’s Modified Eagle Medium containing 10% (v/v) FCS (foetal calf serum), 1% (v/v) penicillinstreptomycin and 1% (v/v) L-glutamine. Cells were seeded in 96-well plates containing 200 μL microtitre wells at a density of 5000 cells/200 μL of medium and were incubated at 37 °C for 24 h to allow for exponential growth. Then the compounds used for the testing were dissolved in the minimal amount of DMSO (dimethylsulfoxide) possible and diluted with medium to obtain stock solutions of 5 × 10−4 M in concentration and less than 0.7% of DMSO. The cells were then treated with varying concentrations of the compounds and incubated for 48 h at 37 °C. Then, the solutions were removed from the wells and the cells were washed with PBS (phosphate buffer solution) and fresh medium was added to the wells. Following a recovery period of 24 h incubation at 37 °C, individual wells were treated with 200 μL of a solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) in medium. The solution consisted of 30 mg of MTT in 30 mL of medium. The cells were incubated for 3 h at 37 °C. The medium was then removed and the purple formazan crystals were dissolved in 200 μL DMSO per well. Absorbance was then measured at 540 nm by a Wallac Victor (Multilabel HTS Counter) Plate Reader. Cell viability was expressed as a percentage of the absorbance recorded for control wells. The values used for the dose response curves represent the values obtained from four consistent MTT-based assays for each compound tested. Titanocenes2a–d were found to have IC50 values of 24 (±8), 101 (±14), 54 (±21) and 27 (±4) μM respectively.3. Results and discussion
3.1 Synthesis
Titanocene Y was synthesised according to the known literature method by the hydridolithiation of 6-(p-methoxyphenyl)fulvene to give an isolable lithium cyclopentadienide intermediate which can then be transmetallated to TiCl4 (Scheme 1).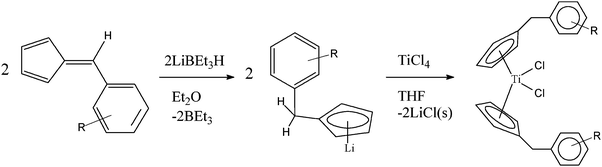 | ||
| Scheme 1 Synthesis of benzyl substituted titanocenes from fulvenes using the hydridolithiation reaction. | ||
Titanocene Y was then used in an anion exchange reaction in THF. Previously it had been seen in the literature that a relatively short reflux time was required for the exchange of the chloride ligand with cyanate and thiocyanate with ansa-substituted titanocenes.20 This procedure did not result in the desired product when using TitanoeneY. Prolonged reaction times whilst stirring at room temperature were necessary to synthesise the pseudo-halide substituted titanocenes and the insoluble sodium or potassium chloride. Titanocenes2a–2d were isolated in yields of 29–87% (Scheme 2).
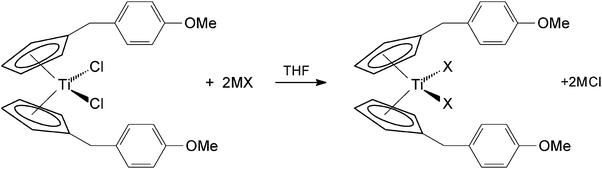 | ||
| Scheme 2 Synthesis of pseudo-halide substituted titanocenes. | ||
3.2 Structural discussion
Suitable crystals for X-ray crystallography to determine the molecular structure of 2b and 2c were grown from saturated trichloromethane solutions with slow infusion of pentane. 2b crystallised with 2 molecules in the unit cell, whilst 2c crystallised with 4 molecules in the unit cell. In both structures the titanium atom is placed on a twofold axis. Despite the addition of two cyanates and thiocyanates in replacement of the two chlorines on the titanium centre there is almost no apparent change in the molecular structures of 2b and 2c in comparision to TitanoceneY.The length of the bonds between the titanium centre and the carbon atoms of the cyclopentadienide rings are very similar for both TitanoceneY and 2b and 2c. They vary from 2.336(3) Å to 2.413(3) Å for TitanoceneY, from 2.353(2) Å to 2.403(2) Å for 2b and from 2.316(2) Å to 2.463(2) Å for 2c. The titanium–centroid distances are highly comparable for 2b (2.052(1) Å) and 2c (2.058(1) Å) in comparison to TitanoceneY (2.061(1) Å and 2.063(1) Å). The centroid–titanium–centroid bond angle is 133.77(1)° for both 2b and 2c, which compares to 130.68(2)° for the corresponding angle in TitanoceneY. The widening of the centroid–titanium–centroid bond angle is facilitated by the smaller Van der Waals radius of the nitrogen atom of the pseudo-halide in comparison to the larger chloride anions. The titanium–nitrogen bond lengths for 2b and 2c are very similar: 2.015(2) Å (2b) and 2.019(1) Å (2c), whilst the nitrogen–titanium–nitrogen bond angles are 93.0(1)° and 91.00(7)° for 2b and 2c, respectively. The titanium–nitrogen–carbon bond angle is bent slightly out of linear geometry for 2b (166.7(2)°) and 2c (170.7(1)°). However, the nitrogen–carbon–oxygen bond angle remains linear in 2b (179.6(2)°) and the same is seen for the nitrogen–carbon–sulfur bond angle in 2c (179.1(2)°) (Fig. 2 and 3Tables 1 and 2).
![X-Ray diffraction structure of bis-[(4-methoxybenzyl)cyclopentadienyl] titanium(iv) di-iso-cyanate2b (thermal ellipsoids are drawn on the 50% probability level); symmetry operations: I 1 − x, y, −z.](/image/article/2009/MT/b911753a/b911753a-f2.gif) | ||
| Fig. 2 X-Ray diffraction structure of bis-[(4-methoxybenzyl)cyclopentadienyl] titanium(IV) di-iso-cyanate2b (thermal ellipsoids are drawn on the 50% probability level); symmetry operations: I 1 − x, y, −z. | ||
![X-Ray diffraction structure of bis-[(4-methoxy-benzyl)cyclopentadienyl]titanium(iv) di-iso-thiocyanate2c (thermal ellipsoids are drawn on the 30% probability level); symmetry operations: I − x, y, ½ − z.](/image/article/2009/MT/b911753a/b911753a-f3.gif) | ||
| Fig. 3 X-Ray diffraction structure of bis-[(4-methoxy-benzyl)cyclopentadienyl]titanium(IV) di-iso-thiocyanate2c (thermal ellipsoids are drawn on the 30% probability level); symmetry operations: I − x, y, ½ − z. | ||
| Identification code | 2b | 2c |
|---|---|---|
| Bond lengths (Å) | ||
| Ti–C(1) | 2.4030(15) | 2.4633(14) |
| Ti–C(2) | 2.3924(16) | 2.4027(13) |
| Ti–C(3) | 2.3528(18) | 2.3161(14) |
| Ti–C(4) | 2.3583(18) | 2.3361(14) |
| Ti–C(5) | 2.3794(18) | 2.3883(14) |
| C(1)–C(2) | 1.409(2) | 1.405(2) |
| C(2)–C(3) | 1.416(3) | 1.414(2) |
| C(3)–C(4) | 1.413(3) | 1.412(2) |
| C(4)–C(5) | 1.402(3) | 1.398(2) |
| C(5)–C(1) | 1.412(2) | 1.419(2) |
| C(1)–C(6) | 1.498(2) | 1.507(2) |
| C(6)–C(7) | 1.515(2) | 1.502(2) |
| Ti–N | 2.0149(15) | 2.0188(12) |
| Ti–N#1 | 2.0149(15) | |
| Ti–Cent | 2.052(1) | 2.058(1) |
| N–C(14) | 1.179(2) | 1.1678(18) |
| C(14)–O(2) | 1.192(2) | |
| C(14)–S | 1.6082(14) | |
| Bond angles (°) | ||
| Cent–Ti–Cent#1 | 133.77(1) | 133.78(1) |
| N–Ti–N#1 | 92.97(9) | 91.00(7) |
| N–Ti–Cent | 105.69(4) | 104.47(3) |
| N#1–Ti–Cent | 105.67(4) | 107.49(3) |
| C(14)–N–Ti | 166.70(14) | 170.72(11) |
| N–C(14)–O(2) | 179.6(2) | |
| N–C(14)–S | 179.12(14) |
Further evidence for the titanium to nitrogen bond for titanocenes2a–d can be seen by infrared spectroscopy, which agrees with the obtained crystal structures. 2a shows the antisymmetric and symmetric stretching bands of two non-linear azide groups at 2083 and 2060 cm−1 consistent with IR absorptions seen in the literature for dicyclopentadienyl titanium diazide.21 The IR spectrum of 2b features characteristic bands of iso-cyanate at 2229 and 2207 cm−1.20 Within the IR spectrum of 2c, the two stretching modes are overlapping and a broad signal can be seen at 2051 cm−1, which is consistent with the range of iso-thiocyanates (2140–1990 cm−1), whereas sulfur bound thiocyanate would give absorption bands between 2175 and 2160 cm−1. 2d features a band at 2071 cm−1 in the IR spectrum typical of a nitrogen bound iso-selenocyanate .22 This band is again broadened, which is an indication of an overlap of the two expected stretching vibrations of the iso-selenocyanate groups.
In the 1H NMR of the pseudo-halide substituted titanocenes significant shifts can be seen for the singlet representing the benzyl protons (C5H4-CH2-C6H4OCH3) in comparison to the parent titanocene dichloride; TitanoceneY, which has a resonance of 4.02 ppm8 for these benzyl protons. Also the movement of this signal also clearly reflects the electron withdrawing capabilities of the pseudo-halide ligands (N3− > NCO− > NCS− > NCSe− ). The chemical shifts are 3.65, 3.84, 3.89 and 3.91 ppm respectively for titanocenes2a–2d. In the 13C-NMR it is not possible to distinguish between the noise and signal referring to the tertiary carbon for iso-cyanate, iso-thiocyanate and iso-selenocyanate for compounds 2b–2d.
3.3 Cytotoxicity studies
The pseudo-halide exchanged titanocenes were tested on LLC-PK cells, which have been shown to be a good in vitro model for kidney cancer, in order to determine their cytotoxicity values. Respectively titanocenes2a, 2b, 2c and 2d show IC50 values of 24 (±8) μM, 101 (±14) μM, 54 (±21) μM and 27 (±4) μM. This shows a significant improvement of up to 14-fold with respect to titanocene dichloride, which has an IC50 value of 2000 μM on the LLC-PK cell line .23 In comparison to TitanoceneY, these species do not show an improvement in cytotoxicities with species 2a and 2d showing similar cytotoxic behaviour but a worsening of cytotoxicity for 2b and 2c. Cisplatin has an IC50 value of 3.3 μM on this particular cell line .8 (Fig. 4 and 5)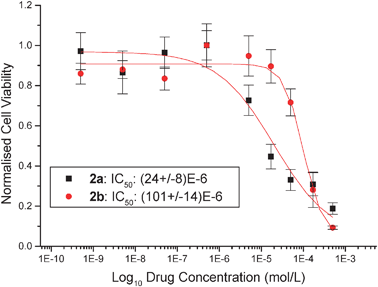 | ||
| Fig. 4 Cytotoxicity curves from typical MTTassays showing the effect of compounds 2a and 2b on the viability of LLC-PK cells. | ||
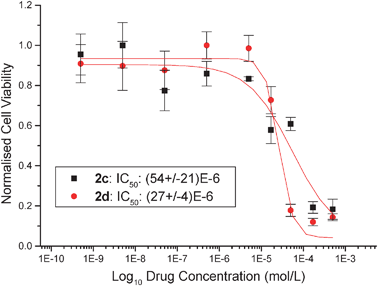 | ||
| Fig. 5 Cytotoxicity curves from typical MTTassays showing the effect of compounds 2c and 2d on the viability of LLC-PK cells. | ||
4. Conclusions and outlook
The synthesis of compounds 2a–d was significantly more difficult than expected when compared against previous literature syntheses. In this respect, reaction time and temperature are crucial for the synthesis of pure samples of the pseudo-halide substituted derivatives of TitanoceneY. In MTT-based assays , compounds 2a–d showed significant improvement in in vitro cytotoxicities against LLC-PK cells compared to unsubstituted titanocene dichloride, for which Phase I/II clinical trials have been performed. Compounds 2b and 2c showed an unexpected decrease in cytotoxic behaviour in comparison to the parent compound TitanoceneY, whereas compounds 2a and 2d have comparable in vitro cytotoxicities with respect to TitanoceneY. It is probably necessary to do some in vivo testing on compounds 2a and 2d to fully evaluate and realise their cytotoxic potential and to see whether the exchange of chlorine versus the pseudo-halide, which possibly leads slower hydrolysis, is beneficial in comparsion to TitanoceneY.Acknowledgements
The authors thank the Higher Education Authority (HEA), the Centre for Synthesis and Chemical Biology (CSCB), University College Dublin (UCD) and COST D39 for funding.References
- B. Rosenberg, L. van Camp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385–386 CrossRef CAS.
- T. Schilling, B. K. Keppler, M. E. Heim, G. Niebch, H. Dietzfelbinger, J. Rastetter and A. R. Hanauske, Invest. New Drugs, 1996, 13, 327–332 CAS.
- E. Melendez, Crit. Rev. Oncol. Hematol., 2002, 42, 309–315 CrossRef.
- F. Caruso and M. Rossi, Metal Ions in Biological Systems, 2004, 42, 353–384 CAS.
- G. Lummen, H. Sperling, H. Luboldt, T. Otto and H. Rubben, Cancer Chemother. Pharmacol., 1998, 42, 415–417 CrossRef CAS.
- N. Kröger, U. R. Kleeberg, K. Mross, L. Edler, G. Saß and D. Hossfeld, Onkologie, 2000, 23, 60–62 CrossRef.
- O. R. Allen, L. Croll, A. L. Gott, R. J. Knox and P. C. McGowan, Organometallics, 2004, 23, 288–292 CrossRef CAS.
- N. J. Sweeney, O. Mendoza, H. Müller-Bunz, C. Pampillón, F.-J. K. Rehmann, K. Strohfeldt and M. Tacke, J. Organomet. Chem., 2005, 690, 4537–4544 CrossRef CAS.
- K. Strohfeldt and M. Tacke, Chem. Soc. Rev., 2008, 37, 1174–1187 RSC.
- J. Bannon, I. Fichtner, A. O’Neill, C. Pampillón, N. J. Sweeney, K. Strohfeldt, R. W. G. Watson, M. Tacke and M. M. Mc Gee, Br. J. Cancer, 2007, 97, 1234–1241 CrossRef CAS.
- P. Köpf-Maier and H. Köpf, Struct. Bonding, 1988, 70, 103–185.
- P. Köpf-Maier, B. Hesse, R. Voigtländer and H. Köpf, J. Cancer Res. Clin. Oncol., 1980, 97, 31–39 CrossRef CAS.
- P. Köpf-Maier, T. Klapötke and H. Köpf, Inorg. Chim. Acta, 1988, 153, 119–122 CrossRef.
- M. Valadares and M. Queiroz, Eur. J. Pharmacol., 2002, 439, 35–42 CrossRef CAS.
- P. Ghosh, O. D’Cruz, R. K. Narla and F. Uckun, Clin. Cancer Res., 2000, 6, 1536–1545 CAS.
- J. Claffey, M. Hogan, H. Müller-Bunz, C. Pampillón and M. Tacke, ChemMedChem, 2008, 3, 729–731 CrossRef CAS.
- I. Fichtner, J. Claffey, B. Gleeson, M. Hogan, D. Wallis, H. Weber and M. Tacke, Lett. Drug Des. Discovery, 2008, 5, 489–493 Search PubMed.
- G. M. Sheldrick, SADABS Version 2.03, University of Göttingen, Germany, 2002 Search PubMed.
- G. M. Sheldrick, SHELXS-97 and SHELXL-97, University of Göttingen, Germany, 1997 Search PubMed.
- S. Fox, J. P. Dunne, M. Tacke and J. F. Gallagher, Inorg. Chim. Acta, 2004, 357, 225–234 CrossRef CAS.
- M. Tacke, Ch. Klein, D. J. Stufkens and A. Oskam, J. Organomet. Chem., 1993, 444, 75–81 CrossRef CAS.
- A. Turco, C. Pecile and M. Nicolini, J. Chem. Soc., 1962, 3008–3015 RSC.
- L. T. Allen, L. P. Cuffe, W. M. Gallagher, Y. Lou, O. Mendoza, H. Müller-Bunz, F.-J. K. Rehmann, N. Sweeney and M. Tacke, J. Organomet. Chem., 2004, 689, 3203–3209 CrossRef CAS.
Footnote |
| † CCDC reference numbers 735708 for 2b and 735709 for 2c, respectively. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b911753a |
| This journal is © The Royal Society of Chemistry 2009 |
