Hijacking transferrin bound iron: protein–receptor interactions involved in iron transport in N. gonorrhoeae†
Claire J.
Parker Siburt‡
a,
Petra L.
Roulhac‡
a,
Katherine D.
Weaver‡
a,
Jennifer M.
Noto
b,
Timothy A.
Mietzner
c,
Cynthia N.
Cornelissen
b,
Michael C.
Fitzgerald
*a and
Alvin L.
Crumbliss
*a
aDepartment of Chemistry, Duke University, Durham, NC 27708-0346, USA. E-mail: alvin.crumbliss@duke.edu; michael.c.fitzgerald@duke.edu
bDepartment of Microbiology and Immunology, Virginia Commonwealth University, Richmond, VA, 23298-0678, USA
cDepartment of Molecular Genetics and Biochemistry, University of Pittsburgh School of Medicine, Pittsburgh, PA, 15261, USA
First published on 9th April 2009
Abstract
Neisseria gonorrhoeae has the capacity to acquire iron from its human host by removing this essential nutrient from serum transferrin. The transferrin binding proteins, TbpA and TbpB, constitute the outer membrane receptor complex responsible for binding transferrin, extracting the tightly bound iron from the host-derived molecule, and transporting iron into the periplasmic space of this Gram-negative bacterium. Once iron is transported across the outer membrane, ferric binding protein A (FbpA) moves the iron across the periplasmic space and initiates the process of transport into the bacterial cytosol. The results of the studies reported here define the multiple steps in the iron transport process in which TbpA and TbpB participate. Using the SUPREX technique for assessing the thermodynamic stability of protein–ligand complexes, we report herein the first direct measurement of periplasmic FbpA binding to the outer membrane protein TbpA. We also show that TbpA discriminates between apo- and holo-FbpA; i.e. the TbpA interaction with apo-FbpA is higher affinity than the TbpA interaction with holo-FbpA. Further, we demonstrate that both TbpA and TbpB individually can deferrate transferrin without energy supplied from TonB resulting in sequestration by apo-FbpA.
Introduction
Almost all living organisms must acquire iron from their environment. Neisseria gonorrhoeae employs a complex system of proteins to strip iron from the human host protein transferrin (Tf) and transport iron across its membranes. Transferrin binding protein A (TbpA), a 100 kDa outer membrane protein, is required for utilization of transferrin-bound iron and functions synergistically with transferrin binding protein B (TbpB).1–3 While the surface-exposed lipoprotein TbpB is not absolutely required for in vitro growth by N. gonorrhoeae on transferrin as a sole iron source, TbpB makes iron utilization more efficient.2 In the periplasm, ferric binding protein A (FbpA) binds iron with a synergistic anion and delivers it to the cytoplasmic membrane.4–9 Although the important components of this transport process have been identified, the contributions of TbpA and TbpB to removal of iron from transferrin have not been individually evaluated and the biological process of how FbpA acquires transferrin-derived iron in intact cells remains unclear.The mechanism(s) of iron transport in other Gram-negative bacteria has been extensively studied (see ref. 10–19) and the crystal structures of several key proteins have been described.4,20–22 Iron acquisition can be accomplished by hijacking the iron bound by host heme proteins or metalloproteins, as is the case for N. gonorrhoeae discussed here, or by incorporating extracellularly chelated iron in the form of ferric siderophores. Acquisition of siderophore bound iron involves binding to, and passage through, integral outer membrane proteins called TonB-dependent transporters.23–27 These transporters share a similar architecture, being comprised of a 22-stranded β-barrel and an amino-terminal plug domain, which is sequestered within the lumen of the β-barrel.20,28 The receptorproteins in Neisseria (TbpA/TbpB), which acquire iron from the human metalloprotein transferrin, have not yet been crystallized. However, by analogy with the other TonB-dependent transporters, it is expected that the integral membrane, TonB-dependent protein TbpA forms a β-barrel, which is periplasmically-occluded by a plug domain.1,29,30
TonB-dependent transporters require TonB and metabolic energy to accomplish transport across the outer membrane. TonB, in a complex with ExbB and ExbD, is energized by the proton motive force across the cytoplasmic membrane.31 TonB-derived energy is then conveyed to the outer membrane transporter proteins to accomplish iron internalization.32 Energy transduction from TonB to accomplish vectorial transport of the ligand into the periplasm involves an interaction between the carboxy-terminal domain of TonB and a short amino acid sequence near the amino-terminus of TonB-dependent transporters, known as the TonB-box.33 The TonB box of gonococcal TbpA has been defined and is similarly required for interaction with TonB and iron internalization from transferrin.34 Dislocation or extraction of the plug from within the β-barrel of the transporter and subsequent presentation of transported ligand to the periplasmic binding protein is thought to be the general mechanism of transport by TonB-dependent outer membrane receptors.35 However in N. gonorrhoeae, TonB-derived energy is also required for efficient release of transferrin from the gonococcal cell surface, as transferrin dissociation from intact gonococci is dramatically inhibited in the absence of TonB.36,37
A model, as depicted conceptually in Scheme 1, for the transport of iron in N. gonorrhoeae involves several protein–protein interactions and is likely to follow these steps: (1) holo-Tf interaction with the TbpA/B receptor, (2) iron release from holo-Tf, (3) iron movement across the outer membrane, (4) apo-Tf dissociation from the TbpA/B receptor, and (5) iron insertion into apo-FbpA.38,39 Since iron hydrolysis/precipitation and redox reactions that produce toxic radicals must be prevented in a biological system, it is a reasonable assumption that the first coordination shell of iron is always controlled by sequestration in protein binding sites. Therefore, we hypothesize additional steps are involved in the above model: (i) TbpA/B receptor interacts with apo-FbpA and (ii) holo-FbpA dissociates from the TbpA/B receptor. The exact role(s) of TbpA and TbpB in each of these steps, especially (i) and (ii), remain unclear. In addition, the point(s) at which the energy requirement fulfilled by TonB is imposed on this step-wise process has not been completely defined.36 Conceptually, the seven steps outlined above can be treated separately in order to investigate the proteins and detailed mechanism involved in iron transport.
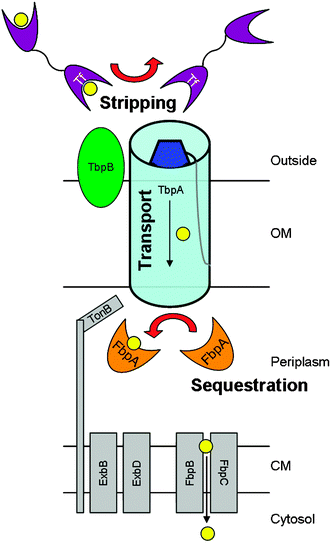 | ||
| Scheme 1 Utilization of transferrin-bound iron by live, wild-type Neisseria gonorrhoeae involves many proteins. The transferrin binding proteins, TbpA and TbpB constitute the outer membrane receptor complex responsible for binding transferrin, stripping the tightly-bound iron from the host-derived molecule, and transporting iron into the periplasmic space. In the periplasm, iron is sequestered by ferric binding protein A, which delivers it to the cytoplasmic membrane. After crossing the cytoplasmic membrane, iron is metabolized according to the needs of the cell. Energy derived from TonB, ExbB, and ExbD is required for utilization of transferrin-bound iron. | ||
Previously, we used SUPREX (Stability of Unpurified Proteins from Rates of H/D Exchange) to demonstrate that phosphate serves as a synergistic anion in the FeFbpA-PO4 complex in vivo.7 Using SUPREX, we have also investigated the change in protein folding stability as the synergistic anion is varied.40 Here we used the well-characterized SUPREX behaviors of apo-FbpA and FeFbpA-PO4 as probes to investigate the transferrin-iron transport system of N. gonorrhoeae. These studies illustrate the power of SUPREX to probe protein–protein interactions and receptor–substrate binding in the presence of other proteins and in crude membrane preparations.
Here we report the first direct detection and analysis of the apo-FbpA binding interaction with TbpA. We also show that TbpA can discriminate between apo- and holo-FbpA. We demonstrate that TbpB does not bind either apo- or holo-FbpA. Finally, these studies help to delineate the functions of TbpA and TbpB by showing that either TbpA or TbpB can facilitate the release of iron from holo-Tf without the participation of TonB. This investigation provides key experimental data that significantly extends our understanding of the distinctive roles that TbpA and TbpB play and provides insight into the point at which energy requirements are imposed on this multi-step iron transport process across the outer membrane.
Experimental methods
Human apo- and holo-transferrin were purchased from Sigma Aldrich. Recombinant FbpA (N. gonorrhoeae) was expressed in E. coli and purified as previously reported.41,42 Holo-FbpA was reconstituted in either the phosphate or citrate form as reported previously.6Horseradish peroxidase (HRP)-labeled transferrin (Jackson ImmunoResearch) was used in competitive solid-phase, transferrin-binding assays (data not shown) as described previously.3Total membrane preparations were isolated from gonococcal strains FA19 (TbpA+/TbpB+), FA6905 (TbpA+/TbpB−), FA6747 (TbpA−/TbpB+), and FA6815 (TbpA−/TbpB−). Gonococcal strains were subjected to iron-stressed growth to promote maximal expression of the iron-regulated proteins, including transferrin binding proteins. Following growth, cells were harvested and bacterial pellets were resuspended in 10 mM HEPES at pH 7.4 and subjected to French Press at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi. The resulting lysate was centrifuged to remove cellular debris. The supernatant was collected and then subjected to ultracentrifugation at 140
000 psi. The resulting lysate was centrifuged to remove cellular debris. The supernatant was collected and then subjected to ultracentrifugation at 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g to isolate total membrane fractions. Total membranes were suspended in 10 mM HEPES and BCA (bicinchoninic acid) assays (Pierce Biochemicals) were performed to quantify total protein concentration. TbpA and TbpB were estimated to comprise approximately 1% of the total membrane protein concentration.43 TonB was not functional in the total membrane preparations because the membrane potential of the inner membrane is not preserved, uncoupling TonB from its energy source.
000 × g to isolate total membrane fractions. Total membranes were suspended in 10 mM HEPES and BCA (bicinchoninic acid) assays (Pierce Biochemicals) were performed to quantify total protein concentration. TbpA and TbpB were estimated to comprise approximately 1% of the total membrane protein concentration.43 TonB was not functional in the total membrane preparations because the membrane potential of the inner membrane is not preserved, uncoupling TonB from its energy source.
The SUPREX analyses of FbpA in the absence and presence of transferrin and/or membrane preparations were initiated by 10-fold dilution into a series of deuterated H/D exchange buffers. The deuterated exchange buffers contained 50 mM MES, 200 mM KCl (pD = 6.5), and concentrations of deuterated GdmCl that ranged from 0 to 6 M. The concentration of FbpA in the exchange buffers was 0.3 μM. A high-sensitivity SUPREX protocol was used on these complex mixtures.44 SUPREX curves were generated using an H/D exchange time of 1 h. The H/D exchange reactions in each SUPREX experiment were quenched with the addition of TFA (final concentration 0.3% v/v). Protein samples were concentrated and desalted using C4 ZipTipsTM (Millipore, Inc.) and subsequently mixed with 5 μL of a saturated aqueous solution of sinapinic acid. Data analyses were performed as described previously for FbpA.40
To evaluate surface binding by FbpA, whole iron-stressed gonococci were applied to nitrocellulose membranes as previously described.1,38 After blocking, membranes were probed with his-tagged, recombinant FbpA. Specifically bound apo- or ferrated-FbpA was detected with his-tag specific antibodies, followed by a secondary antibody conjugated to either alkaline phosphatase or horseradish peroxidase. In an indirect assay , we also tested whether recombinant FbpA (apo or ferrated) interfered with binding of human transferrin to intact gonococci. As described previously,1,3 whole, iron-stressed gonococci were applied to nitrocellulose and then probed with a mixture of HRP-labeled Tf and unlabeled competitor apo- or ferrated-FbpA. Transferrin that remained bound in the presence of excess competitor was detected by development with the commercially-available substrate for HRP, Opti-4CN (Bio-Rad).
Results and discussion
TbpA directly binds apo-FbpA
When apo-FbpA was exposed to membranes containing TbpA and TbpB (TbpA+/TbpB+), the SUPREX curve transition midpoint (i.e.C1/2 value) of apo-FbpA was shifted to a higher concentration of denaturant and the pre-transition baseline was lowered to a smaller Δ mass (Fig. 1A). This change in SUPREX behavior indicated an increase in the stability of apo-FbpA. The shift in the pre-transition baseline was consistent with a larger population of globally protected amide protons in the apo-FbpA/TbpA+/TbpB+ complex compared to that of apo-FbpA alone. As expected, the post-transition regions were within the uncertainty of Δ mass values which was typically 10 Da. The SUPREX behavior of apo-FbpA in the presence of membranes containing only TbpA (TbpA+/TbpB−) was similar to that observed for membranes containing TbpA and TbpB (TbpA+/TbpB+; Fig. 1A). | ||
| Fig. 1 Apo-FbpA binds to TbpA. A) SUPREX analysis of apo-FbpA (●) alone or in the presence of (○) TbpA+/B+ or (■) TbpA+/B− membrane preparations. Dotted lines indicate the C1/2SUPREX values for each curve. A shift in C1/2SUPREX of ∼0.5 M corresponds to a ΔΔG value of ∼1 kcal mol−1 using an m-value of 2.4.44 B) SUPREX analysis of apo-FbpA in the presence of (○) TbpA−/B+. The data for apo-FbpA (●) alone is reprinted for ease of comparison. The dotted line indicates the C1/2SUPREX value. Notice the scale of Fig. 1A and 1B are different. No binding event between apo-FbpA and TbpB is detected within the limits of the experiment (0.5 kcal mol−1). All data were collected using an H/D exchange time of 1 h at pD 6.5. Data not shown for apo-FbpA in the presence of membranes containing TbpA−/TbpB− has same midpoint as apo-FbpA alone. | ||
In contrast, the SUPREX behavior of apo-FbpA in the presence of membranes containing only TbpB (TbpA−/TbpB+) was unchanged from that of apo-FbpA alone (Fig. 1B). Also, SUPREX analysis of apo-FbpA in the presence of null membrane preparations (TbpA−/TbpB−) exhibited the same behavior as purified apo-FbpA (data not shown). These data suggest that within the limits of our binding assay , which would be expected to detect binding events with binding free energies >0.5 kcal mol−1 (see Fig. 1), there was no evidence of a binding interaction between apo-FbpA and TbpB. Taken together, these data suggest that apo-FbpA was stabilized by TbpA and not TbpB. We therefore conclude that apo-FbpA only binds to TbpA.
The N. gonorrhoeae membrane preparations used in our experiments consisted of both inner and outer membranes and contained many different proteins. Membrane vesicles, generated by French Press of intact, iron-starved gonococci, were heterogeneous with respect to protein orientation and possibly dynamic in solution. Some vesicles were oriented with the periplasmic face of the outer membrane exposed to the solution. This means that the extracellular face of the receptor (TbpA and TbpB) and the periplasmic face of TbpA were exposed to the solvent. Additionally, the stoichiometry of TbpA and TbpB required for a functioning receptor complex has not been unequivocally resolved.3,45–48 Consequently, the interpretations of the experiments reported here are complicated by the heterogeneity of protein orientation in any observed binding event.
We hypothesize that apo-FbpA interacts with TbpA on the periplasmic face of the outer membrane in intact cells. In our SUPREX experiments however, due to the heterogeneity of the membrane vesicle orientation, exogenously added FbpA is exposed to both extracellular and periplasmic faces of the outer membrane. To determine whether FbpA could interact with the extracellullar face of TbpA- or TbpB-containing membranes, we measured direct binding of FbpA to whole cells. His-tagged FbpA (both apo and holo) were applied to whole cells and an anti-his antibody was utilized to visualize binding to the cell surface. The results for holo-FbpA and apo-FbpA were identical. Neither apo- nor holo-FbpA bound to the surface of gonococcal cells in a specific manner. Background levels of reactivity were detected in all strains tested, regardless of whether TbpA was expressed or not (data not shown). This result indicates that FbpA does not bind to surface exposed epitopes of TbpA, at least to an extent that is detectable in this assay format. Therefore, we conclude that any binding event detected between FbpA and TbpA-containing membrane preparations using SUPREX likely occurs at the periplasmic interface.
We also tested whether recombinant apo- or holo-FbpA could compete with horseradish peroxidase labeled transferrin (HRP-Tf) for binding to the gonococcal Tbps on whole cells. The concentration of HRP-Tf remained constant, while the concentration of FbpA was varied from a molar ratio of 0 : 1 (Fbp : HRP-Tf) to a molar ratio of 1000 : 1 (data not shown). Binding of HRP-Tf to the Tbp receptor was not diminished in the presence of either excess apo-FbpA or holo-FbpA. This result strengthens our conclusion that FbpA does not bind to the external surface of TbpA directly, nor does it interfere with the interaction between TbpA and transferrin.
Our SUPREX results show that apo-FbpA was stabilized by the presence of TbpA and that apo-FbpA was not affected by the presence of TbpB or membranes lacking both of these proteins. Using SUPREX we have demonstrated a direct binding event between apo-FbpA and TbpA. These data support our model in which apo-FbpA interacts in intact cells with the periplasmic face of TbpA and is separated from TbpB by the outer membrane.
TbpA discriminates between apo- and holo-FbpA
As we have shown previously, FbpA is more stable when it is in the FeFbpA-PO4 form rather than the apo-FbpA form with respect to protein folding.40 Evidence for this stabilization comes from the observed changes in SUPREX behavior: relative to apo-FbpA, FbpA in the FeFbpA-PO4 form has a lower Δ mass baseline and the C1/2SUPREX value for FbpA in the FeFbpA-PO4 form is shifted to the right of the C1/2SUPREX value of apo-FbpA.40 This characteristic SUPREX behavior of FeFbpA-PO4 can be used to probe the protein–protein interactions in which FeFbpA-PO4 participates.When FeFbpA-PO4 was exposed to gonococcal membrane preparations, whether devoid of Tbp receptor complex (TbpA−/TbpB−) or containing the complete Tbp receptor complex (TbpA+/TbpB+), the SUPREX behavior was the same as FeFbpA-PO4 alone (Fig. 2). The presence of TbpA or TbpB containing membranes neither increased nor decreased the thermodynamic stability of FbpA in the FeFbpA-PO4 form with respect to protein folding, to a degree which could be detected within the limits of our experimental system.
 | ||
| Fig. 2 SUPREX analysis of FeFbpA-PO4 (●) alone, (○) in the presence of TbpA+/B+ and (▲) in the presence of null (TbpA−/B−) membrane preparations using an H/D exchange time of 1 hour at pD 6.5. | ||
We hypothesize that in a living bacterium TbpA would have a higher binding affinity for apo-FbpA than for holo-FbpA, ensuring the vectorial transport of iron through the outer membrane into the periplasm. The observed change in SUPREX behavior of apo-FbpA in the presence of TbpA (Fig. 1A) and the lack of any observed change in SUPREX behavior of FeFbpA-PO4 in the presence of TbpA containing membranes (Fig. 2) provide the first direct experimental evidence for the ability of TbpA to discriminate between apo- and holo-FbpA. Further, this discriminatory binding may indicate a thermodynamic sink which imposes directional transport of iron across the outer membrane.
Our conclusion that TbpA can discriminate between apo- and holo-FbpA, corresponds well with the seven-step transport process described in the Introduction, in which holo-Tf binds to the receptor, iron is released and apo-Tf dissociates, while apo-FbpA binds to TbpA, iron is inserted into apo-FbpA, and holo-FbpA dissociates.
TbpA and TbpB facilitate iron transfer between transferrin and FbpA
When apo-FbpA and holo-Tf were mixed together, no exchange of iron was observed between the two protein binding sites. The SUPREX curve resulting from the mixture of apo-FbpA and holo-Tf was coincident with that of purified apo-FbpA (data not shown). This was also true when apo-FbpA, holo-Tf, and TbpA−/B− membranes were mixed together (data not shown). However, when the mixture of apo-FbpA and holo-Tf was combined with membrane preparations containing TbpA and TbpB (TbpA+/TbpB+), the resulting SUPREX curve matched that of purified FeFbpA-PO4 (Fig. 3). These data suggest that the Tbp receptor complex facilitates the transfer of iron from holo-Tf to apo-FbpA. Interestingly, when apo-FbpA and holo-Tf were reacted in the presence of either TbpA+/TbpB− or TbpA−/TbpB+ membranes, the SUPREX curves also resembled FeFbpA-PO4 (Fig. 3). These further findings suggest that either TbpA or TbpB, individually or together can facilitate the transfer of iron from holo-Tf to apo-FbpA. It is interesting to note that this transfer of iron can be facilitated by TbpB even though we cannot detect a binding event between TbpB and FbpA. | ||
| Fig. 3 Exchange of iron between transferrin and FbpA. SUPREX analysis of apo-FbpA in the presence and absence of holo-Tf and Tbp receptor membrane constructs. SUPREX data obtained for (○) apo-FbpA, (●) apo-FbpA/TbpA+/TbpB+, (■) apo-FbpA/TbpA+/TbpB+/holo-Tf, (▲) apo-FbpA/TbpA+/TbpB−/holo-Tf, (grey diamonds) apo-FbpA/TbpA−/TbpB+/holo-Tf and (□) FeFbpA-PO4 using an H/D exchange time of 1 h at pD 6.5. Data are not shown for apo-FbpA with null membrane preparations; however, data are coincident with data for apo-FbpA alone. | ||
Previous studies with the closely related N. meningitidis (an organism that is nearly 80% identical by genetic sequence to N. gonorrhoeae),49,50 indicate the Tbp complex is able to facilitate the transfer of iron from holo-Tf to apo-FbpA.51,52 Here using different techniques we were able to confirm that in the presence of membranes derived from N. gonorrhoeae, iron can be transferred from holo-Tf to apo-FbpA in the presence of TbpA or TbpB or both. From these data, we cannot discern whether or not iron is transported through the receptor barrel. However, we were able to conclude that either TbpA or TbpB can facilitate the stripping of iron from holo-Tf in an energy independent process.
Equivalent behavior of the phosphate and citrate forms of FbpA
As we have shown previously, citrate and phosphate act as synergistic anions in vitro.6,40In vitro the synergistic anion is labile and the identity of the anion affects the thermodynamic properties of FbpA.6,53 Although we have shown that phosphate is present in the iron binding site of FbpA in crude cell lysates, it is important to understand the protein–protein interactions in which the citrate form of FbpA participates, since the anion is labile.7We observed parallel behavior, with respect to protein–protein interactions, when citrate was the synergistic anion rather than phosphate. These observations are illustrated by data presented in the ESI.† In the presence of TbpA+/TbpB+ apo-FbpA-citrate was stabilized in the SUPREX experiment, indicating a binding event between apo-FbpA-citrate and the bacterial receptor. As well, when apo-FbpA-citrate, TbpA+/TbpB+, and holo-Tf were combined, the SUPREX behavior observed matched that of holo-FbpA-citrate. This later observation is consistent with the bacterial receptor stripping the iron from holo-Tf, and apo-FbpA-citrate acting as the iron chelator. We conclude therefore that both the phosphate and citrate forms of apo-FbpA bind to the bacterial receptor. Further, both the phosphate and citrate forms of apo-FbpA are able to chelate iron when it is released from holo-Tf by TbpA and TbpB.
The roles of TbpA, TbpB, and TonB
TonB derived energy is required for transferrin iron utilization in intact cells.34 When considering the role of TonB, we regard the mechanistic steps of deferrating holo-Tf, releasing apo-Tf from the receptor, and transporting iron across the membrane as separate functions. Each function may have different energy requirements. Cornelissen et al. have shown previously that energy supplied from TonB is required to release apo-Tf from the Tbp complex.36 However, iron-utilization studies with live, intact bacteria do not allow differentiation between the separate activities of stripping iron from holo-Tf and transporting iron through the membrane. The data shown here allow us to separate the functions of stripping iron from transferrin and transporting iron across the membrane. We report here that either TbpA or TbpB can facilitate the release of iron from holo-Tf without energy supplied by TonB. Further studies are necessary to define the energy requirement and the role of TonB in the separate step of transporting iron across the gonococcal outer membrane.In addition to the TonB literature, a significant body of knowledge has been published about the role(s) of TbpA and TbpB in transferrin bound iron acquisition. It has been shown previously that the Tbp receptor is required for initiation of infection in vivo, and in vitro studies have shown that only TbpA is required for iron utilization.1,54 We specifically show here that TbpA can independently facilitate the release of iron from holo-Tf, an individual mechanistic step required for iron utilization. It has also been shown previously, that amino acids located in the proposed plug domain of TbpA are required for iron utilization.55 Consequently, when those amino acids are replaced with alanines, the ability of gonococci to acquire transferrin bound iron via TbpA (in a TbpB− construct) is lost. Similarly, the presence of small epitope insertions in putative loops 2, 9, or 11 also render TbpA incapable of iron utilization from transferrin in the absence of a functional TbpB protein.38 However, the presence of TbpB can compensate for these defects in TbpA and restore the ability of gonococci to utilize transferrin derived iron.38,55
Although not required and not able to sustain iron utilization alone, we demonstrate here that TbpB can remove the iron from transferrin in the absence of TbpA. Therefore, we propose that while the separate step of iron transport through the membrane requires TbpA, stripping of iron from transferrin can occur independently of TbpA. More studies are necessary to identify how the various structural domains of TbpA (the plug and the β-barrel) are involved in each step of the iron transport model proposed here. However, our SUPREX results suggest that both TbpA and TbpB can interact with holo-Tf and strip the iron from transferrin independently of one another or in concert.
Further, we have shown that TbpA and TbpB each play a specific discriminatory role, with respect to protein–protein interactions essential to our hypothesized multi-step transport process. It has been shown previously that only TbpB discriminates between apo-Tf and holo-Tf, preferentially binding holo-Tf.3,56–58 Herein we demonstrate that only TbpA is able to discriminate the iron status of FbpA, preferentially binding apo-FbpA.
Conclusions
While the proteins required for the transport of iron from human transferrin to the periplasmic binding protein FbpA have been identified, there have been questions about how the tightly-bound iron is released from transferrin via the membrane-bound receptor complex and how iron is transferred to FbpA. The SUPREX studies described here allowed us to explore the protein–protein interactions involved in iron transport by N. gonorrhoeae and to address these questions. In the current study, we have shown that (1) TbpA binds to apo-FbpA, (2) TbpA can discriminate between apo- and holo-FbpA, (3) TbpB does not interact with apo- or holo-FbpA, and (4) both TbpA and TbpB can facilitate the release of iron from holo-Tf in an energy-independent fashion resulting in sequestration by apo-FbpA. These studies help to clarify the role(s) that each protein plays in our expanded hypothesis for the multi-step transferrin-mediated iron transport mechanism in N. gonorrhoeae.Acknowledgements
ALC thanks the National Science Foundation (CHE 0418006 and CHE 0809466) for financial support. Funding to CNC was provided by Public Health Service grant R01 AI047141 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. JMN was supported by the Training in Molecular Pathogenesis grant (T32 AI07617) from the National Institutes of Health. The authors thank Heather Strange, VCU, for her excellent technical contributions and Patrick DeArmond, Duke, for helpful discussions.References
- C. N. Cornelissen, G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson and P. F. Sparling, J. Bacteriol., 1992, 174, 5788–5797 CAS.
- J. E. Anderson, P. F. Sparling and C. N. Cornelissen, J. Bacteriol., 1994, 176, 3126–3170.
- C. N. Cornelissen and P. F. Sparling, J. Bacteriol., 1996, 178, 1437–1444 CAS.
- C. M. Bruns, A. J. Nowalk, A. S. Arvai, M. A. McTigue, K. G. Vaughan, T. A. Meitzner and D. E. McRee, Nat. Struct. Biol., 1997, 4, 919–924 CrossRef CAS.
- T. A. Mietzner, S. B. Tencza, P. Adhikari, K. G. Vaughan and A. J. Nowalk, in Current Topic in Microbiology and Immunology, vol. 225 Bacterial Infection: Close Encounters at the Host Pathogen Interface, ed. P. K. Vogt and M. J. Maham, Springer-Verlag, Berlin, Heidelberg, Germany, 1998 Search PubMed.
- S. Dhungana, C. H. Taboy, D. S. Anderson, K. G. Vaughan, P. Aisen, T. A. Mietzner and A. L. Crumbliss, PNAS, 2003, 100, 3659–3664 Search PubMed.
- P. L. Roulhac, K. D. Weaver, P. Adhikari, D. S. Anderson, P. D. DeArmond, T. A. Mietzner, A. L. Crumbliss and M. C. Fitzgerald, Biochemistry, 2008, 47, 4298–4305 CrossRef CAS.
- C. M. Bruns, D. S. Anderson, K. G. Vaughan, P. A. Williams, A. J. Nowalk, D. E. McRee and T. A. Mietzner, Biochemistry, 2001, 40, 15631–15637 CrossRef CAS.
- M. Guo, I. Harvey, W. Yang, L. Coghill, D. J. Campopiano, J. A. Parkinson, R. T. A. MacGillivary, W. R. Harris and P. J. Sadler, J. Biol. Chem., 2003, 278, 2490–2502 CrossRef CAS.
- T. J. Brickman, M. T. Anderson and S. K. Armstrong, Biometals, 2007, 20, 303–320 Search PubMed.
- D. Perkins-Balding, M. Ratliff-Griffin and I. Stojiljkovic, Microbiol. Mol. Biol. Rev., 2004, 68, 154–171 CrossRef CAS.
- E. E. Wyckoff, A. R. Mey and S. M. Payne, Biometals, 2007, 20, 405–416 Search PubMed.
- K. D. Krewulak and H. J. Vogel, Biochim. Biophys. Acta, 2008, 1778, 1781–1804 CAS.
- I. C. Boulton, A. R. Gorringe, B. Gorinsky, M. D. Retzner, A. B. Schryvers, C. L. Joannou and R. W. Evans, Biochem. J., 1999, 339, 143–149 CrossRef CAS.
- A. G. Khan, S. R. Shouldice, S. D. Kirby, R.-h. Yu, L. W. Tari and A. B. Schryvers, Biochem. J., 2007, 404, 217–225 CrossRef CAS.
- T. Krell, G. Renauld-Mongenie, M.-C. Nicolai, S. Fraysse, M. Chevalier, Y. Berard, J. S. Oakhill, R. W. Evans, A. Gorringe and L. Lissolo, J. Biol. Chem., 2003, 278, 14712–14722 CrossRef CAS.
- J. S. Oakhill, C. L. Joannou, S. K. Buchanan, A. Gorringe and R. W. Evans, Biochem. J., 2002, 364, 613–616 CrossRef CAS.
- A. Ekins, A. G. Khan, S. R. Shouldice and A. B. Schryvers, BioMetals, 2004, 17, 235–243 Search PubMed.
- S. Letoffe, K. Wecker, M. Delepelaire, P. Delepelaire and C. Wandersman, J. Bacteriol., 2005, 187, 4637–4645 CrossRef CAS.
- S. K. Buchanan, B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitka, R. Chakraborty, D. van der Helm and J. Deisenhofer, Nat. Struct. Biol., 1999, 6, 56–63 CrossRef CAS.
- A. D. Ferguson, R. Charkraborty, B. S. Smith, L. Esser, D. van der Helm and J. Deisenhofer, Science, 2002, 295, 1715–1719 CrossRef CAS.
- K. P. Locher, B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch and D. Moras, Cell, 1998, 95, 771–778 CrossRef CAS.
- C. Wandersman and P. Delepelaire, Annu. Rev. Microbiol., 2004, 58, 611–647 CrossRef CAS.
- G. S. Moeck and J. W. Coulton, Mol. Microbiol., 1998, 28, 675–681 CrossRef CAS.
- K. Brillet, L. Journett, H. Celia, L. Paulus, A. Stahl, F. Pattus and D. Cobessi, Structure, 2007, 15, 1383–1391 CrossRef CAS.
- S. Devanathan and K. Postle, Mol. Microbiol., 2007, 65, 441–453 CrossRef CAS.
- I. J. Schalk, P. Kyslik, D. Prome, A. van Dorsselaer, K. Poole, M. A. Abdallah and F. Pattus, Biochemistry, 1999, 38, 9357–9365 CrossRef CAS.
- A. D. Ferguson and J. Deisenhofer, Biochim. Biophys. Acta, 2002, 1565, 318–332 CAS.
- C. N. Cornelissen and P. F. Sparling, Mol. Microbiol., 1994, 14, 843–850 CrossRef CAS.
- I. C. Boulton, M. K. Yost, J. E. Anderson and C. N. Cornelissen, Infect. Immun., 2000, 68, 6988–6996 CrossRef CAS.
- V. Braun, ACS Chem. Biol., 2006, 1, 352–354 CrossRef CAS.
- K. Postle and R. A. Larsen, Biometals, 2007, 20, 453–465 Search PubMed.
- D. D. Shultis, M. D. Purdy, C. N. Banchs and M. C. Wiener, Science, 2006, 312, 1396–1399 CrossRef CAS.
- C. D. Kenney and C. N. Cornelissen, J. Bacteriol., 2002, 184, 6138–6145 CrossRef CAS.
- J. Gumbart, M. C. Wiener and E. Tajkhorshid, Biophys. J., 2007, 93, 496–504 CrossRef CAS.
- C. N. Cornelissen, J. E. Anderson and P. F. Sparling, Mol. Microbiol., 1997, 26, 25–36 CrossRef CAS.
- A. J. DeRocco, M. K. Yost-Daljev, C. D. Kenney and C. N. Cornelissen, Biometals, 2008 DOI:10.1007/s10534-008-9179-y , epub ahead of print.
- M. K. Yost-Daljev and C. N. Cornelissen, Infect. Immun., 2004, 72, 1774–1785.
- C.-Y. Chen, S. A. Berish, S. A. Morse and T. A. Mietzner, Mol. Microbiol., 1993, 10, 311–318 CrossRef CAS.
- P. L. Roulhac, K. D. Powell, S. Dhungana, K. D. Weaver, T. A. Mietzner, A. L. Crumbliss and M. C. Fitzgerald, Biochemistry, 2004, 43, 15767–15774 CrossRef CAS.
- T. A. Mietzner, G. Bolan, G. K. Schoolnik and S. A. Morse, J. Exp. Med., 1987, 165, 1041–1057 CrossRef CAS.
- C. H. Taboy, K. G. Vaughan, T. A. Mietzner, P. Aisen and A. L. Crumbliss, J. Biol. Chem., 2001, 276, 2719–2724 CrossRef CAS.
- T. A. Mietzner and S. A. Morse, in Pathog. Neisseriae. Proc. Int. Symp. 4th, Washington, DC, 1985, pp. 406–414 Search PubMed.
- K. D. Powell and M. C. Fitzgerald, Anal. Chem., 2001, 73, 3300–3304 CrossRef CAS.
- I. C. Boulton, A. R. Gorringe, R. J. G. Carr, B. Gorinsky, C. L. Joannou and R. W. Evans, FEBS, 1997, 414, 409–413 CrossRef CAS.
- N. B. L. Powell, K. Bishop, H. M. Palmer, D. A. Ala'aldeen, A. R. Gorringe and S. P. Borriello, J. Med. Microbiol., 1998, 47, 257–264 CrossRef CAS.
- C. Ronpirin, A. E. Jerse and C. N. Cornelissen, Infect. Immun., 2001, 69, 6336–6347 CrossRef CAS.
- I. C. Boulton, A. R. Gorringe, J. K. Shergill, C. L. Joannou and R. W. Evans, J. Theor. Biol., 1999, 198, 497–505 CrossRef CAS.
- C. N. Cornelissen, J. E. Anderson, I. C. Boulton and P. F. Sparling, Infect. Immun., 2000, 68, 4725–4735 CrossRef CAS.
- C. N. Cornelissen, J. E. Anderson and P. F. Sparling, Infect. Immun., 1997, 65, 822–828 CAS.
- J. A. Gomez, M. T. Criado and C. Ferreiros, Res. Microbiol., 1998, 149, 381–387 CrossRef CAS.
- S. W. Irwin, N. Averil, C. Y. Cheng and A. B. Schryvers, Mol. Microbiol., 1993, 8, 1125–1133 CrossRef CAS.
- H. Boukhalfa, D. S. Anderson, T. A. Mietzner and A. L. Crumbliss, J. Biol. Inorg. Chem., 2003, 8, 881–892 CrossRef CAS.
- C. N. Cornelissen, M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen and P. F. Sparling, Mol. Microbiol., 1998, 27, 611–616 CrossRef CAS.
- J. M. Noto and C. N. Cornelissen, Infect. Immun., 2008, 76, 1960–1969 CrossRef CAS.
- G. Renauld-Mongenie, M. Latour, D. Poncet, S. Naville and M.-J. Quentin-Millet, FEMS, 1998, 169, 171–177 Search PubMed.
- I. C. Boulton, A. R. Gorringe, N. Allison, A. Robinson, B. Gorinsky, C. L. Joannou and R. W. Evans, Biochem. J., 1998, 334, 269–273 CAS.
- M. D. Retzer, R.-H. Yu, Y. Zhang, G. C. Gonzalez and A. B. Schryvers, Microb. Pathog., 1998, 25, 175–180 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2; additional SUPREX data. See DOI: 10.1039/b902860a |
| ‡ These authors made equal contributions to this manuscript. |
| This journal is © The Royal Society of Chemistry 2009 |
