In vivo phytochelatins and Hg–phytochelatin complexes in Hg-stressed Brassica chinensis L.
Liqin
Chen
a,
Limin
Yang
a and
Qiuquan
Wang
*ab
aDepartment of Chemistry & the MOE Key Laboratory of Analytical Sciences, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China. E-mail: qqwang@xmu.edu.cn; Fax: +86 5922181796; Tel: +86 5922181796
bState Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen, 361005, China
First published on 27th November 2008
Abstract
In vivo phytochelatins (PCs) and their corresponding Hg–PC complexes were characterized using RPLC-ESI-MS/MS in the roots of Brassica chinensis L. under the stress of a mercury cysteine complex (HgCys2) and/or a mercury humic acid complex (Hg–HA). Results indicated that the presence of Cys and/or HA decreased the Hg uptake in both the roots and shoots of B. chinensis but increased the generation of PCs in the roots compared with those where only HgCl2 was in the culture solutions. A series of Hg–PC complexes were synthesized in vitro for predicting the possible Hg–PC formed in vivo in the HgCys2 and/or Hg–HA stressed roots of B. chinensis. The discovery of in vivo oxidized PC2, PC3 and PC4 and their corresponding HgPC2, HgPC3, HgPC4 and Hg2PC4, which were confirmed by their specific isotope distribution, provided definite evidence for understanding the defense and accumulation mechanism of B. chinensis to Hg, in which the induced PCs play an important role not only in Hg detoxification through forming Hg–PC complexes but also for reducing the oxidative stress induced by Hg2+.
Introduction
Mercury (Hg) pollution is a ubiquitous problem resulting both from natural events and anthropogenic activities. The amount of Hg mobilized and released into the biosphere has gradually increased since the beginning of the industrial age. It has caused deep concern because of its toxicity, mobility, bioaccumulation, methylation process and transport in the biosphere .1 When absorbed by human bodies, it causes neurological toxicity, kidney damage and even death due to its strong and specific interaction with sulfhydryls in proteins.2,3 Hg-contaminated soil is believed to contribute to human health risks and major environmental problems. Many studies have shown that plant roots accumulate Hg when exposed to Hg-contaminated soils.4–6 As a typical soft Lewis acid, Hg2+ complexes strongly with reduced sulfur-containing ligands resulting in the predominant Hg chemical form in aquatic and soil environments.7,8 Humic acids (HA), the predominant fraction of humic substances and well known Hg ligands with cysteine (Cys) in their peptide fragments, tend to increase Hg solubility and mobility, and alter its availability to plants.9,10 When exposed to heavy metal ions including Hg2+, higher plants and Schizosaccharomyces pombe respond by synthesizing Cys sulfhydryl residue-rich peptides and phytochelatins (PCs) in the cytoplasm to defend against their phytotoxicity.11,12 PCs have the general structure of γ-(Glu–Cys)n–Gly (n = 2–11), and are synthesized from glutathione (GSH) through a constitutively present PC synthase. Subsequently, heavy metal ions such as Hg2+ are complexed and sequestered by the induced PCs viathiolate coordination due to their high affinity with SH groups.13 Previous in vitro studies demonstrate that Hg2+ is facile to transfer from shorter- to longer-chain PCs. The strength of Hg2+ binding to GSH and PCs follows the order γ-Glu–Cys–Gly < γ-(Glu–Cys)2–Gly < γ-(Glu–Cys)3–Gly < γ-(Glu–Cys)4–Gly,12 and GSH and PCs play important roles in resistance in Hydrilla verticillata (l.f.) Royle and Vallisneria spiralis L. under Hg2+ stress.14 On the other hand, it has been shown that a mutant having a defect in PC synthesis shows significantly enhanced sensitivity to Hg2+.15 In addition, overexpression of Escherichia coli γ-GlyCys synthetase and GSH synthetase in Arabidopsis thaliana plants provides significant increases in tolerance and accumulation of Hg2+.16 However, definite evidence of in vivo Hg–PC complexes and their corresponding PC precursors in support of the plant’s defense and accumulation mechanisms is still scarce, and this makes it difficult to understand the nature of the Hg–PC complexes present in plant tissues. Among the few studies found in the literature,12,14,17 only one reports the detection of in vivo Hg–PC complexes in Brassica napus, but only PC2 and its Hg complexes are observed in the case of adding chelating agents.17Since HA is the active fraction of soil organic substances and Cys is the most active component towards Hg in HA,18 in this study we investigated the behavior of Cys and HA on Hg accumulation in Brassica chinensis L., and the subsequent synthesis of PCs and the formation of their corresponding Hg complexes for the first time. The hyphenation of RPLC with ESI-MS/MS was used to characterize not only in vitro synthesized Hg–PC complexes so as to predict the possible Hg–PC complexes and their binding stoichiometry, but also in vivo Hg–PC complexes present in the Hg-treated plant tissues to provide clear and definite evidence of detoxifying and/or deactivating Hg species by PCs in plant tissues.
Experimental
Chemicals
The HPLC-grade acetonitrile (ACN) and trifluoroacetic acid (TFA) used as the components of the mobile phase in the RPLC experiments were purchased from Merck (Darmstadt, Germany). Ultrapure water (18 Ω) was prepared with a Milli-Q system (Millipore, Bedford, MA, USA), and used throughout this study. Reduced glutathione (GSH) was purchased from Sino-American Technology (Shanghai, China), and 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) and HA were purchased from Sigma-Aldrich. All other reagents used in this study were at least of analytical-reagent grade. PC standards and a mixture of PCs (100 μM GSH, 30 μM PC2, 60 μM PC3 and 15 μM PC4) used were purified and prepared in our own laboratory from the shoots of B. chinensis L. under cadmium stress.19 The PC mixture was incubated with Hg2+ with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of SH and Hg2+ to prepare in vitro synthesized Hg–PC complexes.
1 molar ratio of SH and Hg2+ to prepare in vitro synthesized Hg–PC complexes.
Plant material
B. chinensis seeds (F1 Beauty Crown from Japan) were germinated on filter papers in Petri dishes. Three days after germination, seedlings were carefully transferred to 100 mL pots filled with modified Hoagland nutrient solution.20B. chinensis seedlings were allowed to grow in hydroponics for one week before treatment with Hg started. Three Hg substrates were added into the nutrient solution to achieve 200 μM HgCl2, (200 μM HgCl2 + 1.5 mg g−1 HA), and 200 μM HgCys2 for the studies on the effects of different Hg species on Hg accumulation in B. chinensis and on the degree of in vivo PC synthesis. The seedlings were grown at a controlled temperature (25 ± 1 °C) with a 16 h per day white light (photon flux, 700 μmol m−2 s−1) and humidity of about 60%. After three days of stress, seedling fresh weights were measured and then the roots were immersed in ice-cold 20 mmol L−1EDTA solution for 15 min to displace extracellular Hg. The seedlings were then rinsed with ultrapure water, and blotted to remove excess water before further examination.Hg determination in B. chinensis
Appropriate amounts of cultured roots and shoots of B. chinensis were first dried at 40 °C in a conventional electric oven21 until constant weight was obtained, and then digested in 5 mL HNO3 in 50 mL closed polypropylene centrifuge tubes in a water bath at 95–100 °C for 2 h. After natural cooling to room temperature (25 °C), the digests were diluted with 2% HNO3 to 25 mL for Hg determination using inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer Elan-DRC II, SCIEX, Canada). Certified reference material BCR 463 (tuna fish) was used for quality control.Extraction of demetallized PCs and Hg–PC complexes in Hg-stressed B. chinensis
Fresh roots and shoots were ground in liquid nitrogen and homogenized in ice-cold 1 M NaOH–0.5% (w/w) NaBH4. Homogenates were centrifuged at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 4 °C, and then the supernatants obtained were acidified to pH 1.0 with 6 mol L−1HCl. The precipitated materials were centrifuged again and clean extracts were collected for demetallized PC analysis. For the analysis of Hg–PC complexes, the roots of Hg–HA and Hg–Cys treated B. chinensis were ground in liquid nitrogen and homogenized in ultrapure water. Homogenates were centrifuged at 30
000 g for 15 min at 4 °C, and then the supernatants obtained were acidified to pH 1.0 with 6 mol L−1HCl. The precipitated materials were centrifuged again and clean extracts were collected for demetallized PC analysis. For the analysis of Hg–PC complexes, the roots of Hg–HA and Hg–Cys treated B. chinensis were ground in liquid nitrogen and homogenized in ultrapure water. Homogenates were centrifuged at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 4 °C, and then the supernatants were collected for in vivo Hg–PC analysis.
000 g for 15 min at 4 °C, and then the supernatants were collected for in vivo Hg–PC analysis.
PC analysis using RPLC
PC analysis was performed with RPLC using a system similar to the method of Grill et al.11 Briefly, PCs were separated on a C18 reverse phase column (2.0 mm I.D. × 150 mm in length; Shimadzu, Japan) at 0.15 mL min−1 using a 2 to 20% ACN linear gradient containing 0.02% (v/v) TFA over 25 min. The content of PCs was measured at 410 nm using on-line postcolumn derivatization with a solution including 1.8 mM DTNB, 15 mM EDTA, 0.3 M K2HPO4 (pH = 7.88). Total PCs (∑PCs) were reported as the molar concentration of a sum of γ-GluCys units of PC variants with n from 2 to 4. The assignments of the respective peaks were performed with electrospray ionization mass spectrometry (ESI-MS) and ESI-MS/MS (ESQUIRE-LC, Bruker Daltonik, Germany) after RPLC separation without postcolumn DTNB derivatization.Analysis of in vitro synthesized and in vivo Hg–PC complexes
In vitro synthesized and in vivo Hg–PC complexes were analyzed using RPLC-ESI-MS/MS. The RPLC parameters were used as described above for PC analysis. The eluate was introduced on-line into ESI-MS/MS. The instrument was used as a molecular-specific detector for the detection of Hg–PC complexes via their molecular peaks, Hg isotopic distribution assignment and their MS/MS spectra. The parameters used were capillary voltage +3500 V, nebulizer gas (N2) 11 L min−1, dry gas 21 psi, dry temperature 350 °C, trap drive 70.0, capillary exit offset 70.0 V, skim 1 45.0 V and fragment amplitude 1.0 V.Results and discussion
Hg accumulation and PC generation in B. chinensis under the stress of different Hg species
Concerning the bio-geo-chemical cycle of Hg, most Hg is bound to thio-organics and humic substances in the soil.22 Growth of B. chinensis and uptake of Hg by B. chinensis in the culture solutions containing different Hg species of HgCl2, HgCys2 and Hg–HA were investigated first in this study. The biomass and Hg concentrations in roots and shoots of B. chinensis were related to the different Hg species in which the total amount of Hg is constant (Table 1). It should be noted that we tried to wash Hg off the epidermis of the roots with EDTA. However, EDTA seemed to be not very effective. It was thus difficult to distinguish between the amount of Hg which was adsorbed onto the epidermis and that taken up into root cells.17 In this case, the Hg content determined in the roots was thus the sum of that taken up by the cells and that adsorbed onto the epidermis. The results obtained indicated that the presence of Cys and HA depressed the Hg uptake in both the roots and shoots of B. chinensis (Table 1). This might be ascribed to the less free Hg2+ in the culture solutions because of the high stability contents of HgCys2 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 40)23 and Hg–HA (log
K = 40)23 and Hg–HA (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K > 30).24 But, significantly increased generation of PCs was detected in the HgCys2 and/or Hg–HA stressed roots compared with those of HgCl2-stressed ones, as shown in Table 1. It is reported that PC synthesis produces a detectable depletion in GSH content, and Cys, the GSH precursor thiol molecule, is produced at higher rates in order to support GSH biosynthesis under heavy metal treatment.25 The low concentration of PCs in the roots of B. chinensis under exposure to HgCl2 might have been due to the unavailable Cys, but not to the ineffective activation of PC synthase by Hg2+.
K > 30).24 But, significantly increased generation of PCs was detected in the HgCys2 and/or Hg–HA stressed roots compared with those of HgCl2-stressed ones, as shown in Table 1. It is reported that PC synthesis produces a detectable depletion in GSH content, and Cys, the GSH precursor thiol molecule, is produced at higher rates in order to support GSH biosynthesis under heavy metal treatment.25 The low concentration of PCs in the roots of B. chinensis under exposure to HgCl2 might have been due to the unavailable Cys, but not to the ineffective activation of PC synthase by Hg2+.
| Hg species | Biomassb /10−2 g FW | Hg in rootc /μg g−1 DW | Hg in shootc /μg g−1 DW | PCs in the rootc /nmol g−1 FW | ∑PC/nmol g−1 | ||
|---|---|---|---|---|---|---|---|
| PC2 | PC3 | PC4 | |||||
| a DW, dry weight. n.d., not detected. b Mean ± SD of 9 repetitions from triplicate cultivations. c Mean ± SD of 6 repetitions from triplicate cultivations. ∑PC, molar concentrations of the sum of γ-Glu–Cys units of the detected PC variants. | |||||||
| Control | 8.02 ± 1.38 | 0.33 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. |
| HgCl2 | 6.10 ± 0.89 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 089 ± 4931 089 ± 4931 |
2839.0 ± 184.7 | <2.36 | <1.36 | <0.88 | <12.29 |
| HgCys2 | 7.53 ± 1.64 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 605 ± 3545 605 ± 3545 |
2740.4 ± 774.3 | 24.86 ± 7.17 | 60.88 ± 6.08 | 15.00 ± 1.88 | 292.4 ± 40.1 |
| Hg–HA | 8.33 ± 1.36 | 2279.1 ± 606.7 | 1946.4 ± 913.5 | 30.67 ± 4.46 | 31.30 ± 1.44 | 7.55 ± 0.42 | 185.5 ± 14.9 |
The fact that considerable PCs [(γ-Glu–Cys)n–Gly] with an n value from 2 to 4 have been detected in the cases of HgCys2 and Hg–HA suggests that Cys and HA offer the sulfur source to support the GSH biosynthesis as well as the subsequent PCs.25 It should be noted also that PCs have not been detected in the shoots of B. chinensis, indicating that PCs are not the compounds for long-distance root-to-shoot Hg translocation.26
RPLC-ESI-MS analyses of in vitro synthesized Hg–PC complexes
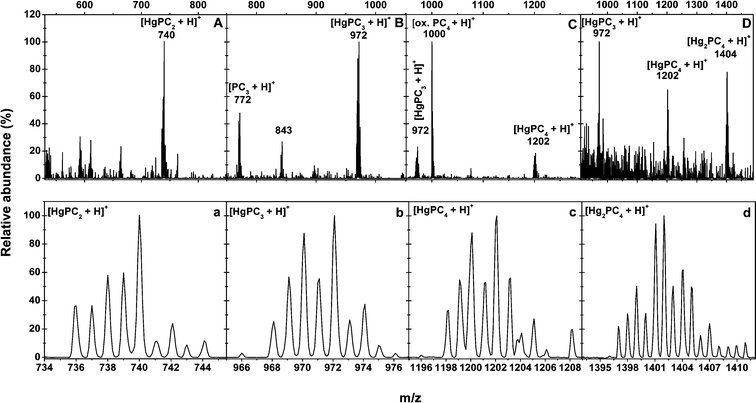
Immobilization and deactivation of heavy metal by natural compounds in plants, such as PCs, is the major mechanism in counteracting heavy metal toxicity. Structural studies of PC–metal complexes by extended X-ray absorption fine structure spectroscopy27,28, nuclear magnetic resonance spectroscopy29 and conventional optical spectroscopy have already documented a ligandation of Cd2+, Ag2+, Pb2+ and Hg2+ by thiolate coordination, as is known for the corresponding metallothionein–metal complexes.3013C-NMR spectroscopy was also employed to demonstrate the formation and connectivity of S,S′-bis(glutathionyl)–Hg2+ species in solution; Hg2+ bonding is exclusive to sulfur, and the amino and carboxyl moieties are remote from Hg2+ on account of an extended molecular chain configuration.31–33 As Hg2+ has a linear configuration in coordination compounds, the formation of Hg–PC complexes seems to be through the sequential substitution of the protons of two SHs of PCs by one Hg. Mehra et al. also showed that, by UV-VIS detection, the in vitro synthesized Hg–PC complexes are in different forms.12,34 However, just the presence of HgPC2 is ensured by ESI-MS17 and, for others, more were deduced than definitively detected.Although our previous results show the presence of in vitro synthesized Cd–PC complexes by direct-injection-ESI-MS/MS,19 those results do offer the possibility for the existence of Cd–PC complexes, but might not indicate the true speciation of Cd–PC complexes in solutions due to the possible generation of mixed complexes of different stoichiometry during the ESI process. In this study, since the Hg–PC complex was highly stable in a wide pH range (even at an acidic condition of pH 2.0),31 RPLC could be used for the mutual separation of different Hg–PC complexes (Fig. 1). RPLC coupled on-line with ESI-MS/MS was proposed to characterize the stoichiometry of the in vitro synthesized Hg–PC complexes, avoiding the possibility of unintelligible mixed Hg–PC complex formation during the ESI process. The in vitro synthesized Hg–PC complexes were characterized exactly by their own retention times and by mass spectrometric information. Fig. 1 and 2 show good separation of the Hg–PC complexes under the chromatographic conditions described in the experimental section in their corresponding mass spectra, indicating that HgGS2, HgPC2, HgPC3, HgPC4 and Hg2PC4 were formed under in vitro conditions. The main signals (as 202Hg) were assigned as follows: [HgGS2 + H]+ (m/z 815) and [HgGS2 + Na]+ (837) in Fig. 2A; [HgPC2 + H]+ (740) and [HgPC2 + Na]+ (762) in Fig. 2B; [HgPC3 + H]+ (972) and [PC3 + H]+ (772) in Fig. 2C; [HgPC4 + H]+ (1202), [HgPC4 + Na]+ (1224) and [PC4 + Na]+ (1022) in Fig. 2D; and [Hg2PC4 + H]+ (1404) and [Hg2PC4 + Na]+ (1426) in Fig. 2E. The lower part of each figure (Fig. 2a–e) shows the magnification of the corresponding [HgGS2 + H]+ and [HgPCn + H]+ (n = 2–5) ions due to the natural isotopic distribution of Hg (196Hg, 0.15%; 198Hg, 10.02%; 199Hg, 16.84%; 200Hg, 23.13%; 201Hg, 13.22%; 202Hg, 29.8%; and 204Hg, 6.85%.). The detected isotopic distribution of HgPC2 (736, 30.4%; 737, 47.3%; 738, 67.7%; 739, 52.4%; 740, 100.0%; 741, 35.1%; 742, 25.7%; 743, 10.6%; and 744, 8.6%), for example, was quite consistent with the theoretical isotopic distribution (734, 0.4%; 736, 27.8%; 737, 53.2%; 738, 78.2%; 739, 58.2%; 740, 100.0%; 741, 26.3%; 742, 31.1%; 743, 7.0%; 744, 3.2%; 745, 0.5%; and 746, 0.2%), which was calculated using an isotope pattern calculator.35 It is worth noting that the formation of HgPC4 implied that a disulfide bond formed between two Cys residues in the PC4 monomer. In addition, the capillary voltage was quite important in maintaining the stability of Hg–PC complexes; for example, the intensity of HgPC3 was 2.5 × 105 at 3500 V, while it was 4.8 × 104 at 3600 V. Thus, the high capillary voltage should be fairly tuned, otherwise this would lead to the dissociation or inexplicable formation of Hg–PC complexes.
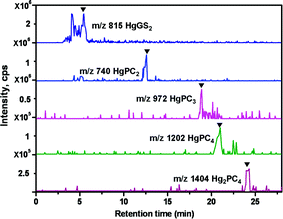 | ||
| Fig. 1 Analysis of in vitro synthesized Hg–PC complexes using RPLC-ESI-MS. Total ion chromatogram (TIC) and extracted ion chromatograms (EIC) of HgGS2, HgPC2, HgPC3, HgPC4 and Hg2PC4 analyzed by ESI-MS. ▼, species identified as mentioned in trace. | ||
 | ||
| Fig. 2 ESI-MS spectra of the in vitro synthesized HgGS2 (A), HgPC2 (B), HgPC3 (C), HgPC4 (D) and Hg2PC4 (E) and their isotopic peak distributions of HgGS (a), HgPC2 (b), HgPC3 (c), HgPC4 (d) and Hg2PC4 (e). | ||
In vivo Hg–PC complexes induced in the roots of B. chinensis under Hg–HA and HgCys2 stress
At the beginning of this study, we found that when the in vivo PCs and their corresponding Hg–PC complexes were extracted from the B. chinensis roots, they were partly subject to decomposition during the 24 h in the extraction solutions, while they were stable in the intact plant for at least one month at −20 °C. To determine the existence of the in vivo PCs and the Hg–PC complexes in the roots of both Hg–HA and HgCys2, stressed B. chinensis analysis should be performed within 24 h using RPLC-ESI-MS/MS after extraction. However, only oxidized PC2 (m/z 538), PC3 (m/z 770) and PC4 (m/z 1000) were detected besides the unequivocal identification of in vivo HgPC2, HgPC3, HgPC4 and Hg2PC4, as shown in Fig. 3A and 3B. The production of active oxygen species (such as H2O2) and the oxidative stress generated by Hg2+,36 which in turn oxidizes the induced PCs, might be responsible for the oxidation of the in vivo PCs, since the stronger reaction of PCs with H2O2 rather than with GSH or ascorbate has been suggested before.37 The existence of [HgPC2 + H]+ (m/z 740), [HgPC3 + H]+ (972), [HgPC4 + H]+ (1202), [Hg2PC4 + H]+ (1404) in the mass spectrum (Fig. 4) of the extract of the HgCys2 stressed B. chinensis roots was observed and confirmed by their corresponding isotopic distribution pattern. Similar results were obtained in the Hg–HA stressed B. chinensis roots. Moreover, Fig. 5 (as an example of an MS/MS spectrum of the Hg–PC complexes) shows there was a match between the fragment ions obtained for the in vitro synthetic HgPC2 standard (Fig. 5A) and that for the in vivo HgPC2 in the HgCys2 stressed B. chinensis roots (Fig. 5B), in addition to the identical retention time of 13.0 min recorded in the corresponding chromatograms shown in Fig. 1 and 3, respectively. | ||
| Fig. 3 Analysis of the in vivo Hg–PC complexes and PCs in the roots of B. chinensis under the stress of Hg–HA (A) and HgCys2 (B) using RPLC-ESI-MS. Total ion chromatogram (TIC) and extracted ion chromatograms (EIC) of oxidized PC2, oxidized PC3, oxidized PC4, HgPC2, HgPC3, HgPC4 and Hg2PC4 analyzed by ESI-MS. ▼, species identified as mentioned in trace. | ||
 | ||
| Fig. 4 ESI-MS spectra of the in vivo HgPC2 (A), HgPC3 (B), HgPC4 (C) and Hg2PC4 (D) and their isotopic peak distributions of HgPC2 (a), HgPC3 (b), HgPC4 (c) and Hg2PC4 (d) in the HgCys2 stressed B. chinensis roots. | ||
 | ||
| Fig. 5 ESI-MS/MS spectra of the in vitro HgPC2 standard (A) and the in vivo HgPC2 (B) in the HgCys2 stressed B. chinensis roots at m/z 740. | ||
In conclusion, our results demonstrated that Hg accumulation and in vivo PC production in B. chinensis were related to the kind of Hg species. Although the presence of HA and/or Cys reduced the Hg accumulation in the roots of B. chinensis, they improved the generation of in vivo PCs in these roots. Positive identification of in vivo oxidized PCs and Hg–PC complexes in the HgCys2 and Hg–HA stressed B. chinensis roots provided insight into the defense and accumulation mechanisms, elucidating the important roles of PCs in Hg tolerance, not only for their sequestration of free Hg2+ but also for reducing the oxidative stress in cells.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (20535020, 20775062), the National 863 Hi-Tech Project (2006AA06Z404) and the National Basic Research Program of China (2009CB416000). We thank Prof. John Hodgkiss, The University of Hong Kong, for assistance with the English in this paper.References
- L. Rodríguez, F. López-Bellido, A. Carnicer and V. Alcalde, Fresenius Environ. Bull., 2003, 12, 967 CAS.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149 CrossRef CAS.
- Environmental Health Criteria 1, World Health Organization, Geneva, 1976, pp. 1–132 Search PubMed.
- A. Bersenyi, S. Fekete, I. Hullar, I. Kadar, M. Szilagyi, R. Glavits, M. Kulcsar, M. Mezes and L. Zoldag, Acta Vet. Hung., 1999, 47, 181 Search PubMed.
- P. Kalac and L. Svoboda, Food Chem., 2000, 69, 273 CrossRef CAS.
- M. Conquery and P. M. Welbourn, Arch. Environ. Contam. Toxicol., 1994, 26, 335.
- T. L. Leonard, G. E. Taylor, Jr, M. S. Gustin and G. C. J. Fernandez, Environ. Toxicol. Chem., 1998, 17, 2063 CrossRef CAS.
- R. G. Person, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- D. Hesterberg, J. W. Chou, K. J. Hutchison and D. E. Sayers, Environ. Sci. Technol., 2001, 35, 2741 CrossRef CAS.
- D. Y. Wang, T. Y. Qing and Y. J. Guo, Water, Air, Soil Pollut., 1997, 95, 35 CAS.
- E. Grill, E.-L. Winnacker and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 439 CAS.
- R. K. Mehra, J. Miclat, R. Kodati, R. Abdullah, T. C. Hunter and P. Mulchandani, Biochem. J., 1996, 314, 73 CAS.
- R. A. Goyer, in Casarett and Doull’s toxicity: The Basic Science of Poisons, ed. C. D. Klaassen, M. O. Amdur and J. Doull, McGraw-Hill, New York, 2001, p. 111 Search PubMed.
- M. Gupta, R. D. Tripathi, U. N. Rai and P. Chandra, Chemosphere, 1998, 37, 785 CrossRef CAS.
- R. Howden and C. S. Cobbett, Plant Physiol., 1992, 99, 100.
- Y. J. Li, A. C. P. Heaton, L. Carreira and R. B. Meagher, Physiol. Plant., 2006, 128, 48 CrossRef CAS.
- S. Iglesia-Turiño, A. Febrero, O. Jauregui, C. Caldelas, J. L. Araus and J. Bort, Plant Physiol., 2006, 142, 742 CrossRef CAS.
- A. R. Khwaja, P. R. Bloom and P. L. Brezonik, Environ. Sci. Technol., 2006, 40, 844 CrossRef CAS.
- L. Q. Chen, Y. F. Guo, L. M. Yang and Q. Q. Wang, J. Anal. At. Spectrom., 2007, 22, 1403 RSC.
- K. A. Feldmann and M. D. Marks, Mol. Gen. Genet., 1987, 208, 1 CrossRef CAS.
- A. I. C. Ortiz, Y. M. Albarrán and C. C. Rica, J. Anal. At. Spectrom., 2002, 17, 1595–1601 RSC.
- T. Barkay, R. Turner, E. Saouter and J. Horn, Biodegradation, 1992, 3, 147 CrossRef CAS.
- J. Stary and K. Kratzer, J. Radioanal. Nucl. Chem., 1988, 126, 69 CrossRef CAS.
- A. R. Khwaja, P. R. Bloom and P. L. Brezonik, Environ. Sci. Technol., 2006, 40, 844 CrossRef CAS.
- C. Xiang and D. J. Oliver, Plant Cell, 1998, 10, 1539 CAS.
- J. M. Gong, D. A. Lee and J. I. Schroeder, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10118 CrossRef CAS.
- H. Strasdeit, A.-K. Duhme, R. Kneer, M. H. Zenk, C. Hermes and H.-F. Nolting, J. Chem. Soc., Chem. Commun., 1991, 16, 1129–1130 RSC.
- I. J. Pickering, R. C. Prince, M. J. George, W. E. Rauser, W. A. Wickramasinghe, A. A. Watson, C. T. Dameron, I. G. Dance, D. P. Fairlie and D. E. Salt, Biochim. Biophys. Acta, 1999, 1429, 351 CrossRef CAS.
- V. Dorĕák and A. Krężel, Dalton Trans., 2003, 11, 2253 RSC.
- J. H. R. Kägi, Methods Enzymol., 1991, 205, 613 CAS.
- B. J. Fuhr and D. L. Rabenstein, J. Am. Chem. Soc., 1973, 95, 6944 CrossRef CAS.
- B. Birgersson, T. Drakenberg and G. A. Neville, Acta Chem. Scand., 1973, 27, 3953 CrossRef CAS.
- G. A. Neville and T. Drakenberg, Can. J. Chem., 1974, 52, 616 CrossRef CAS.
- W. Bae and R. K. Mehra, J. Inorg. Biochem., 1997, 68, 201 CrossRef.
- J. H. Yan, Isotope Pattern Calculator v4.0, http://www.geocities.com/junhuayan/pattern.htm Search PubMed.
- B. Heidenreich, K. Mayer, H. J. R. Sandermann and D. Ernst, Plant, Cell Environ., 2001, 24, 1227 CrossRef CAS.
- N. Tsuji, N. Hirayanagi, M. Okada, H. Miyasaka, K. Hirata, M. H. Zenk and K. Miyamoto, Biochem. Biophys. Res. Commun., 2002, 293, 653 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
