Photomobile polymer materials—various three-dimensional movements†
Munenori
Yamada
a,
Mizuho
Kondo
a,
Ryo
Miyasato
a,
Yumiko
Naka
a,
Jun-ichi
Mamiya
a,
Motoi
Kinoshita
a,
Atsushi
Shishido
a,
Yanlei
Yu
b,
Christopher J.
Barrett
c and
Tomiki
Ikeda
*a
aChemical Resources Laboratory, Tokyo Institute of Technology, R1-11, 4259 Nagatsuta, Midori-ku, Yokohama 226-8503, Japan. E-mail: tikeda@res.titech.ac.jp; Web: http://www.res.titech.ac.jp/~polymer/index-e.html Fax: +81-45-924-5275; Tel: +81-45-924-5240
bDepartment of Materials Science, Fudan University, 220 Handan Road, Shanghai 200433, China
cDepartment of Chemistry, McGill University, 801 Sherbrooke Street West, Montreal, Quebec H3A 2K6, Canada
First published on 14th October 2008
Abstract
The composition of a crosslinked azobenzene liquid-crystalline polymer and a flexible polymer film can provide a variety of simple devices that can walk in one direction like an ‘inchworm’ and move like a ‘robotic arm’ induced by light.
Biomimetic actuators are receiving great interest for use as novel devices, such as humanoid robots and micro(nano)-machines, with stimulus-responsive gels and polymers.1 In particular, soft materials driven by light could play an important role as efficient energy conversion systems because light is a good energy source that can be controlled remotely, instantly and precisely. Polymers incorporating photochromic molecules like an azobenzene, which photoisomerizes reversibly between a rod-like trans and a bent cis isomer upon irradiation, respond to light irradiation by either expanding or contracting by a few percent.2
Crosslinked liquid-crystalline polymers (CLCPs) are unique materials possessing the properties of both liquid crystals (LCs) and elastomers.3,4 CLCPs exhibit a contraction along the alignment direction of mesogens when heated above the LC–isotropic (I) phase transition temperature.5 By incorporating azobenzene moieties into CLCPs, a larger deformation can be induced by photochemical reactions of these azobenzene chromophores.6 Furthermore, bending of CLCP films composed only of azobenzene mesogens has been observed by irradiation with UV light.7 It has also been reported that some unique movements of CLCP films, such as oscillating, twisting and swimming, can be induced by light.8 Most recently, photoinduced rotational motions have been demonstrated, including a first light-driven plastic motor with CLCP laminated films.9
In this communication, we demonstrate new three-dimensional movements of architectures prepared from these photoresponsive materials. We were able to induce large and sophisticated motions of laminated films composed of a CLCP layer containing azobenzene moieties and a plastic sheet by photoirradiation, which leads to three-dimensional movements such as an inchworm walk and a flexible robotic arm motion.
Homogeneously aligned CLCP films used in this study were prepared by photopolymerization of a mixture of LC monomers (1 and 2) shown in Fig. 1a with a ratio of 20 : 80 mol : mol containing 2 mol% of a photoinitiator in a glass cell coated with rubbed polyimide alignment layers. The mixture shows a smectic phase on cooling from 89 °C, and photopolymerization was conducted at a temperature where the mixture exhibited a smectic phase. The freestanding CLCP films were taken off from the cells after polymerization. The CLCP films prepared in the smectic phase showed a higher order of azobenzene mesogens with their alignment along the rubbing direction of the polyimide alignment layers and produced a larger mechanical force by irradiation with UV light than those prepared in the nematic phase.7f Furthermore, the glass-transition temperature of the CLCP films appeared around room temperature as detected by differential scanning calorimetry, allowing the films to work at room temperature. The CLCP laminated films were prepared by thermal compression bonding of a CLCP layer and an unstretched low-density polyethylene (PE) film with an adhesion layer (Fig. 1b).
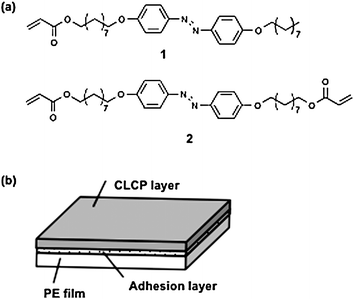 | ||
| Fig. 1 Chemical structures of the LC monomer (molecule 1) and LC diacrylate (molecule 2) used in this study. On cooling from 92 °C, molecule 1 changes from an isotropic to a smectic phase, and at 60 °C it becomes crystalline; on cooling from 91 °C, molecule 2 changes from an isotropic to a smectic phase, and at 74 °C it becomes crystalline; on cooling from 89 °C, the mixture of molecule 1 + molecule 2 (20 : 80 mol : mol) changes from an isotropic to a smectic phase, and at 60 °C it becomes crystalline. | ||
Fig. 2a shows a photoinduced motion of a CLCP laminated film having a curved shape, partly laminated with a CLCP layer. In this CLCP laminated film, the azobenzene mesogens were aligned along the long axis of the film. The CLCP laminated parts are curled due to the difference in the thermal expansion coefficients between the two layers. Upon irradiation with UV light, the laminated film extended to a flat shape and reverted to the initial bent shape upon irradiation with visible light, which could be repeated at room temperature just by changing the wavelength of the actinic light. We measured the change in length of the CLCP layer by alternate irradiation with UV and visible light (Fig. 2b). Upon exposure to UV light, the trans–cisphotoisomerization of the azobenzene moieties in the CLCP layer occurs and a contraction force is generated along the alignment direction of the azobenzene mesogens of the CLCP layer through photoinduced reduction of LC order. The contraction of the CLCP layer results in extension of the whole laminated film. On the other hand, upon irradiation with visible light, the cis--trans back-isomerization occurs and the film reverts to the initial bent state.
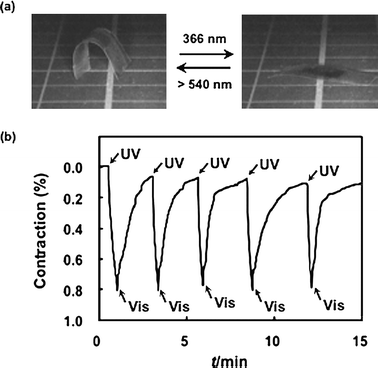 | ||
| Fig. 2 (a) Photographs showing the photoinduced extension and retraction motion of the CLCP laminated film at room temperature. The film extended upon irradiation with UV light and reverted to the initial shape upon irradiation with visible light. Size of the CLCP laminated film: 16 mm × 8 mm; the CLCP laminated part: 6 mm × 5 mm. Thickness of the layers of the film: PE, 50 µm; CLCP, 18 µm. (b) Change in length of the CLCP layer by alternate irradiation with UV (366 nm, 20 mW cm−2) and visible light (>540 nm, 40 mW cm−2) at 30 °C. | ||
Fig. 3a demonstrates a unidirectional motion, an inchworm walk, of the CLCP laminated film with asymmetric end shapes. The film moves forward upon alternate irradiation with UV and visible light at room temperature (see Movie 1 in the ESI† for real-time movement). A close inspection of this inchworm walk has revealed that upon exposure to UV light, the film extends forward because the sharp edge acts as a stationary point, and the film retracts from the rear side upon irradiation with visible light because the flat edge acts as the stationary point, which enables the film to move in one direction only (Fig. 3b).
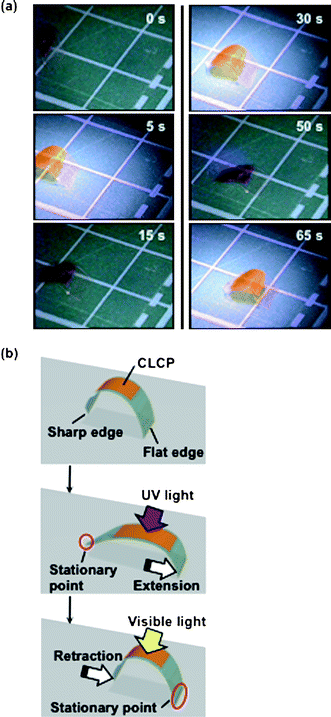 | ||
| Fig. 3 (a) Series of photographs showing time profiles of the photoinduced inchworm walk of the CLCP laminated film by alternate irradiation with UV (366 nm, 240 mW cm−2) and visible light (>540 nm, 120 mW cm−2) at room temperature. The film moved on the plate with 1 cm × 1 cm grid. (b) Schematic illustrations showing a plausible mechanism of the photoinduced inchworm walk of the CLCP laminated film. Upon exposure to UV light, the film extends forward because the sharp edge acts as a stationary point (the second frame), and the film retracts from the rear side by irradiation with visible light because the flat edge acts as a stationary point (the third frame). Size of the film: 11 mm × 5 mm; the CLCP laminated part: 6 mm × 4 mm. Thickness of the layers of the film: PE, 50 µm; CLCP, 18 µm. | ||
Additionally, as CLCP laminated films are readily fabricated, we can easily make many devices in arbitrary shapes and sizes. In other words, we can make plastic films without photoresponsive properties to move freely at any place by partially laminating with CLCP layers. For example, we prepared a rolled-up film by laminating with CLCP layers at two places as shown in the first frame of Fig. 4. The azobenzene mesogens here were aligned along the long axis of the film. Fig. 4 shows a sequential and flexible motion of the laminated film by light at room temperature (see Movie 2 in the ESI† for real-time movement). Upon exposure to UV light, the CLCP laminated parts extend from a curved shape to a flat one and revert to the initial state upon irradiation with visible light, working as a ‘hinge joint’, which leads to a large and flexible movement of the whole film. By controlling the irradiation position and the intensity of the actinic light, one can drive the film in a chosen manner, which enables us to use it as a flexible ‘robotic arm’ to manipulate objects just by light irradiation.
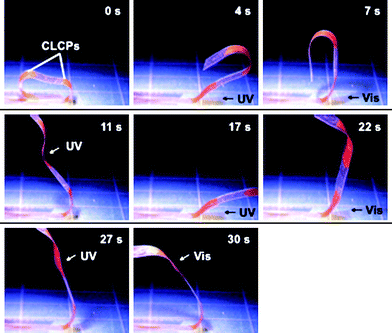 | ||
| Fig. 4 Series of photographs showing time profiles of the flexible robotic arm motion of the CLCP laminated film induced by irradiation with UV (366 nm, 240 mW cm−2) and visible light (>540 nm, 120 mW cm−2) at room temperature. Arrows indicate the direction of light irradiation. Spot size of the UV light irradiation is about 60 mm2. Size of the film: 34 mm × 4 mm; the CLCP laminated parts: 8 mm × 3 mm and 5 mm × 3 mm. Thickness of the layers of the film: PE, 50 µm; CLCP layers, 16 µm. | ||
In summary, laminated films composed of a homogeneously aligned CLCP layer and a PE film have been prepared. Large and rapid-responsive motions with the CLCP composite materials are successfully induced by photoirradiation, which leads to novel three-dimensional movements such as an inchworm walk and a flexible robotic arm motion. These results show the high possibility of numerous applications based on CLCP composites that can convert light energy directly into mechanical work and move without any batteries or electric wires.
References
- (a) M. V. Gandhi and B. S. Tomson, Smart Materials and Structures, Chapman and Hall, London, 1992 Search PubMed; (b) Polymer Sensors and Actuators, ed. Y. Osada and D. E. DeRossi, Springer, Berlin, 2000 Search PubMed; (c) Y. Osada, H. Okuzaki and H. Hori, Nature, 1992, 355, 242–244 CrossRef CAS; (d) S. Maeda, Y. Hara, T. Sakai, R. Yoshida and S. Hashimoto, Adv. Mater., 2007, 19, 3480–3484 CrossRef CAS.
- (a) A. Natansohn and P. Rochon, Chem. Rev., 2002, 102, 4139–4175 CrossRef CAS; (b) G. Smets and F. De Blauwe, Pure Appl. Chem., 1974, 39, 225–238 CrossRef CAS; (c) C. D. Eisenbach, Polymer, 1980, 21, 1175–1179 CrossRef CAS; (d) L. Matéjka, M. Ilavsky, K. Dusek and O. Wichterle, Polymer, 1981, 22, 1511–1515 CrossRef CAS.
- (a) P.-G. de Gennes, M. Hébert and R. Kant, Macromol. Symp., 1997, 113, 39–49 CAS; (b) R. Zentel, Adv. Mater., 1989, 1, 321–329; (c) M. Warner and E. M. Terentjev, Liquid Crystal Elastomers, Oxford University Press, Oxford, UK, 2003 Search PubMed; (d) P. Xie and R. Zhang, J. Mater. Chem., 2005, 15, 2529–2550 RSC.
- T. Ikeda, J. Mamiya and Y. Yu, Angew. Chem., Int. Ed., 2007, 46, 506–528 CrossRef CAS.
- (a) J. Küpfer and H. Finkelmann, Macromol. Chem. Phys., 1994, 195, 1353–1367 CrossRef; (b) M. Warner and E. M. Terentjev, Prog. Polym. Sci., 1996, 21, 853–891 CrossRef CAS; (c) G. N. Mol, K. D. Harris, C. W. M. Bastiaansen and D. J. Broer, Adv. Funct. Mater., 2005, 15, 1155–1159 CrossRef CAS; (d) H. Wermter and H. Finkelmann, e-polymers, 2001 Search PubMed , no. 13; (e) D. L. Thomsen III, P. Keller, J. Naciri, R. Pink, H. Jeon, D. Shenoy and B. R. Ratna, Macromolecules, 2001, 34, 5868–5875 CrossRef; (f) J. Naciri, A. Srinivasan, H. Jeon, N. Nikolov, P. Keller and B. R. Ratna, Macromolecules, 2003, 36, 8499–8505 CrossRef CAS.
- (a) H. Finkelmann, E. Nishikawa, G. G. Pereira and M. Warner, Phys. Rev. Lett., 2001, 87, 015501 CrossRef CAS; (b) P. M. Hogan, A. R. Tajbakhsh and E. M. Terentjev, Phys. Rev. E, 2002, 65, 041720 CrossRef CAS; (c) M.-H. Li, P. Keller, B. Li, X. Wang and M. Brunet, Adv. Mater., 2003, 15, 569–572 CrossRef CAS.
- (a) T. Ikeda, M. Nakano, Y. Yu, O. Tsutsumi and A. Kanazawa, Adv. Mater., 2003, 15, 201–205 CrossRef CAS; (b) Y. Yu, M. Nakano and T. Ikeda, Nature, 2003, 425, 145 CrossRef CAS; (c) Y. Yu, M. Nakano and T. Ikeda, Pure Appl. Chem., 2004, 76, 1435–1445; (d) Y. Yu, M. Nakano, A. Shishido, T. Shiono and T. Ikeda, Chem. Mater., 2004, 16, 1637–1643 CrossRef CAS; (e) M. Kondo, Y. Yu and T. Ikeda, Angew. Chem., Int. Ed., 2006, 45, 1378–1382 CrossRef CAS; (f) Y. Yu, T. Maeda, J. Mamiya and T. Ikeda, Angew. Chem., Int. Ed., 2007, 46, 881–883 CrossRef CAS; (g) J. Mamiya, A. Yoshitake, M. Kondo, Y. Yu and T. Ikeda, J. Mater. Chem., 2008, 18, 63–65 RSC.
- (a) K. D. Harris, R. Cuypers, P. Scheibe, C. L. van Oosten, C. W. M. Bastiaansen, J. Lub and D. J. Broer, J. Mater. Chem., 2005, 15, 5043–5048 RSC; (b) T. J. White, N. V. Tabiryan, S. V. Serak, U. A. Hrozhyk, V. P. Tondiglia, H. Koerner, R. A. Vaia and T. J. Bunning, Soft Matter, 2008, 4, 1796–1798 RSC; (c) M. Camacho-Lopez, H. Finkelmann, P. Palffy-Muhoray and M. Shelley, Nat. Mater., 2004, 3, 307–310 CrossRef CAS; (d) N. Tabiryan, S. Serak and X. M. Dai, Opt. Express, 2005, 13, 7442–7448 CrossRef CAS.
- M. Yamada, M. Kondo, J. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986–4988 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization details; movies showing inchworm walk and flexible robotic arm motions of CLCP laminated films. See DOI: 10.1039/b815289f |
| This journal is © The Royal Society of Chemistry 2009 |
