Smelting in the age of nano: iron aerogels†
Nicholas
Leventis
*a,
Naveen
Chandrasekaran
a,
Chariklia
Sotiriou-Leventis
a and
Arif
Mumtaz
b
aDepartment of Chemistry, Missouri University of Science & Technology, Rolla, MO 65409, USA. E-mail: leventis@mst.edu; Fax: +1-573-341-6033; Tel: +573-341-4391
bDepartment of Physics, Quaid-i-Azam University, Islamabad, Pakistan. E-mail: arif@qau.edu.pk; Tel: +92-51-96042127
First published on 17th October 2008
Abstract
Smelting of interpenetrating networks of resorcinol-formaldehyde (RF) and iron oxide (FeOx) aerogels yields porous ferromagnetic and superparamagnetic materials in monolithic form with compositions closely resembling that of pig iron.
About 3000 years ago humankind entered the iron age by inventing the smelting process, where an iron ore (e.g., hematite) is reduced by carbon to metallic iron according to:
| Fe2O3 + C → Fe + CO2 |
The condensation of resorcinol (R) and formaldehyde (F) is catalyzed effectively by HCl.7 Thus, by moving from the typical Na2CO3/H2O/80 °C system,8 to a HCl/CH3CN/room temperature gelation protocol it has been possible to accelerate the RF gelation process from 7 days at 80 °C to a couple of hours at room temperature.7 Meanwhile, hydrated metal oxides such as [Fe(H2O)6]3+, are fairly strong Brønsted acids (pKa ∼ 3.5).9 That property is used successfully in conjunction with the irreversible proton-consuming ring opening of epoxides (e.g., epichlorohydrin) for the formation of metal–oxygen–metal networks leading to gelation and ultimately to metal oxide aerogels.9,10 Reasoning that the acidity of a [Fe(H2O)6]3+ sol gelling towards iron oxide could be utilized for the co-gelation of a RF network, we prepared sols by mixing solution A containing 0.337 g (3.06 mmol) R and 0.455 mL of a commercial 37% w/w solution of F (6.11 mmol) in 5.75 mL CH3CN, and solution B containing 0.89 g (3.3 mol) FeCl3·6H2O, and 2.75 mL (33 mmol) of epichlorohydrin in 5 mL EtOH. That sol was poured into glass molds and it was allowed to react at 80 °C for 1 h followed by room temperature aging for 24 h. Gelation occurs in 10 min, but there is no way of knowing which network is formed first, because the red color of the iron salt obscures the red color of o-quinone methide that develops upon gelation of the RF system.7 Thus, the duration of the post-gelation aging was decided by monitoring of the pH during gelation, and on control experiments with RF-hafnia (see ESI†).
The HCl/CH3CN : EtOH (1 : 1 v/v)/RF system gels at 80 °C in 10 min and its pH remains around zero throughout the process. The [Fe(H2O)6]3+/CH3CN : EtOH (1 : 1 v/v)/RF system gels at 80 °C in ∼50 min (confirming the role of the hydrated iron salt as a catalyst for the RF gelation), and its pH remains very acidic rising from 0.2 to 0.6 during the process. The [Fe(H2O)6]3+/CH3CN : EtOH (1 : 1 v/v)/epichlorohydrin system (no RF) gels in ∼10 min and consumes protons.9 Indeed the pH starts at 0.6, gelation takes place at pH ∼ 4.2 and it reaches pH ∼ 7.0 in ∼50 min where it remains constant thereafter. The complete [Fe(H2O)6]3+/CH3CN : EtOH (1 : 1 v/v)/RF/epichlorohydrin system gels also in ∼10 min; the pH starts at 0.6, gelation occurs at pH ∼ 3.8 and it keeps rising reaching a value of ∼5.5 after 70 min. The analogous RF-hafnia control system gels also in ∼10 min, but the initial gels are almost colorless, allowing observation of the red color of the RF network developing in ∼40 min (see ESI†). The pH data and the RF-hafnia system together indicate that the iron oxide network is formed first. Since at about neutral pHs the RF system is expected to gel in a day,11RF/FeOx wet gels were aged for another 24 h in their molds.
RF-FeOx wet gels were washed with CH3CN : EtOH (1 : 1 v/v, 3×) and solvent-exchanged with acetone (4×). They were either dried supercritically to native n-RF-FeOx aerogels, or in order to increase their mechanical integrity they were crosslinked with a hexamethylene diisocyanate oligomer (Desmodur N3300A, supplied by Bayer) that yields a conformal polyurea coating over the entire skeletal framework according to procedures well established in our laboratory.12 The crosslinked samples are designated X-RF-FeOx. All wash solvents were collected, dried and the solid residue was pyrolyzed at 800 °C in air yielding a small amount of pure Fe2O3, corresponding to 98.9% mol/mol of the initial iron in the sol retained in the gel. Both n- and X-RF-FeOx were characterized thermogravimetrically (TGA), microscopically (SEM), physically (N2 sorption porosimetry), chemically (energy dispersive spectroscopy: EDS or EDAX, and XRD) and magnetically (MH curves).
By TGA in air (see ESI†) n-RF-FeOx loses 65% of its mass by 375 °C, showing that it consists of about 65% w/w RF. Under the same conditions X-RF-FeOx loses 90% of its mass by 500 °C, consistent with a massive polymer uptake during crosslinking. SEM (Fig. 1) confirms that both n- and X-RF-FeOx aerogels consist of networks of nanoparticles. In X-RF-FeOx, polymer coats clusters of nanoparticles leaving a vast interstitial space open in agreement with our previous studies.12 Porosities (as % v/v empty space) were calculated from bulk and skeletal densities (ρb = 0.046 g cm−3 and ρs = 2.86 ± 0.12 g cm−3 for n-RF-FeOx; ρb = 0.418 ± 0.015 g cm−3 and ρs = 1.42 ± 0.031 g cm−3 for X-RF-FeOx) and they are equal to 98.2 ± 0.2% and 70.6 ± 1.0% for n- and X-RF-FeOx, respectively. The corresponding BET surface area of n- and X-RF-FeOx are 410 m2g−1 and 80 m2g−1, respectively.
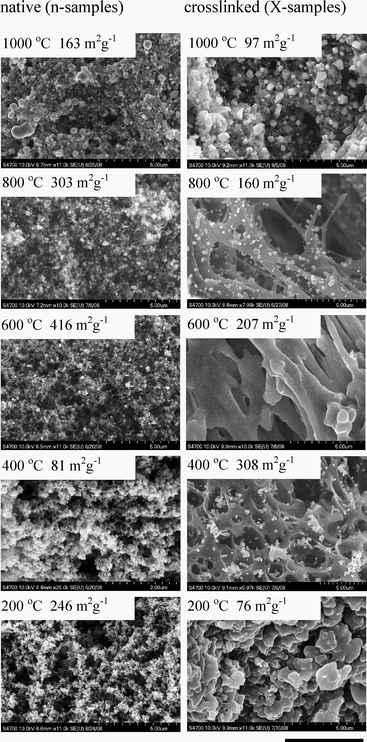 | ||
| Fig. 1 SEMs of native (n-RF-FeOx) and crosslinked (X-RF-FeOx) monoliths after pyrolysis under Ar at different temperatures. The length bar is 5 µm. BET surface areas for each sample are also provided. | ||
Pyrolysis under a flowing stream of Ar in the 200–1000 °C range yields different chemical and structural evolution for the two materials. By SEM, X-RF-FeOx display macroporosity as early as 400 °C, consistently with previous findings with crosslinked RF aerogels:13 based on extensive correlation of SEM, 13C-NMR, TGA and DSC data, the chemical bonding between the crosslinking polymer (polyurea) and the RF network breaks at ∼250 °C; the polymer, finding itself above its melting point (123 °C), melts and exerts surface tension forces to the surrounding network that cause partial collapse creating macropores.13 That morphology survives after pyrolysis at 1000 °C and the material resulting from X-RF-FeOx (called X-C-Fe) is clearly macroporous with a BET surface area σ = 97 m2 g−1. By comparison n-C-Fe is mesoporous with σ = 163 m2 g−1 (see ESI†).
By EDS (see ESI† and Fig. 2) the O content as a function of the pyrolysis temperature in both n- and X-RF-FeOx samples decreases monotonically to zero. The C content in both cases reaches a maximum at ∼35% after pyrolysis at 600 °C and then decreases to <5% w/w after pyrolysis at 1000 °C. The Fe content, however, behaves differently in the two materials: it increases monotonically in n-RF-FeOx, but it shows a local minimum at 400 °C in X-RF-FeOx. Clearly, n- and X-RF-FeOx have different chemical evolutions as confirmed by XRD (Fig. 3 and Table 1).
| 200 °C | 400 °C | 600 °C | 800 °C | 1000 °C | |
|---|---|---|---|---|---|
| n-RF-FeOx | |||||
| γ-Fe2O3 | 100 (4.1) | 100 (16) | 100 (28) | 4.3 (19) | |
| Fe3C | 48.8 (33) | ||||
| Fe | 10.9 (36) | 100 (35) | |||
| Fe15.1C | 18.2 | ||||
| C | 17.8 (7.8) | ||||
| X-RF-FeOx | |||||
|---|---|---|---|---|---|
| γ-Fe2O3 | 100 (4.6) | 49.5 (27) | 11.3 (23) | 4.8 (19) | |
| Fe3C | 44.3 (30) | 74.5 (36) | 43.7 (33) | 75.0 (30) | |
| Fe | 14.2 | 13.3 (9.6) | 25.0 (25) | ||
| Fe15.1C | 9.9 | ||||
| C | 28.3 (3.2) | ||||
| FeO | 6.1 (35) | ||||
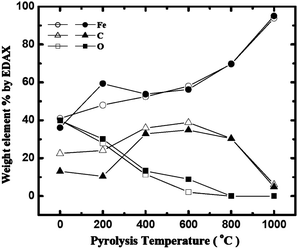 | ||
| Fig. 2 EDS analysis of n-RF-FeOx (open symbols) and X-RF-FeOx (dark symbols) after pyrolysis at different temperatures. | ||
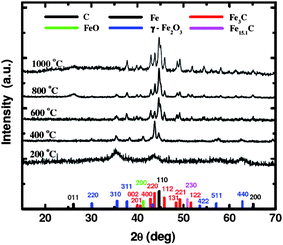 | ||
| Fig. 3 XRD data of X-RF-FeOx aerogels after pyrolysis under Ar at different temperatures (for the XRD data of n-RF-FeOx, see ESI†). | ||
As-made n- and X-RF-FeOx are both amorphous. At 200 °C, we observe broad reflections corresponding to microcrystalline maghemite (γ-Fe2O3) with crystallite size of only 4.1 nm. At 400 and 600 °C, n-RF-FeOx continues to show γ-Fe2O3 as the only crystalline phase present while the reflections become sharper and the crystallite size increases. At 800 °C, a small amount of γ-Fe2O3 is still present in both samples, but their main crystalline make up includes Fe3C, iron, austenite (Fe15.1C) and graphite. After pyrolysis at 1000 °C, the only crystalline phase of n-C-Fe is iron, even though EDS shows a small amount of (presumably amorphous) carbon (4.9%). The behavior of X-RF-FeOx is quite different. As early as 400 °C, we note the formation of Fe3C and a small amount of wustite (FeO). At 600 °C, wustite is no longer present and we note the formation of Fe. At 800 °C, the two main crystalline components of X-C-Fe are Fe3C and Fe, while at 1000 °C those are the only ones. Based on the Fe3C : Fe w/w ratio of 3 : 1 (see Table 1) the amount of carbon in the crystalline components of X-C-Fe is calculated at 5.0%. By EDS, the total amount of carbon after pyrolysis at 1000 °C was 6.08%. Therefore, X-C-Fe produced at 1000 °C consists almost exclusively of crystalline Fe3C and Fe with about the same crystallite sizes (∼25–30 nm, Table 1), and presumably a small amount (about 1.1%) of amorphous carbon. Basically, the chemical composition of both n- and X-C-Fe is very similar to that of pig iron. Obviously, however, the thermolytic behavior of the two materials has been different and this is attributed to the molten crosslinker that first causes partial collapse of the RF and FeOx networks and subsequently it plays the role of a “solvent” where nanoparticles undergo more efficient mixing and reaction.
After pyrolysis at 800 or 1000 °C n-C-Fe and X-C-Fe are both electrically conducting and magnetic (attracted by small laboratory magnets). Analysis of MH loops, however, reveals that their magnetic properties are also quite different. After pyrolysis at 800 °C, n-C-Fe shows ferromagnetic behavior with a hysteresis loop and nonvanishing magnetization at zero field (Fig. 4A). The demagnetization curve follows a concave shape (rather than the more conventional convex shape), a behavior that is enhanced as the temperature is raised to 325 K, where a pinched-shaped hysteresis loop is evident. Finally, the magnetization at 20 kOe does not reach saturation, which is a typical behavior of small particles with modified surfaces.14 On the other hand, after pyrolysis at 800 °C X-C-Fe is superparamagnetic at 300 K (it shows zero coercivity and remanence at zero field, Fig. 4B) consistent with smaller particles (refer to the corresponding SEM) and a lower blocking temperature (at around 140 K, see ESI†). It is noted, however, even though the loop of X-C-Fe has collapsed at the origin, there is yet significant hysteresis at higher fields. The anomalous behavior of both n- and X-C-Fe samples, in one case the pinched hysteresis loop and in the other case a nonvanishing hysteresis at high fields, most probably stems from the presence of two ferromagnetic phases namely, the soft Fe phase and a relatively hard Fe3C phase.
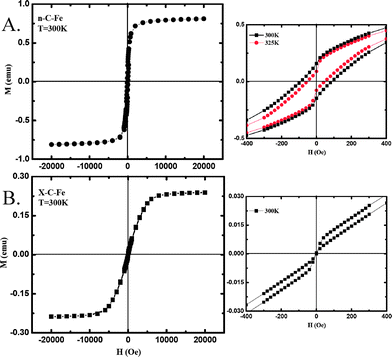 | ||
| Fig. 4 Magnetization loops of the materials obtained by pyrolysis at 800 °C of n-RF-FeOx (A), and of X-RF-FeOx (B). Figures on the right magnify the region around the origin. n-C-Fe shows ferromagnetic and X-C-Fe superparamagnetic behavior. | ||
In conclusion, porous pig iron is produced by smelting interpenetrating RF and FeOx aerogels. Crosslinking alters the thermolytic behavior leading to macropores, but most importantly by melting it mixes intimately the skeletal RF and FeOx nanoparticles depressing their reaction temperature by as much as 400 °C. This is explored further with other interpenetrating networks of nanoparticles.
Acknowledgements
We thank NSF for financial support under CHE-0809562 and CMMI-0653919.References
- S. A. Al-Muhtaseb and J. A. Ritter, Adv. Mater., 2003, 15, 101 CrossRef CAS.
- C. Moreno-Castilla and F. J. Maldonado-Hódar, Carbon, 2005, 43, 455 CrossRef CAS.
- C. Moreno-Castilla, F. J. Maldonado-Hódar and A. F. Perez-Cadenas, Langmuir, 2003, 19, 5650 CrossRef CAS.
- T. F. Baumann, G. A. Fox, J. H. Satcher, N. Yoshizawa, R. Fu and M. S. Dresselhaus, Langmuir, 2002, 18, 7073 CrossRef.
- S. A. Steiner III, T. F. Baumann, J. King, J. H. Satcher Jr. and M. S. Dresselhaus, Langmuir, 2007, 23, 5161 CrossRef.
- L. C. Cotet, M. Gich, A. Roig, I. C. Popescu, V. Cosoveanu, E. Molins and V. Danciu, J. Non-Cryst. Solids, 2006, 352, 2772 CrossRef CAS.
- S. Mulik, C. Sotiriou-Leventis and N. Leventis, Chem. Mater., 2007, 19, 6138 CrossRef CAS.
- R. W. Pekala, J. Mater. Sci., 1989, 24, 3221 CAS.
- A. E. Gash, T. M. Tillotson, J. H. Satcher, Jr., J. F. Poco, L. W. Hrubesh and R. L. Simpson, Chem. Mater., 2001, 13, 999 CrossRef CAS.
- N. Leventis, P. Vassilaras, E. F. Fabrizio and A. Dass, J. Mater. Chem., 2007, 17, 1502 RSC.
- E. Bekyarova and K. Kaneko, Adv. Mater., 2000, 12, 1625 CrossRef CAS.
- N. Leventis, Acc. Chem. Res., 2007, 40, 874 CrossRef CAS; N. Leventis, C. Sotiriou- Leventis, G. Zhang and A.-M. M. Rawashdeh, Nano Lett., 2002, 2, 957 CrossRef CAS.
- S. Mulik, C. Sotiriou-Leventis and N. Leventis, Chem. Mater., 2008 DOI:10.1021/cm801428p.
- R. H. Kodama, A. E. Berkowitz, E. J. McNiff, Jr. and S. Foner, Phys. Rev. Lett., 1996, 77, 394 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Photographs of aerogel monoliths, pH variation during gelation, photographs of gelling RF-FeOx and RF-hafnia, TGA data for n-RF-FeOx and X-RF-FeOx, typical EDS data, XRD experimental and data for n-RF-FeOx, N2 sorption isotherms and temperature dependence of zero-field-cooled magnetization of X-C-Fe. See DOI: 10.1039/b815985h |
| This journal is © The Royal Society of Chemistry 2009 |
