Novel catalyst support materials for PEM fuel cells: current status and future prospects
Yuyan
Shao
,
Jun
Liu
,
Yong
Wang
and
Yuehe
Lin
*
Pacific Northwest National Laboratory, Richland, WA, 99352, USA. E-mail: yuehe.lin@pnl.gov
First published on 10th November 2008
Abstract
Catalyst support materials exhibit great influence on the cost, performance, and durability of polymer electrolyte membrane (PEM) fuel cells. This feature article summarizes several important kinds of novel support materials for PEM fuel cells (including direct methanol fuel cells): nanostructured carbon materials (carbon nanotubes, carbon nanofibers, mesoporous carbon), conductive doped diamonds and nanodiamonds, conductive oxides (tin oxide/indium tin oxide, titanium oxide, tungsten oxide), and carbides (tungsten carbides). The advantages and disadvantages, the acting mechanism to promote electrocatalytic performance, and the strategies to improve present catalyst support materials and search for new ones are discussed. This is expected to shed light on future development of catalyst supports for PEM fuel cells.
 Yuyan Shao | Dr Yuyan Shao received his PhD in Applied Chemistry from Harbin Institute of Technology in 2006. He is currently a postdoctoral research associate in the Chemical & Materials Sciences Division at the Pacific Northwest National Laboratory. His main research interests are in the fields of electrochemical energy conversion and storage, and his current research has focused on durable catalytic materials for PEM fuel cells. |
 Yuehe Lin | Dr. Yuehe Lin is a Laboratory Fellow at the Pacific Northwest National Laboratory. His research interests include electrochemistry, nanobiotechnology, nanomaterials, and biosensors. He has more than 10 patents and over 200 publications. He serves as editor and editorial advisory board member of 16 international journals; additionally, he guest-edited a special issue on Nanomaterials for Sensing and Electrocatalysis for Journal of Nanoscience and Nanotechnology. He also co-edited the Handbook of Electrochemical Nanotechnology and was a section editor of the Encyclopedia of Microfluidics and Nanofluidics. |
1. Introduction
Polymer electrolyte membrane (PEM) fuel cells are being developed as electrical power sources for vehicles and for stationary and portable applications as an alternative to conventional internal combustion engines, secondary batteries, and other conventional power sources. Much effort from government, industry, and academy has been devoted to developing PEM fuel cells in the last few decades, and great advances have been achieved.1 However, PEM fuel cells, including direct methanol fuel cells (DMFCs), are still far from market launch, which is hindered by two main issues:1–3 the prohibitive production cost and poor durability and reliability.Catalysts exhibit great influence on both the cost and the durability of PEM fuel cells.4 Most of the presently used catalysts are Pt-based and are usually supported on porous conductive materials with a high specific surface area. The support materials are necessary to obtain a high dispersion and a narrow distribution of Pt and Pt-alloy nanoparticles, which is the prerequisite to obtain a high catalytic performance of catalysts. The support materials can also interplay with catalytic metals, which influences the catalytic activity. The durability of the catalyst is also greatly dependent on its support. Generally, the requirements for catalyst support materials can be summarized as:2 1) high specific surface area, which is necessary for improving the dispersion of catalytic metals, 2) low combustive reactivity under both dry and humid air conditions at low temperatures (150 °C or less), 3) high electrochemical stability under fuel cell operating conditions, 4) high conductivity, and 5) easy-to-recover Pt in the used catalyst. In addition, the interaction between catalytic metals and the support materials5 should be considered in improving the catalytic activity and durability. The most popular support material is Vulcan XC-72 carbon black.
In addition to the recently increased emphasis on electrocatalytic metals,6,7 much effort has been devoted to developing novel catalyst supports, including novel nanostructured carbons (such as carbon nanotubes [CNTs],8–10 carbon nanofibers [CNFs]11 and mesoporous carbon12), oxides,13 carbides,14 nitrides,15 etc. Nanostructured carbon can also be doped by other atoms16,17 or compounds to enhance both the catalytic activity and durability of the resultant catalysts. These support materials can be roughly classified into two categories: 1) the primary supports, such as novel nanostructured carbons and conductive diamonds, and 2) the secondary supports, such as oxides, which are mainly used to modify and promote the primary supports, even though they can also be used as independent supports. Recent progress in several important support materials, both primary and secondary, is briefly summarized. The problems associated with current support materials and future challenges and possible approaches are discussed.
2. Novel nanostructured carbons
2.1 Carbon nanotubes and carbon nanofibers
Novel nanostructured carbon materials (CNTs8–10,18,19 and CNFs20) have been receiving attention for a long time as catalyst supports for PEM fuel cells because of their unique structure and properties.21 For example, CNTs provide overwhelming advantages as compared with conventional carbon black for PEM fuel cell electrocatalyst supports:22 1) the unique structure and electrical properties of CNTs provide a high electrical conductivity and a specific interaction between catalytic metals and the CNT supports (the delocalized π electrons of CNTs and Pt d-electrons), resulting in a higher catalytic activity, 2) CNTs have few impurities, while carbon black (e.g., Vulcan carbon XC-72) contains significant quantities of organosulfur impurities, which can poison Pt metal, 3) CNTs are free from deep cracks that exist in carbon black in which Pt nanoparticles deposited will lose catalytic activity because the electrochemical triple-phase boundary (TPB) does not form. CNTs make it possible to construct an ordered catalyst layer in PEM fuel cells,10,19 which might provide benefits for mass/electron transport, resulting in higher cell performance. These are also true for CNFs.Some review articles on CNT/CNF-supported catalysts have already been reported,23,24 primarily focusing on the synthesis and characterization of CNT/CNF-supported electrocatalysts. Here, we put the emphasis on the influence of CNT/CNF nanostructures on the performance and durability of resultant electrocatalysts, such as single-wall carbon nanotubes (SWNTs),25,26 double-wall carbon nanotubes (DWNTs),27 multi-wall carbon nanotubes (MWNTs),8,18,28,29 hollow-structured MWNT/CNFs, bamboo-structured MWNT/CNFs,30 cup-stacked CNTs,31 herringbone CNFs,32,33 and so on. Fig. 1 and Fig. 2 show representative transmission electron microscopy (TEM) images of these nanostructured carbons.
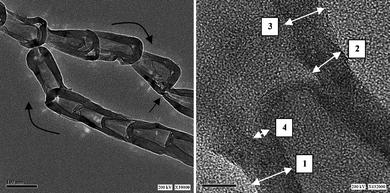 | ||
| Fig. 1 TEM images of bamboo-shaped CNTs:34 1) wall graphitic layers, 2) dangling graphitic layers, 3) compartmentalization of original graphitic layers, and 4) compartment graphitic layers. Reprinted by permission of ref. 34. Copyright the American Chemical Society 2006. | ||
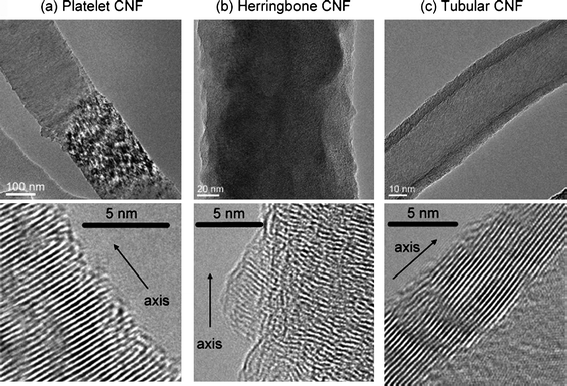 | ||
| Fig. 2 HRTEM images of (a) platelet, (b) herringbone, and (c) tubular CNFs.35 Reprinted by permission of ref. 35. Copyright the American Chemical Society 2007. | ||
First, the number of carbon shells and diameters (SWNTs, DWNTs, and MWNTs) exhibits a great influence on the performance of electrocatalysts. In terms of specific surface area, SWNTs are better than DWNTs and MWNTs as PEM fuel cell catalyst supports because generally the smaller the CNT diameter, the larger the specific surface area, which is beneficial for highly dispersing Pt nanoparticles. This is also confirmed in some experimental studies on CNT-supported catalysts. Wu et al.36 and Tang et al.37 reported that SWNT-supported Pt catalysts show better performance toward methanol oxidation than those supported by DWNTs37 and MWNTs.36,37 They attributed the enhanced catalytic activity to the higher utilization and activation of Pt metals on SWNTs because of the higher specific surface area of SWNTs, leading to a higher dispersion of Pt nanoparticles.36,37 They also proposed that a better charge transfer at the electrode/electrolyte interface due to a sounder graphic crystallinity of SWNTs36 and a lower bulk resistance of CNTs with the single wall than that of CNTs with multiwalls37 also contributed to the enhanced catalytic activity of Pt/SWNTs. However, these claims regarding the properties of SNWTs were in conflict with some other available literature in which parts of the SWNTs are claimed to be semiconductors, depending on their chirality and structure.38 This exhibits a slower electron transfer rate than MWNTs.10 There is also a controversial report concerning the catalytic activity of these three kinds of CNT-supported catalysts. Li et al.27 reported that, among the catalysts of PtRu supported on SWNTs, DWNTs, MWNTs, and carbon black, the PtRu/DWNT catalyst shows the highest specific activity for methanol oxidation reaction (MOR) in rotating disk electrode experiments and the highest performance as an anode catalyst in DMFC single-cell tests. Chen et al.39 also reported that Pt/DWNTs show the highest catalytic activity toward the oxygen-reduction reaction (ORR). The difference between the results of Li et al.,27 Chen et al.,39 Wu et al.,36 and Tang et al.37 might be due to the different structures of their respective CNTs and the synthesis process of the catalysts. Whatever the cause, the enhancement of CNTs as the support material on the catalytic activity of the resultant catalysts toward fuel cell reactions, as compared with Vulcan XC-72 carbon black, has been widely observed.24,40–42 But the conflicting reports as described above led us to pursue more investigations on the structure effect of catalyst support and the open possibility to further improve the catalyst performance. For examples, parts of SWNTs are known to be semiconductors, depending on their chirality,38 and they exhibit low electronic conductivity, which must negatively influence the catalytic activity of the supported electrocatalysts. So the question arises: in the case of Pt/SWNTs, are Pt nanoparticles deposited on semiconductive SWNTs or conductive SWNTs, or both? This should make a great difference in promoting electrocatalysis. How about metal-enriched SWNTs43 as electrocatalyst supports? These are still open questions for CNTs as electrocatalyst supports.
In addition to the number of carbon shells of CNTs, the catalytic activity of catalysts supported by CNTs is also influenced by the CNTs' carbon nanostructures.32,44 For example,30 bamboo-structured MWNTs, in which the axis of the graphite planes is at an angle to the axis of nanotubes, which, like bamboo, are periodically closed along their lengths into compartments and formed “bamboo-knots”(Fig. 1), was observed to show faster electron transfer in electrochemical electrode processes,30 as compared with hollow-structured MWNTs in which the axis of the graphite planes is parallel to the axis of the nanotubes.45 So it is reasonable to speculate that bamboo-structured MWNT-supported catalysts show a higher performance than those supported by hollow-structured MWNTs. This has been confirmed for the reduction of H2O2.45 The enhanced catalytic activity of catalysts supported by bamboo-structured MWNTs is attributed to its greater proportion of edge plane-like defect sites in the structure of bamboo-structured MWNTs.45
The support nanostructure influences catalyst performance, and this was also found in CNF-supported catalysts.11,32,35 It was found46 that platelet CNF-supported Pd (Pd/p-CNF) is more active toward ORR than fish-bone CNF-supported Pd (Pd/f-CNF), as can be seen from the onset reduction potential and ORR current peak potentials. This is a result of the higher ratio of edge atoms to basal atoms of p-CNF. This is consistent with Tsuji et al.'s report35 in which PtRu/CNF catalysts with platelet-CNF as support were observed to exhibit higher activity toward methanol oxidation than those supported by tubular-CNFs and herringbone-CNFs.35 Steigerwalt et al. found that PtRu/graphitic carbon nanofiber (GCNF) nanocomposites with narrow tubular herringbone GCNFs exhibited a higher performance as a DMFC anode than SWNTs, MWNTs, and wide herringbone GCNF-supported PtRu catalysts.32 The authors attributed this to the difference in the electrical conductivity of these four carbons.32 It was proposed that electrons produced on the surfaces of PtRu catalyst particles could flow across the graphene sheets of the herringbone layers to the highly conducting tubular graphitic core without encountering significant ohmic barriers in the narrow tubular herringbone GCNFs,32 which, however, has not been confirmed by an independent experiment. It was also found that twisted CNFs performed better than straight CNFs for PEM fuel cell catalyst support, and the performance of CNTs and CNFs with a smaller diameter was better than that of larger ones.47
From the above, it can be concluded that the structure defects in CNTs or CNFs play an important role in improving catalytic activity, which is mainly due to the enhanced electron transfer rate.48 This provides guidance for developing PEM fuel cell electrocatalyst supports. However, as is known, the corrosion of carbon materials always initiates at defect sites.49,50 The influence of structure defects on the durability of electrocatalysts supported by CNTs and CNFs is still an open question. Few reports are available in this area of research.
The durability of the supports and the catalysts is another important issue, which has received more attention in recent years.1,2,51 The support materials exhibit great influence on electrocatalyst durability. Carbon support can be oxidized into CO2/CO or surface oxygen-containing species under PEM fuel cell conditions. In the former case, Pt or other catalytic metal nanoparticles will detach from the support and lose activity; in the latter case, Pt–carbon interactions will be weakened. More detailed information about the influence of the support on electrocatalyst durability can be found in an article by Shao et al.2 CNTs have been shown to improve the durability of catalysts for PEM fuel cells as compared with Vulcan XC-72 carbon black.2
The resistance to electrochemical corrosion of bare MWNTs has recently been compared with that of widely used Vulcan XC-72 carbon black under simulated PEM fuel cell conditions using the accelerated degradation test (ADT) methods,49,52 i.e., to degrade the CNT electrode and carbon black electrode in a diluted acid solution at a high potential (1.2V reversible hydrogen electrode [RHE]), which was used to mimic PEM fuel cell conditions. It was found that, after a certain period of degradation test, the increase in surface oxygen content was much higher for Vulcan carbon than that for MWNTs, indicating that CNTs are more resistant to electrochemical corrosion.49,52 The durability of MWNTs also depends on their diameters, with medium-size MWNTs exhibiting higher stability under electrochemical stressing.53
CNT-supported Pt catalyst (Pt/CNT) was found to be more durable under the ADT conditions than the carbon black-supported Pt/C,50,52,54 which corresponds to the durability of the supports. The degradation in the electrochemical surface area of Pt nanoparticles is greatly suppressed when Pt is supported on CNTs as compared with that on Vulcan XC-72 carbon black.50,54 As is known,2,3 the electrochemical surface area of Pt is one of the most important factors that influences the performance of a fuel cell electrode. The enhanced durability of Pt/CNTs is attributed to the strong resistance to corrosion of CNTs50,54 and the specific interaction between Pt nanoparticles and CNTs.5,50 The durability of Pt/CNTs can be further improved by high-temperature graphitization of CNTs.55
The nanostructures and carbon shells in CNTs (i.e., SWNTs, DWNTs, and MWNTs) also influence the durability of the supported Pt catalysts. It has been shown that the durability of MWNT-supported Pt is better than that of SWNT-supported Pt,39,56 and DWNT-supported Pt has a slightly higher stability than Pt/MWNTs.39 They all have better durability than Pt/C.39
It is obvious that these novel nanostructured carbon materials (CNT/CNFs) achieve promising performance in terms of both catalytic activity and durability. However, there is still room to improve using a properly nanostructured carbon. A synthesis strategy for carbon materials with controlled nanostructures needs to be developed and the catalytic performance–nanostructure relationships need to be established.
2.2 Mesoporous carbon
Mesoporous carbon (with pore sizes from 2 nm to 50 nm) can be classified into two categories according to their structures and morphologies:21 1) ordered mesoporous carbon (OMC), which is usually synthesized by nanocasting ordered mesoporous silica (OMS) templates or by directly templating triblock copolymer structure-directing species and 2) disordered mesoporous carbon (DOMC) with irregular pore structures. Although DOMC shows mesoporosity, the mesopores are, in most cases, isolated or irregularly interconnected, which results in low conductivity, and the distribution of pore sizes is relatively wider than those of OMC. So OMC is preferred as catalyst support in terms of specific surface area, electrical conductivity, and mass transport.Mesoporous carbon materials as catalyst supports for PEM fuel cells have been extensively studied.57–59 In contrast to conventional carbon supports, mesoporous carbon exhibits an attractive property structure as a catalyst support, i.e., a large surface area with mono-dispersed three-dimensionally interconnected mesopores. The mesoporous carbon-supported Pt60,61 and Pt alloy catalysts62,63 and even non-precious metal catalysts64 have shown excellent performance in PEM fuel cell electrode reactions. This is attributed to the high and uniform dispersion of catalytic metals, the high electrical conductivity, and the enhanced mass transfer due to the specific pore structure of mesoporous carbon.65 As is known, a high-performance PEM fuel cell electrode requires an efficient reaction zone at nanoscale, i.e., the TPB, which is the zone accessible for mass (reactants and products), protons, and electrons, in which only the electrochemical reactions can occur. As mentioned above, conventional carbon support (Vulcan XC-72 carbon black) has more micropores (<2 nm) and deep cracks, making it difficult to form TPB and resulting in decreased Pt utilization when Pt nanoparticles are deposited in it because it is difficult for the reactants and proton-conducting polyelectrolyte (e.g., Nafion) to access the micropores. Carbon supports with a pore size larger than 50 nm have a lower surface area and a higher electrical resistance.63 So it is also expected that the performance of the carbon-supported catalysts is influenced by both the pore structures and sizes as well as the electrical conductivity of mesoporous carbon. Yu et al.59,66 studied the influence of the pore size of porous carbon (in the range of 10 to 1000 nm) on the catalytic activity of the supported PtRu catalysts under DMFC conditions. They found that the porous carbon with a mesopore size (25 nm) showed the highest performance, which corresponds to a 43% increase in activity as compared to that of a commercially available PtRu/C catalyst (E-TEK).59,66 This higher performance was attributed not only to the higher surface areas and larger pore volumes, which allows a higher degree of catalyst dispersion, but also to the highly integrated interconnected pore systems with periodic order, which allows an efficient transport of reactants and products.59,66
Another factor that influences the catalyst performance is the electrical conductivity of mesoporous carbon; for example, some Pt catalysts supported by high-resistance mesoporous carbon perform more poorly than those using Vulcan XC-72 carbon black as a support.67 There are several ways to decrease the electrical resistance of mesoporous carbon (the inherent resistance and the contact resistance between carbon particles):68 the use of aromatic carbon precursors in the preparation of mesoporous carbon, high-temperature annealing, and catalytic graphitization. Recent studies69,70 have shown that high-temperature annealing and catalytic graphitization can enhance the graphitization degree of mesoporous carbon, which exhibits electrical conductivity up to two orders higher than that obtained for the non-graphitized samples.70 However, the graphitization strategies have to be implemented at the expense of scarifying the porosity and the specific surface area of the resultant carbon materials, making it difficult to deposit metal nanoparticles. Recently, new strategies have been developed to decrease the resistance without the above problems. Su et al.68 developed an approach to largely increase the electrical conductivity of ordered mesoporous carbon by bridging the OMC particles with CNTs (from 138 S·m−1 [before bridging] to 645 S·m−1 [after bridging]). The synthesis procedure is illustrated in Fig. 3: an ordered mesoporous SBA-15 silica template was first infiltrated with carbon using a chemical vapor deposition (CVD) method with benzene as the carbon precursor to yield a carbon/SBA-15 silica composite. Second, a metal nanoparticle catalyst was supported on the surface of the composite. Third, the growth of CNTs was carried out on the composite surface. Finally, the silica framework and metal catalyst were removed to leave behind OMC particles bridged with CNTs. Another new strategy is to modify the mesoporous carbon with polypyrrole (PPy). After OMC selectively covered the outer surface with PPy, it was found that71 the electrical resistance of PPy-OMC showed a lower value than that of pristine OMC; however, at a high loading amount of PPy, the resistance rose to the value of pristine OMC. But it is known that the long-term stability of PPy in an acid environment is poor, so this method for improving the electrical conductivity of OMC still needs reconsideration if used in PEM fuel cells. But this “bridge strategy” provides a novel way to improve electronic conductivity of nanostructured porous carbon materials.
 | ||
| Fig. 3 Schematic illustration of preparing a CNT-bridged mesoporous carbon (OMC/CNT): (a) SBA-15 silica template, (b) carbon/SBA-15 silica composite after infiltration of the pores of the template with carbon using the CVD method, (c) impregnation of a metal catalyst on the external surface of the carbon/SBA-15 silica composite, (d) growth of CNTs catalyzed by the metal catalyst, (e) OMC/CNT after removal of the silica template and catalyst, (f) the carbon network of OMC/CNT showing the bridging of OMC by CNTs.68 Reprinted by permission of ref. 68. Copyright Elsevier Science BV. 2007. | ||
Nanostructured carbons (CNT, CNF, and mesoporous carbon) are usually used as primary supports for PEM fuel cells. However, there are still some challenges to their widespread commercial application as PEM fuel cell electrocatalyst supports. The production cost of these materials, especially for SWNT and DWNT, is still much higher than that of Vulcan XC-72 carbon black. The durability of the nanostructured carbons, even though some of them performed better than Vulcan XC-72, still needs improvement. The doping strategy with a secondary element (such as nitrogen16) and the composites of nanostructured carbons and other compounds seem promising in this aspect, which is discussed below.
3. Conductive diamonds
Conductive diamonds, usually produced by doping diamonds with a secondary element such as boron-doped diamond (BDD), have been studied for about two decades as excellent electrodes in many electrochemical fields because of their attractive properties, such as an extremely wide potential window, a very low background current, and in particular a high chemical and dimensional stability.72 Recently, more attention has been paid to the durability issues to find durable component materials for PEM fuel cells.1–3 Conductive doped diamond is intrinsically attractive for application as a durable catalyst support because of its above-mentioned specific properties.73,74 Furthermore, the fact that all of the Pt nanoparticles are usually deposited on its outer surface and not inside micropores is also advantageous for catalyst supports.75 Moreover, the mechanical and chemical stability of doped diamond also allows the use of “severe” methods, such as thermal decomposition, for preparing catalyst nanoparticles without significant modification of the surface properties of the substrate,76,77 which provides more chances to obtain catalysts with new structures and properties. Recently, much effort has been devoted to developing doped diamond supported catalysts for PEM fuel cells.78–80Platinum and platinum alloy (Pt/Sn,81 Pt/Ru,76 Pt80Ru10Sn10,80 Pt-RuO2-RhO2,82 etc.) were often deposited on the doped diamond support by the microemulsion method,80,81 the sol-gel method,82 the sputtering method,83 or electrochemical deposition83–86 to produce electrocatalysts or model electrocatalysts. The doped diamond supported catalysts have shown excellent performance toward ORR83 and the electrochemical oxidation of methanol,87 ethanol,82 and ethylene glycol,88 which are the widely used fuels for direct-oxidation fuel cells. The bi-metallic or tri-metallic catalysts supported on doped diamond show similar behavior toward alcohol oxidation, which means that, for example, for both methanol and ethanol electrooxidation, the tri-metallic Pt80Ru10Sn10 catalyst exhibited lower onset potentials80 than either pure Pt or the corresponding bimetallic catalysts (Pt/Ru76 and Pt/Sn81). The Pt-RuO2-RhO2/BDD electrode82 exhibits an enhanced anti-CO poisoning effect for the methanol and the ethanol oxidation reactions as compared with Pt/BDD and Pt-RuO2/BDD electrodes, similar to the case in common sp2 carbon-supported catalysts.89 This means that conductive doped diamond as a support material can act in the same way as the widely used sp2 carbon, which also indicates its potential application as a catalyst support.
The electrically conducting nanocrystalline boron-doped diamond was also used to modify sp2 carbon to improve its chemical resistance and mechanical stability,90,91 which could be used as a PEM fuel cell catalyst support.91 It was found that there were no microstructural changes in nanocrystalline boron-doped diamond with or without Pt on it even if it was stressed at about 1.6 V. This confirmed the high stability of doped diamond-supported catalysts.91
Wang and Swain84,92,93 developed boron-doped diamond stabilized Pt catalysts via a sequential fabrication procedure of diamond growth/Pt deposition/diamond growth, in which Pt nanoparticles were incorporated into a carbon matrix, as shown in Fig. 4. The surrounding carbon matrix is expected to reduce the mobility of Pt nanoparticles,92 which will improve the stability of the resultant catalysts. For example, no loss in activity was observed after 2000 potential cycles between the oxygen and hydrogen evolution regimes in 0.1 M HClO4,92 and no evidence of morphological or microstructural damage and no loss of catalyst activity for hydrogen evolution or oxygen reduction was observed after harsh electrolysis in hot 85% H3PO4 (170 °C),73 while a Pt-impregnated sp2 carbon cloth electrode was observed to catastrophically fail under the same stressing conditions.73 The deposition of Pt nanoparticles can be carried out using magnetron sputtering92 and electrochemical deposition,84,85 with the electrochemical deposition providing a relatively high dispersion of Pt nanoparticles,79,84 but the size of the Pt nanoparticles (10 to 300 nm) is still too large for fuel cell applications.84 Furthermore, about 1/3 of the active Pt surface is lost after the secondary diamond deposition because of the combination of complete coverage of some smaller particles and partial coverage of the base of larger particles.84 Further work is still needed to improve the dispersion of Pt nanoparticles and to alleviate the negative influence of the coverage of Pt nanoparticles from the secondary diamond deposition. The oxygen-reduction behavior on the diamond-supported electrode is similar to that on clean polycrystalline Pt,84 which exhibits the fastest kinetics in 0.1 M HClO4 as compared with 0.1 M H2SO4 and 0.1 M H3PO4. In addition, it was found that the diamond matrix exerts little negative influence on the oxygen-reduction response. Whatever may be the explanation, Swain et al.'s work84,92,93 again confirmed the promising application of doped diamond as a PEM fuel cell catalyst support and also provided a new strategy to produce durable catalysts for PEM fuel cells: to stabilize the catalytic metal nanoparticles with the post-deposited durable carbon matrix surrounding it (Fig. 4).84
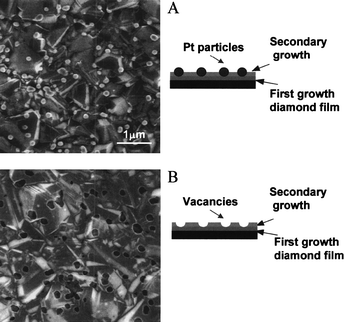 | ||
| Fig. 4 SEM images of Pt/diamond composite electrode (A) before and (B) after acid etching in aqua regia. Pt particles with diameters of 50 to 300 nm are seen on the surface before the acid etching (A). Numerous pits are observed in the diamond film after acid etching (B). These pits are the voids left after dissolving the metal and have diameters of 50 to 300 nm. This observation confirms that the diamond film does surround the base of some of the metal particles, entrapping and anchoring them in the surface microstructure. The depth of the pits depends on the secondary diamond-growth time.84 Reprinted by permission of ref. 84. Copyright the Electrochemical Society, 2003. | ||
Boron-doped diamond is also considered as a good substrate for studying the intrinsic properties of deposited catalytic nanoparticles,76,77 avoiding the problems encountered with other common substrates, i.e., surface corrosion, oxide formation, or electronic interactions with the deposit. This is beneficial for the fundamental study of electrocatalysis. The very high stability of conductive boron-doped diamond also allowed the study of the catalytic properties of noble-metal oxide modifications from the level of submonolayer to a few monolayers, avoiding the serious interference of the reactivity of the generally adopted metal supports.94
However, there are still some problems with doped diamonds as electrocatalyst supports:95 the low conductivity, the low surface area, and the poor dispersion of the catalytic metals. In addition, it is still difficult to realize a homogeneous and controllable boron doping level in diamond powders.96
As is known, pure diamond is an insulator. The conductivity of diamond, on the one hand, can be improved by doping97 because of the increased number of charge carriers and defects; on the other hand, the stability of the doped diamond is negatively influenced by the doping (nitrogen doping,97 boron doping,98 etc.). Several factors influence the electrical conductivity and thus the electrochemical behavior of the doped diamond powder:99,100 1) the concentration and the mobility of charge carriers, which are greatly influenced by the doping level, 2) the particle–particle connectivity,95 and 3) the nondiamond carbon impurity.95 For example, in order to have sufficient electrical conductivity for electrochemical applications (<0.1 Ωcm), diamond films must be doped with boron at a concentration of 1 × 1019 cm−3 or greater to provide sufficient charge-carrier concentration and mobility (typical carrier concentrations are in the range of 1018 to 1020 cm−3 with carrier mobilities [holes] of 10 to 500 cm2/Vs).75
The dispersion of the deposited catalytic particles is still poor, and the size of catalytic metal particles is too large to be used as real catalysts because of the much lower Pt utilization.87,101 This might be improved by modifying the synthesis method76,77,79,102 and pretreating the surface of the diamond;103 the latter will also influence the conductivity.
The specific surface area of doped diamond is still unacceptably low for catalyst supports. Researchers discovered that, besides the doping method, the vacuum annealing of undoped nanocrystalline diamond (also called nanodiamond104) can also make a conducting diamond,105 which is more promising for use as a catalyst support because of its enormous specific surface area. The enhanced conductivity of nanodiamonds is attributed to the features of a large specific surface area and high numbers of surface defects as well as the cluster structure of a dense diamond core and a relatively loose shell with a nondiamond phase and surface functional groups.106 The recently developed hybrid diamond-graphite nanowires are also expected to increase both the surface area and the electrical conductivity.107
Based on the fact that conductive diamonds exhibited significantly enhanced durability under electrochemical stressing conditions as compared with other support materials, we propose that they work better for fuel cell cathodes because cathodes see more severely corrosive conditions than anodes,2 even though conductive diamonds as catalyst support exhibit good performance for both anodes (the electrooxidation of alcohol and hydrogen) and cathodes (oxygen reduction). Researchers who are interested in conductive diamonds for PEM fuel cell catalyst support are recommended to pursue this line of research.
4. Conductive oxides
Many conductive or semi-conductive oxides have been studied as catalyst support materials or as a secondary supports to modify and promote the catalyst support for PEM fuel cells, including indium tin oxide (ITO),13 TiOx,108 WOx,109 IrO2,110 SnO2,111 and so on. These metal oxides have shown promising effects on the catalytic activity109,110,112–114 and durability115,116 of PEM fuel cell catalysts. For example, it has been shown that Pt catalysts supported on tin oxide or indium tin oxide can change the reaction pathways of the electrochemical oxidation of ethanol from two-electron and four-electron partial oxidation to 12-electron complete oxidation.117 The corrosion of the catalyst support could be minimized by modifying the support surface with semiconducting oxides, such as WO3, TiO2, or by coating carbon on metal oxides.115 The following discussion selectively reviews several important oxides that have been used as fuel cell catalyst support materials.4.1 Tin oxide/indium tin oxide (SnOx/ITO)
Metal tin and its oxides have been used for a long time to alloy with Pt supported on carbon black or CNTs as anode catalysts for direct alcohol fuel cells (DAFCs).118 Here we treat the oxides as the support materials.Sun's group111,119 reported the electrochemical deposition of Pt119 and PtRu111 nanoparticles onto the surface of tin oxide (SnO2) nanowires directly grown on carbon paper. The nanowire-based electrode exhibited higher electrocatalytic activity toward both ORR and MOR, as compared with standard Pt/C (30 wt% ETek). It has also been shown that when tin oxide supported platinum catalysts were heat-treated under oxidizing or reducing atmospheres at various temperatures, their catalytic activity for methane combustion and the electrochemical oxidation of CO were enhanced because of their peculiar microstructure and metal–support interactions.120
Tin oxides as the support are also expected to improve the durability of the resultant catalysts. It is known that the degradation of Pt nanoparticle catalysts can be greatly alleviated if the electrochemical oxidation of Pt nanoparticles at high electrode potential (>0.8V RHE) can be suppressed.2,6 The Ota group121 recently found that SnO2 influenced the oxidation and reduction behavior of Pt which is supported on it, in which the adsorption/desorption of oxygen on platinum and the formation/reduction of platinum oxides above 0.6V (RHE) were suppressed. The authors attributed this to the “strong metal–support interaction” between SnO2 and Pt, which might make Pt more resistant to corrosion. This “strong metal–support interaction” was also expected to increase the catalytic activity towards ORR.122
Even though SnO2 is expected to improve the durability of Pt nanoparticles supported on it, it is unstable by itself due to the redox properties121 under electrochemical conditions. It has been recently found by Chhina et al.13 that when tin is combined with indium to form ITO, the ITO has potential as an oxidation-resistant candidate material for catalyst supports in PEM fuel cells. It is much more resistant to oxidation than Vulcan XC-72R, but no results on the catalytic activity towards ORR or MOR are provided in Chhina et al.'s report.13 Chang et al. studied the electrocatalytic properties of Pt nanoparticles attached to ITO (PtNP/ITO) for oxygen reduction, and methanol oxidation123 and Pd nanoparticles on ITO (PdNP/ITO) for oxygen reduction.124 The methanol oxidation behavior on PtNP/ITO is similar to that on bulk Pt,123 with even faster kinetics, and the oxygen reduction on PtNP/ITO123 and PdNP/ITO124 is also similar to that on bulk Pt and Pd, respectively. These indicate that ITO is a promising support for fuel cell catalysts.
4.2 Titanium oxides (TixOy)
Titanium oxide nanomaterials have been widely used in photocatalysis.125 Its application as an electrocatalyst support material has also been pursued recently. Although pure TiO2 is a semiconductor with a band gap of 4.85 eV and an electrical conductivity of only 10−13 Ω−1·cm−1 at 298K,126 it can still be used as an electrode material if defects are introduced by partial reduction to give TiO2 − x or more fully to give the sub-stoichiometric oxide compositions of the general formula TnO2n − 1(the Magnelli phase, where n is between 4 and 10, commercially termed as ‘Ebonex(R)’).13,108,127 For example, Ti4O7 in particular exhibits a high electrical conductivity of 1000 S·cm−1 at room temperature,128 as compared to graphitized carbon with a conductivity of 727 S·cm−1.108 Moreover, these oxides show a high electrochemical stability in acid (Fig. 5),108,129 and can also be used to increase the stability of the electrode.116,130,131 Furthermore, labile protons were observed on titanium oxide, which indicates that this kind of material should have pronounced proton conductivity,132,133 which is quite beneficial for the formation of the electrochemical TPB. This improves the utilization of catalytic metals deposited on it, and titanium oxides can even be used as fuel cell electrolytes.134 So titanium oxides intrinsically are promising fuel cell catalyst supports. Pt/alloys135 and Pd136 deposited on such oxides have shown attractive performance toward ORR137 and MOR.136,138 In the case of PtRu deposited on supporting nanoporous TiO2 − x films for methanol oxidation,135 a lower CO-poisoning effect has been observed for cluster PtRu on a TiO2 − x support film than that for unsupported PtRu or bare Pt catalysts. When a TiO2 thin film is deposited between the Pt and the Nafion membrane, fuel cell performance can be increased compared to a Pt film deposited directly on the Nafion membrane.133 These enhanced performances are attributed to the better dispersion of metal nanoparticles on titanium oxides.133,139 The substrate–catalyst interactions, as shown from quantum mechanical calculations,135 lead to a more facile 2D diffusion of COad from Pt sites to Ru and the supporting TiO2 − x. For example, in the case of methanol oxidation on PtRu/TiO2 − x, this lowers the binding energy of COad and some other intermediates on catalytic metals and also lowers the activation energy for surface mobility of COad.135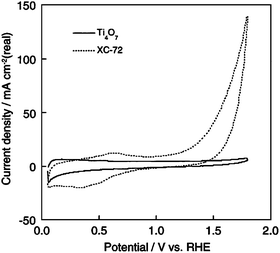 | ||
| Fig. 5 Cyclic voltammograms of the Ti4O7-only and Vulcan XC-72-only electrodes bonded to a Nafion membrane in a single PEM fuel cell configuration under flowing N2. The cell was operated at 80 °C and atmospheric pressure with a humidification temperature of 80 °C. The sweep rate was 100 mV·s−1.108 Reprinted by permission of ref. 108. Copyright Elsevier Science Inc. 2005. | ||
Doping titanium oxide with Nb is another way to produce a conductive support material for electrocatalysts.140,141 It has been shown that the Pt catalysts supported with doped titanium oxide exhibited excellent performance toward ORR compared with those supported with carbon black.141 In addition to the high dispersion of catalytic metal nanoparticles, the interaction between oxide support and metal catalyst, as indicated by the X-ray absorption near edge structure spectroscopy (XANES) spectra of the Pt L edge obtained with the supported catalysts,141 also contributed to the enhanced catalytic activity. Garcia et al.142 reported that the mass activity of PtRu/Nb0.1Ti0.9O2 toward MOR is higher than that of PtRu/C, even though the electrochemical surface area of the former is only about 50% of the latter. These results suggest that the titanium oxides enhance the catalytic activity of the PtRu in a way that carbon cannot,142 which means that the acting mechanisms might be different for these two kinds of catalyst supports. Further investigation is needed to elucidate the underlying nature of the process that will help develop novel support materials. Chen et al.140 reported that, even though Ebonex and Ti4O7 are stable in acid, they can still be oxidized to non-conductive TiO2 at the catalyst/support/electrolyte triple-phase interface after extensive polarization at the positive potentials of the oxygen electrode. On the other hand, Ti0.9Nb0.1O2 remains both thermally and electrochemically stable. This indicates that although conductive oxides can be created either by the formation of a sub-stoichiometric oxide or by doping, doping is the preferred method for enhancing electrical conductivity and stability in oxidizing environments.140
Adding titanium oxides in carbon-supported catalysts can also improve their catalytic performance.143,144 This can be realized through several approaches. Pt/TiOx/C nanocomposites can be synthesized by depositing hydrated titanium oxide on carbon-supported Pt (Pt/C), or by reducing H2PtCl6 on carbon-supported hydrated titanium oxide (TiO2/C), or by one-pot synthesis of hydrated titanium oxide and Pt nanoparticles by reducing H2PtCl6 on carbon support. Among these alternatives, the catalyst that is synthesized by reducing H2PtCl6 on carbon-supported hydrated titanium oxide (TiO2/C) exhibits the highest performance toward oxygen reduction in DMFC and the best methanol tolerance.143 This method has been widely used to synthesize Pt/TiO2-CNT144 and PtRu/TiO2-C,145 both of which also exhibit high catalytic performance toward ethanol/methanol oxidation. Physically mixed titanium oxide and carbon-supported catalysts sometimes also produce catalysts with enhanced performance as compared with carbon-only supported catalysts. For example, adding Au/TiO2 to a PtRu/C electrode has been shown to improve the performance of DMFCs.146 Catalysts from the mixing of TiO2 nanotubes and Pt/C exhibit higher Pt utilization, higher CO tolerance, and higher activity toward ethanol oxidation,147 which are attributed to the following factors:147 the amount of H2O contained in the titanium nanotubes was more than 20 times higher than that in TiO2 before undergoing chemical treatment,148 which makes it easy to form hydroxide species; the tube-like structure of TiO2 nanotubes has a larger specific surface area than TiO2,149 and it is proposed that this will trap more ethanol to increase the local concentration of ethanol around Pt.
Novel nanostructures of titanium oxides have been developed recently, some of which have exhibited promising performance as fuel cell catalyst supports. For example, the carbon-coated anatase titania (CCT) TiO2@C core–shell-like structures developed by Shanmugam et al.115,150 have been employed as Pt nanoparticle supports for ORR and MOR. This exhibits a higher catalytic activity and stability towards MOR and ORR than commercial Pt/C, as shown in Fig. 6. The higher activity of Pt/CCT was attributed to several factors:115 the conductivity of CCT was found to be higher than that of the Vulcan XC-72; Pt nanoparticles deposited on CCT are of high dispersion and good stabilization; the electronic structure of Pt nanoparticles on CCT is expected to be modified by interaction with the oxide interface, which results in a change in the adsorption characteristics of Pt on CCT; the charge-transfer resistance for methanol oxidation on Pt/CCT was almost only half that of commercial Pt/C. Recently developed titania nanotubes have also been used as Pt151 and Pd136 nanocatalyst supports, which exhibit higher activity towards MOR than commercial ETek Pt/C and Pd-TiO2 nanoparticles, respectively.
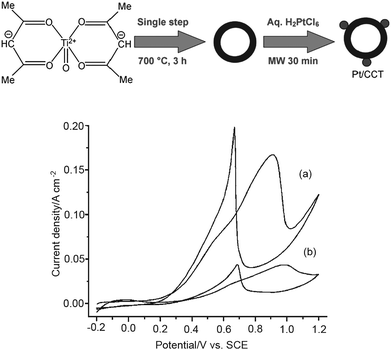 | ||
| Fig. 6 (Upper) Schematic representation of the synthesis of Pt supported on a CCT composite material; (lower) cyclic voltammograms of methanol oxidation with a) Pt/CCT and b) Pt/C (Johnson Matthey) in 1.0 M CH3OH + 0.5M H2SO4 at 25 mV·s−1.115 Reprinted by permission of ref. 115. Copyright Wiley-VCH 2007. | ||
4.3 Tungsten oxides (WOx)
Tungsten oxide is an n-type semiconductor with a reported bandgap of about 2.6 to 2.8 eV.152 Because tungsten has many oxidation states from 2 to 6, it can exist in many forms, making it suitable for electrochromic, photochromic, photocatalyst, and gas-sensor applications.153 The intrinsic electric conductivity in tungsten oxide arises from its nonstoichiometric composition, giving rise to a donor level formed by oxygen-vacancy defects in the lattice.154 Tungsten oxides have been used for a long time to promote Pt/alloy electrocatalysts with an enhanced performance on CO tolerance,155 the electrooxidation of methanol156,157 and ethanol,158 and oxygen reduction.159 It has also been shown that tungsten oxides exhibit considerable proton transfer because of the formation of tungsten trioxide hydrates,160,161 which is an attractive property for fuel cell catalyst support because, as in the case of titanium oxides,132,133 it is beneficial for the formation of electrochemical TPB which increases Pt utilization. This has been confirmed on PtRu-WO3 nanocomposite electrodes for methanol electrooxidation.162 Tungsten oxides can also be used as catalyst supports only by themselves, and they also exhibit enhanced catalytic activity for the electrooxidation of methanol.163,164 In addition to the above-mentioned proton conductivity on tungsten oxides, tungsten oxide can form a hydrogen bronze (HxWO3) that effectively facilitates the dehydrogenation of methanol.164 Tungsten-based electrocatalysts also show high tolerance to CO and H2S contaminants in the fuel stream.165Novel nanostructured tungsten oxide has also been developed for fuel cell catalyst support applications. Maiyalagan et al.166 reported that Pt supported on tungsten oxide nanorods, which are synthesized using an anodic alumina membrane, exhibited good electrocatalytic activity and good stability toward methanol oxidation. This was attributed to a synergistic effect between Pt and WO3 that avoids poisoning of the electrodes.
Tungsten oxides can also promote ORR. It was found that modifying carbon-supported RuSex nanoparticles with ultra-thin WO3 films results in the enhancement of their electrocatalytic properties towards ORR in an acid medium.167 The mixed-valent tungsten(VI,V) oxide support was characterized by good electronic and protonic conductivities. A bifunctional electrocatalytic mechanism was proposed that while RuSex initiates the electrocatalytic reduction of oxygen, WO3 facilitates the decomposition of the undesirable hydrogen peroxide intermediate and makes the overall reduction effectively a 4-electron process.167 These findings are very important for developing non-Pt electrocatalysts, which is the holy grail in the fuel cell community.
Although a Pt-tungsten oxide catalyst usually exhibits high stability,153 tungsten dissolution was still observed.168 The chemical instability of WO3 in an acid medium is still the major issue that hinders its application in fuel cells. The improvement has been attempted by suitably adjusting the conditions of its preparation, for example by incorporating Ti4+ with a low concentration in the WO3 framework, which improves the stability of WO3. This, moreover, decreases its ohmic resistance with the increase in the Ti4+ substitution in the WO3 framework.169
Some other oxides have also been tested as a primary catalyst support or a secondary one to promote carbon support, such as vanadium oxide nanotubes,170 cerium oxide (CeO2),171,172 lead oxide (PbO/PbO2),173 molybdenum oxide (MoOx),174 magnesium oxide (MgO),175 and zirconium oxide(ZrO2),176,177 some of which have also shown promising performances toward the electrooxidation of methanol,170 ethanol172,178 and CO,171 and oxygen reduction.177 All these oxides have a common advantage over carbon supports for extending electrocatalyst life. In addition to the above-mentioned specific properties, when they are oxidized they still remain on the support in another form (e.g., metal hydrates), and Pt nanoparticles are still supported on them and retain some catalytic activity. This is not like the case of carbon supports, which will be gasified into CO/CO2, leading Pt nanoparticles to detach from the support and completely lose activity.179
It is also interesting that some of these oxides are expected to exhibit the property of proton transfer on the surface, as mentioned above for titanium oxides132,133 and tungsten oxides.160,161 Introducing a proton conductor on a catalyst support, which increases Pt utilization, is an attractive way to improve fuel cell performance.180,181 The intrinsic proton transfer property of some oxides provides a new pathway to design electrocatalysts and support materials, which could not only increase the utilization of catalytic metals and enhance the catalytic activity, but also decrease fuel cell cost by lowering the usage of a Nafion ionomer. For example, it has been shown that when no Nafion ionomer was added to the cathode catalyst layer, the PEM fuel cell performance with a Pt/S-ZrO2 cathode was higher than that with a Pt/C cathode,177 which is attributed to the proton conductivity of the S-ZrO2 support.176,177
Even though oxides exhibit promising performance as support for fuel cell catalysts, there are still some problems for currently developed oxide support materials. In general, most oxides have a low surface area and low conductivity, and some are unstable in an acid environment. They are preferred to be used as a secondary support, which works together with the primary catalyst support, usually carbon materials, to improve catalyst performance in terms of both catalytic activity and durability. The oxides of interest are often substoichiometric, whose properties and performance greatly depend on their composition and structures. This provides scientists with more chances and more challenges to search for desirable materials. People have made advances in this direction; for example, novel oxide-carbon nanostructured composites have been developed115,182,183 that exhibited high surface area and conductivity.
5. Carbides
It has been shown since the 1970s184 that the carbides of transition metals (TMCs), especially tungsten carbides, exhibited Pt-like catalytic properties185 because near the Fermi level, the electronic density of states of tungsten carbides resembles that of noble metal platinum.184 This means that these carbides are expected to be used as electrocatalysts by themselves186–189 or as catalyst supports,190–192 which might promote catalytic activity through synergistic effects. In addition, carbides have more chemical flexibility, which makes them more interesting in electrocatalysis. This is because at their surface, the chemical composition and the catalytic properties can be easily modified during synthesis or post-treatments.193 Among all the catalytic TMCs, tungsten carbides are the most promising electrocatalysts/supports194 due to their special properties,195 such as their stability in acid solutions at anodic potentials,185 their selectivity,196 a specially strong resistance to catalytic poisons (carbon monoxide, hydrocarbons, and hydrogen sulfide),14,197 a higher activity toward the dissociation of methanol and water,198 and good electrical conductivity (tungsten carbide [WC] has the lowest electrical resistivity [105 S·cm−1 at 20 °C] of any of the interstitial carbides195,199). In recent years, much work has been done to develop tungsten carbide-based electrocatalysts, which has shown that tungsten carbides work well not only as the electrocatalyst by themselves but also as catalyst supports that exhibit a synergistic effect with Pt203,204 and other catalytic metals (such as Ag205,206), increasing the specific activity of catalytic metals. For example, they serve as electrocatalysts for methanol oxidation,200 H2/CO oxidation,201 and anode catalysts for microbial fuel cells.202The tungsten carbides were observed to promote Pt/C catalysts for oxygen reduction when mixed together.207 When Pt was directly deposited onto tungsten carbides,204 the resultant catalyst Pt-WC/C shows rather better performance toward ORR than that prepared by mixing WC and Pt/C.205 The tremendous enhancement in the activity for ORR on Pt-WC/C prepared by direct reduction of Pt on WC was attributed to the uniform distribution of Pt nanoparticles, which is confirmed from the higher electrochemical surface area of the former.204 According to Nie et al.'s report, the catalytic activity of the Pt-WC/C electrode for ORR is higher than that of Pt/C, even though Pt loading in Pt-WC/C is only half that in Pt/C. This shows a synergistic effect between Pt and WC.204 This indicates that tungsten carbides are promising for catalyst supports. Some other studies were also carried out on tungsten carbide-supported Pt-Ru alloy catalysts,208,209 which also show enhanced CO tolerance.
In addition to the promoting effect on catalytic activity, the electrocatalyst support should also exhibit high durability under fuel cell conditions.2,3 It is known that the most important tungsten carbides are WC and W2C, among which W2C is thermodynamically unstable at low temperatures, while WC is a stable compound.195 It is expected that WC is also more stable under electrochemical conditions. This is confirmed in Zellner et al.'s report210 that in 0.5 M H2SO4, the WC film is stable at anode potentials below 0.6 V. However, W2C has no stable region, causing immediate oxidation to form WxOy species when exposed to air or in an electrochemical environment.210
Chhina et al.199 studied the durability of tungsten carbide-supported Pt catalysts (Pt/WC) under an ADT protocol with multi-potential steps (1.8V for 20s and 0.6V for 60s in each period). After 30 oxidation cycles, degradation in activity by about 20% and 10% was observed for Pt/WC and WC, respectively, while Pt/Vulcan XC was almost completely destroyed at about the 10th oxidation cycle. It is proposed that the oxidation of tungsten carbides produces sub-stoichiometric tungsten oxide (WOx), which is a narrow-bandgap semiconductor with reasonable electrical conductivity. So when the catalyst support WC was oxidized, the structure of the support changed from Pt supported on WC to Pt supported on a WOx shell encapsulating a WC core.199 This was unlike in the case of a carbon support,2,3 which can be gasified to CO and CO2 when oxidized, causing Pt to fall off the support and leading to a lower Pt surface area and rapid and significant degradation in PEM fuel cell performance. Chhina et al.'s investigation was carried out under very rigorous conditions.199 No durability data of tungsten carbides that support Pt catalysts under real PEM fuel cell conditions were available. But according to Zhang et al.'s report,211 tungsten carbides that support Pt catalysts are expected to be quite stable under 1.2V, which is very close to PEM fuel cell conditions.
There are still some issues that should be pointed out for the durability of tungsten carbides. The durability of bare tungsten carbides in the report of Zellner and Chen210 is much lower than that reported by Chhina et al.199 This might be due to the differences in the chemistry and the structure of the two tungsten carbides. So the durability of tungsten carbides, as clearly shown in the work of Zellner and Chen,210 is quite dependent on the chemical structure. This provides an easy way to modify the properties of tungsten carbides. It has also been shown that adding a second metal to the carbides improves both the stability and the electrocatalytic activity. For example, it was found that the corrosion resistance of tungsten carbide was significantly increased by adding Ta to the pure WC catalyst.212 The electrocatalytic activity for the ORR in the WC + Ta catalyst was also observed at 0.8V (versus dynamic hydrogen electrode [DHE]) or less potential. This value was 0.35V higher than that of the pure WC catalyst.212
There are still some challenges for the application of carbides as catalyst materials. For example, the electrochemical surface area of Pt/WC is still quite low,199 which indicates that it is necessary to further optimize the Pt particle size (30 nm for present Pt/WC vs. 3 nm for commercial Pt/C).199 So the feasibility of using WC as a catalyst support depends also on it being capable of producing the powder with smaller particle sizes and on achieving a higher degree of catalyst dispersion on the particles.199
6. Concluding remarks
The catalysts and support materials exhibit great influence on the cost, performance, and durability of PEM fuel cells, which has been a very hot research topic in the past decade. The recent research and development of PEM fuel cell catalyst support materials, especially several novel nanostructured materials exhibiting promising performances, were reviewed. These materials include CNTs, CNFs, mesoporous carbon, conductive doped diamonds and nanodiamonds, conductive oxides (tin oxide/indium tin oxide, titanium oxide, tungsten oxide) and carbides (tungsten carbides), among which carbon materials are usually used as the primary catalyst support and other materials as the secondary support. This promotes the primary carbon support to increase the catalytic activity and the durability (they can also be used as catalyst supports by themselves). It has been pointed out that the application of these materials in fuel cells still needs further improvement. We summarize these materials in Table 1.| Support materialsa | Properties | Application | Future directions | Ref. |
|---|---|---|---|---|
| a See the text for the meanings of the abbreviations and the formulae. | ||||
| CNT/CNF | sp 2 carbon, medium/high surface area, high conductivity, high durability. | Anode and cathode | Nanostructure optimization, doping strategy. | 9,27,46,213,214 |
| Mesopor. Carbon | sp 2/sp3 carbon, high surface area, good pore structures and size distribution. | Anode and cathode | Conductivity and durability improvement, doping strategy. | 16,21,57,60,215 |
| Conduct. Diamond | sp 3 carbon, high stability, low surface area and low conductivity. | Cathode preferred | Conductivity and surface area, nanodiamonds, doping. | 83,84,87 |
| SnOx/ITO | Semiconductor/conductor, low surface area and conductivity, high durability. | Anode and cathode | Conductivity, chemical composition, surface area. | 111,119,123 |
| TixOy | Semiconductor/conductor/proton conductor, low surface area, high durability. | Anode and cathode | Conductivity (e− & H+), chemical composition, surface area, nanocomposite, doping. | 108,134,138 |
| WOx | Semiconductor/proton conductor, low surface area | Anode and cathode | Conductivity (e− & H+), durability, chemical composition, surface area, doping. | 160–162,166 |
| WC/WC2 | Pt-like catalytic properties, synergistic effect | Anode preferred | Durability, surface area. | 198,199,204 |
The development of new catalyst support materials is still an important research topic. Some materials and some findings are very interesting, which are worthy of discussion here.
1. It is well known that, for the conventional electrocatalyst support, adequate electrical conductivity is required. Recently, researchers in the 3M Company developed a novel nonconductive nanostructured organic (pigment) whisker-like material216,217 on which high-aspect ratio elongated Pt nanoparticles were coated to form a conductive nanostructured thin film (NSTF). The coated Pt thin film provides the electronic conduction. This NSTF catalyst was found to survive heavily stressed voltage cycling (33% loss in an electrochemically active surface area in 9000 cycles for NSTF vs. 90% loss in 2000 cycles for Pt/C) and high potential under PEM fuel cell-like conditions.216,217 Silica (SiO2) is also a nonconductor; however, it has also been successfully used as the secondary support to modify electrocatalyst supports or used as a matrix to stabilize Pt nanoparticles, which have been shown to improve Pt utilization218 and the durability of catalysts.219 This indicates that some nonconductive materials can be considered as the secondary catalyst support and even primary support, which extends the candidate materials for applications in fuel cells.
As for carbon materials, it has been widely observed that a second element (such as nitrogen) for doped carbon nanostructures shows promising application as catalyst supports in terms of both catalytic activity and durability.16
All of these observations indicate that there is still much room to improve catalyst performance by developing novel support materials. Therefore, future work should look into a wider range of potential materials and composites with novel structures and properties.
2. Since the emphasis in the fuel cell research community has shifted from improving the short-term performance to improving fuel cell reliability and lifetime and making fuel cells cost competitive,1,2 future work should pay more attention to materials durability under PEM fuel cell conditions, including high-temperature PEM fuel cells, which is more promising for transportation applications.3,220
3. Modern quantum chemistry calculations have been a good resource in searching for novel materials and studying their properties, and this has been a good tool when studying electrocatalysts.221 So the research strategy in materials science (novel structures/materials theoretically predicted from quantum chemistry calculation → real materials synthesized from experiments → modified for specific applications) can be used to search for electrocatalyst supports. For example, C3N4 was first theoretically predicted by quantum chemistry calculations and then experimentally synthesized222 as a nonconductive material; recently, electrically conductive C3N4 materials have been obtained and used as fuel cell catalyst supports that exhibited higher performance in DMFCs than conventional Vulcan XC-72 carbon.223 Quantum mechanical calculations have also been used to study the interaction between catalytic metals and support (for example, Pt-TiO2,135 Pt-CNT,224,225 and Pd-CNT224), which are expected to shed light on understanding the acting mechanism of support materials to promote catalysts. All these indicate that modern quantum chemistry calculation is a powerful tool in developing novel electrocatalyst supports.
In summary, there is still much room to improve catalyst performance with improved catalyst supports, and new strategies are needed in the development of fuel cell catalyst supports.
Acknowledgements
This work is supported by the U.S. DOE-EERE Hydrogen Program. The research was partly performed at the Environmental Molecular Science Laboratory (EMSL), a national scientific user facility sponsored by DOE's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for DOE under Contract DE-AC05-76L01830.References
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K. I. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS.
- Y. Y. Shao, G. P. Yin and Y. Z. Gao, J. Power Sources, 2007, 171, 558–566 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, Z. B. Wang and Y. Z. Gao, J. Power Sources, 2007, 167, 235–242 CrossRef CAS.
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Appl. Catal. B-Environ., 2005, 56, 9–35 CrossRef CAS.
- J. G. Zhou, X. T. Zhou, X. H. Sun, R. Y. Li, M. Murphy, Z. F. Ding, X. L. Sun and T. K. Sham, Chem. Phys. Lett., 2007, 437, 229–232 CrossRef CAS.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220–222 CrossRef CAS.
- V. R. Stamenkovic, B. Fowler, B. S. Mun, G. F. Wang, P. N. Ross, C. A. Lucas and N. M. Markovic, Science, 2007, 315, 493–497 CrossRef CAS.
- Y. Lin, X. Cui and X. Ye, Electrochem. Commun., 2005, 7, 267–274 CrossRef CAS.
- W. Z. Li, C. H. Liang, W. J. Zhou, J. S. Qiu, Z. H. Zhou, G. Q. Sun and Q. Xin, J. Phys. Chem. B, 2003, 107, 6292–6299 CrossRef CAS.
- C. Wang, M. Waje, X. Wang, J. M. Tang, R. C. Haddon and Y. S. Yan, Nano Lett., 2004, 4, 345–348 CrossRef CAS.
- C. A. Bessel, K. Laubernds, N. M. Rodriguez and R. T. K. Baker, J. Phys. Chem. B, 2001, 105, 1115–1118 CrossRef CAS.
- S. H. Joo, S. J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki and R. Ryoo, Nature, 2001, 412, 169–172 CrossRef CAS.
- H. Chhina, S. Campbell and O. Kesler, J. Power Sources, 2006, 161, 893–900 CrossRef CAS.
- R. Ganesan and J. S. Lee, Angew. Chem.-Int. Edit., 2005, 44, 6557–6560 CrossRef CAS.
- H. X. Zhong, H. M. Zhang, G. Liu, Y. M. Liang, J. W. Hu and B. L. Yi, Electrochem. Commun., 2006, 8, 707–712 CrossRef CAS.
- Y. Shao, J. Sui, G. Yin and Y. Gao, Applied Catalysis B: Environmental, 2008, 79, 89–99 Search PubMed.
- G. Wu, D. Y. Li, C. S. Dai, D. L. Wang and N. Li, Langmuir, 2008, 24, 3566–3575 CrossRef CAS.
- (a) Y. Lin, X. Cui, C. Yen and C. M. Wai, Langmuir, 2005, 21, 11474–11479 CrossRef CAS; (b) X. Ye, Y. Lin, C. Wang, M. H. Engelhard, Y. Wang and C. M. Wai, J. Mater. Chem., 2004, 14, 908–913 RSC.
- X. Sun, R. Li, D. Villers, J. P. Dodelet and S. Desilets, Chem. Phys. Lett., 2003, 379, 99–104 CrossRef CAS.
- J. S. Guo, G. Q. Sun, Q. Wang, G. X. Wang, Z. H. Zhou, S. H. Tang, L. H. Jiang, B. Zhou and Q. Xin, Carbon, 2006, 44, 152–157 CrossRef CAS.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, J. J. Wang, Y. Z. Gao and P. F. Shi, J. Power Sources, 2006, 161, 47–53 CrossRef CAS.
- V. Georgakilas, D. Gournis, V. Tzitzios, L. Pasquato, D. M. Guldi and M. Prato, J. Mater. Chem., 2007, 17, 2679–2694 RSC.
- K. Lee, J. J. Zhang, H. J. Wang and D. P. Wilkinson, J. Appl. Electrochem., 2006, 36, 507–522 CrossRef CAS.
- G. Girishkumar, K. Vinodgopal and P. V. Kamat, J. Phys. Chem. B, 2004, 108, 19960–19966 CrossRef CAS.
- J. J. Wang, G. P. Yin, J. Zhang, Z. B. Wang and Y. Z. Gao, Electrochim. Acta, 2007, 52, 7042–7050 CrossRef CAS.
- W. Z. Li, X. Wang, Z. W. Chen, M. Waje and Y. S. Yan, J. Phys. Chem. B, 2006, 110, 15353–15358 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, H. H. Wang, Y. Z. Gao and P. F. Shi, J. Power Sources, 2006, 161, 47–53 CrossRef CAS.
- Y. Lin, X. Cui, C. Yen and C. M. Wai, J. Phys. Chem. B, 2005, 109, 14410–14415 CrossRef CAS.
- S. Shanmugam and A. Gedanken, Electrochem. Commun., 2006, 8, 1099–1105 CrossRef CAS.
- C. Kim, Y. J. Kim, Y. A. Kim, T. Yanagisawa, K. C. Park, M. Endo and M. S. Dresselhaus, J. Appl. Phys., 2004, 96, 5903–5905 CrossRef CAS.
- E. S. Steigerwalt, G. A. Deluga and C. M. Lukehart, J. Phys. Chem. B, 2002, 106, 760–766 CrossRef CAS.
- Y. L. Hsin, K. C. Hwang and C. T. Yeh, J. Am. Chem. Soc., 2007, 129, 9999–10010 CrossRef CAS.
- S. Shanmugam and A. Gedanken, J. Phys. Chem. B, 2006, 110, 2037–2044 CrossRef.
- M. Tsuji, M. Kubokawa, R. Yano, N. Miyamae, T. Tsuji, M. S. Jun, S. Hong, S. Lim, S. H. Yoon and I. Mochida, Langmuir, 2007, 23, 387–390 CrossRef CAS.
- G. Wu and B. Q. Xu, J. Power Sources, 2007, 174, 148–158 CrossRef CAS.
- Y. W. Tang, S. Cao, Y. Chen, J. C. Bao and T. H. Lu, Chem. J. Chin. Univ.-Chin., 2007, 28, 936–939 Search PubMed.
- J. W. G. Wildoer, L. C. Venema, A. G. Rinzler, R. E. Smalley and C. Dekker, Nature, 1998, 391, 59–62 CrossRef CAS.
- Z. Chen, W. Deng, X. Wang and Y. Yan, ECS Transactions, 2007, 11, 1289–1299 Search PubMed.
- J. Y. Cao, C. Du, S. C. C. Wang, P. Mercier, X. G. Zhang, H. Yang and D. L. Akins, Electrochem. Commun., 2007, 9, 735–740 CrossRef CAS.
- J. Narayanamoorthy, S. Durairaj, Y. Song, Y. Xu and J. Choi, Appl. Phys. Lett., 2007, 90, 3.
- G. G. Wildgoose, C. E. Banks and R. G. Compton, Small, 2006, 2, 182–193 CrossRef CAS.
- M. C. Hersam, Nature Nano., 2008, 3, 387–394 Search PubMed.
- E. Yoo, T. Okada, T. Kizuka and J. Nakamura, Electrochemistry, 2007, 75, 146–148 CAS.
- F. Kurusu, H. Tsunoda, A. Saito, A. Tomita, A. Kadota, N. Kayahara, I. Karube and M. Gotoh, Analyst, 2006, 131, 1292–1298 RSC.
- J. S. Zheng, X. S. Zhang, P. Li, J. Zhu, X. G. Zhou and W. K. Yuan, Electrochem. Commun., 2007, 9, 895–900 CrossRef CAS.
- F. L. Yuan and H. J. Ryu, Nanotechnology, 2004, 15, S596–S602 CrossRef CAS.
- P. J. Britto, K. S. V. Santhanam, A. Rubio, J. A. Alonso and P. M. Ajayan, Adv. Mater., 1999, 11, 154–157 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, J. Zhang and Y. Z. Gao, Electrochim. Acta, 2006, 51, 5853–5857 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, Y. Z. Gao and P. F. Shi, J. Electrochem. Soc., 2006, 153, A1093–A1097 CrossRef CAS.
- J. J. Wang, G. P. Yin, Y. Y. Shao, S. Zhang, Z. B. Wang and Y. Z. Gao, J. Power Sources, 2007, 171, 331–339 CrossRef CAS.
- L. Li and Y. C. Xing, J. Electrochem. Soc., 2006, 153, A1823–A1828 CrossRef CAS.
- Y. Y. Shao, G. P. Yin, Y. Z. Gao and P. F. Shi, Electrochemistry (Chinese), 2006, 12, 288–291 Search PubMed.
- X. Wang, W. Z. Li, Z. W. Chen, M. Waje and Y. S. Yan, J. Power Sources, 2006, 158, 154–159 CrossRef CAS.
- J. J. Wang, G. P. Yin, Y. Y. Shao, Z. B. Wang and Y. Z. Gao, J. Phys. Chem. C, 2008, 112, 5784–5789 CrossRef CAS.
- J. Wang, G. Yin, Y. Shao, Z. Wang and Y. Gao, J. Power Sources, 2008, 176, 128–131 CrossRef CAS.
- F. B. Su, J. H. Zeng, X. Y. Bao, Y. S. Yu, J. Y. Lee and X. S. Zhao, Chem. Mat., 2005, 17, 3960–3967 CrossRef CAS.
- W. C. Choi, S. I. Woo, M. K. Jeon, J. M. Sohn, M. R. Kim and H. J. Jeon, Adv. Mater., 2005, 17, 446–451 CrossRef CAS.
- G. S. Chai, S. B. Yoon, J. S. Yu, J. H. Choi and Y. E. Sung, J. Phys. Chem. B, 2004, 108, 7074–7079 CrossRef CAS.
- S. H. Joo, C. Pak, D. J. You, S. A. Lee, H. I. Lee, J. M. Kim, H. Chang and D. Seung, Electrochim. Acta, 2006, 52, 1618–1626 CrossRef CAS.
- J. B. Joo, P. Kim, W. Kim, J. Kim and J. Yi, Catal. Today, 2006, 111, 171–175 CrossRef CAS.
- N. Nakagawa, Y. Suzuki, T. Watanabe, T. Takei and K. Kanamura, Electrochemistry, 2007, 75, 172–174.
- K. Y. Chan, J. Ding, J. W. Ren, S. A. Cheng and K. Y. Tsang, J. Mater. Chem., 2004, 14, 505–516 RSC.
- Y. G. Wang, L. Cheng, F. Li, H. M. Xiong and Y. Y. Xia, Chem. Mat., 2007, 19, 2095–2101 CrossRef CAS.
- H. Chang, S. H. Joo and C. Pak, J. Mater. Chem., 2007, 17, 3078–3088 RSC.
- J. S. Yu, S. Kang, S. B. Yoon and G. Chai, J. Am. Chem. Soc., 2002, 124, 9382–9383 CrossRef CAS.
- K. Wikander, H. Ekstrom, A. E. C. Palmqvist, A. Lundblad, K. Holmberg and G. Lindbergh, Fuel Cells, 2006, 6, 21–25 CrossRef.
- F. Su, X. S. Zhao, Y. Wang and J. Y. Lee, Microporous Mesoporous Mat., 2007, 98, 323–329 CrossRef CAS.
- A. B. Fuertes and S. Alvarez, Carbon, 2004, 42, 3049–3055 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Carbon, 2006, 44, 468–474 CrossRef CAS.
- Y. S. Choi, S. H. Joo, S. A. Lee, D. J. You, H. Kim, C. Pak, H. Chang and D. Seung, Macromolecules, 2006, 39, 3275–3282 CrossRef CAS.
- M. Panizza and G. Cerisola, Electrochim. Acta, 2005, 51, 191–199 CrossRef CAS.
- J. Wang and G. M. Swain, Electrochem. Solid State Lett., 2002, 5, E4–E7 CrossRef CAS.
- J. S. Gao, T. Arunagiri, J. J. Chen, P. Goodwill, O. Chyan, J. Perez and D. Golden, Chem. Mat., 2000, 12, 3495–3500 CrossRef CAS.
- M. Hupert, A. Muck, R. Wang, J. Stotter, Z. Cvackova, S. Haymond, Y. Show and G. M. Swain, Diam. Relat. Mat., 2003, 12, 1940–1949 CrossRef CAS.
- G. Sine, I. Duo, B. El Roustom, G. Foti and C. Comninellis, J. Appl. Electrochem., 2006, 36, 847–862 CrossRef CAS.
- I. Duo, P. A. Michaud, W. Haenni, A. Perret and C. Comninellis, Electrochem. Solid State Lett., 2000, 3, 325–326 CAS.
- B. El Roustom, G. Foti and C. Comninellis, Electrochem. Commun., 2005, 7, 398–405 CrossRef CAS.
- F. Montilla, E. Morallon, I. Duo, C. Comninellis and J. L. Vazquez, Electrochim. Acta, 2003, 48, 3891–3897 CrossRef CAS.
- G. Sine, D. Smida, M. Limat, G. Foti and C. Comninellis, J. Electrochem. Soc., 2007, 154, B170–B174 CrossRef CAS.
- G. Sine, G. Foti and C. Comninellis, J. Electroanal. Chem., 2006, 595, 115–124 CrossRef CAS.
- G. R. Salazar-Banda, H. B. Suffredini, M. L. Calegaro, S. T. Tanimoto and L. A. Avaca, J. Power Sources, 2006, 162, 9–20 CrossRef CAS.
- B. El Roustom, G. Sine, G. Foti and C. Comninellis, J. Appl. Electrochem., 2007, 37, 1227–1236 CrossRef CAS.
- J. Wang and G. M. Swain, J. Electrochem. Soc., 2003, 150, E24–E32 CrossRef CAS.
- J. A. Bennett, Y. Show, S. H. Wang and G. M. Swain, J. Electrochem. Soc., 2005, 152, E184–E192 CrossRef CAS.
- O. Enea, B. Riedo and G. Dietler, Nano Lett., 2002, 2, 241–244 CrossRef CAS.
- G. R. Salazar-Banda, K. I. B. Eguiluz and L. A. Avaca, Electrochem. Commun., 2007, 9, 59–64 CrossRef CAS.
- F. Marken, Y. C. Tsai, A. J. Saterlay, B. A. Coles, D. Tibbetts, K. Holt, C. H. Goeting, J. S. Foord and R. G. Compton, J. Solid State Electrochem., 2001, 5, 313–318 CrossRef CAS.
- E. Antolini, Appl. Catal. B-Environ., 2007, 74, 337–350 CrossRef CAS.
- Y. Show, M. A. Witek, P. Sonthalia and G. M. Swain, Chem. Mat., 2003, 15, 879–888 CrossRef CAS.
- A. E. Fischer, M. A. Lowe and G. M. Swain, J. Electrochem. Soc., 2007, 154, K61–K67 CrossRef CAS.
- J. Wang, G. M. Swain, T. Tachibana and K. Kobashi, Electrochem. Solid State Lett., 2000, 3, 286–289 CrossRef CAS.
- J. Wang, G. M. Swain, T. Tachibana and K. Kobashi, J. New Mat.Electrochem. Syst., 2000, 3, 75–82 Search PubMed.
- A. De Battisti, S. Ferro and M. Dal Colle, J. Phys. Chem. B, 2001, 105, 1679–1682 CrossRef CAS.
- A. E. Fischer and G. M. Swain, J. Electrochem. Soc., 2005, 152, B369–B375 CrossRef CAS.
- J. B. Zang, Y. H. Wang, H. Huang and W. Tang, Electrochim. Acta, 2007, 52, 4398–4402 CrossRef CAS.
- Y. V. Pleskov, M. D. Krotova, V. V. Elkin, V. G. Ralchenko, A. V. Saveliev, S. M. Pimenov and P. Y. Lim, Electrochim. Acta, 2007, 52, 5470–5478 CrossRef CAS.
- I. Gerger and R. Haubner, Diam. Relat. Mat., 2005, 14, 369–374 CrossRef CAS.
- N. R. Wilson, S. L. Clewes, M. E. Newton, P. R. Unwin and J. V. Macpherson, J. Phys. Chem. B, 2006, 110, 5639–5646 CrossRef CAS.
- J. Chane-Tune, J. P. Petit, S. Szunerits, P. Bouvier, D. Delabouglise, B. Marcus and M. Mermoux, ChemPhysChem, 2006, 7, 89–93 CrossRef CAS.
- I. Gonzalez-Gonzalez, D. A. Tryk and C. R. Cabrera, Diam. Relat. Mat., 2006, 15, 275–278 CrossRef CAS.
- G. Sine and C. Comninellis, Electrochim. Acta, 2005, 50, 2249–2254 CrossRef CAS.
- G. R. Salazar-Banda, L. S. Andrade, P. A. P. Nascente, P. S. Pizani, R. C. Rocha and L. A. Avaca, Electrochim. Acta, 2006, 51, 4612–4619 CrossRef CAS.
- E. Mironov, A. Koretz and E. Petrov, Diam. Relat. Mat., 2002, 11, 872–876 CrossRef CAS.
- Y. V. Pleskov, M. D. Krotova, V. G. Ral'chenko, A. V. Khomich and R. A. Khmel'nitskii, Russ. J. Electrochem., 2003, 39, 802–807 CrossRef CAS.
- J. B. Zang, Y. H. Wang, S. Z. Zhao, L. Y. Bian and J. Lu, Diam. Relat. Mat., 2007, 16, 16–20 CrossRef CAS.
- I. I. Vlasov, O. I. Lebedev, V. G. Ralchenko, E. Goovaerts, G. Bertoni, G. V. Tendeloo and V. I. Konov, Adv. Mater., 2007, 19, 4058–4062 CrossRef CAS.
- T. Ioroi, Z. Siroma, N. Fujiwara, S. Yamazaki and K. Yasuda, Electrochem. Commun., 2005, 7, 183–188 CrossRef CAS.
- K. W. Park, K. S. Ahn, Y. C. Nah, J. H. Choi and Y. E. Sung, J. Phys. Chem. B, 2003, 107, 4352–4355 CrossRef CAS.
- A. C. Chen, D. J. La Russa and B. Miller, Langmuir, 2004, 20, 9695–9702 CrossRef CAS.
- M. S. Saha, R. Y. Li and X. L. Sun, Electrochem. Commun., 2007, 9, 2229–2234 CrossRef CAS.
- Z. G. Chen, X. P. Qiu, B. Lu, S. C. Zhang, W. T. Zhu and L. Q. Chen, Electrochem. Commun., 2005, 7, 593–596 CrossRef CAS.
- H. M. Villullas, F. I. Mattos-Costa and L. O. S. Bulhoes, J. Phys. Chem. B, 2004, 108, 12898–12903 CrossRef CAS.
- Z. Jusys, T. J. Schmidt, L. Dubau, K. Lasch, L. Jorissen, J. Garche and R. J. Behm, J. Power Sources, 2002, 105, 297–304 CrossRef CAS.
- S. Shanmugam and A. Gedanken, Small, 2007, 3, 1189–1193 CrossRef CAS.
- J. A. Tian, G. Q. Sun, L. H. Jiang, S. Y. Yan, Q. Mao and Q. Xin, Electrochem. Commun., 2007, 9, 563–568 CrossRef CAS.
- J. Mann, N. Yao and A. B. Bocarsly, Langmuir, 2006, 22, 10432–10436 CrossRef CAS.
- H. S. Liu, C. J. Song, L. Zhang, J. J. Zhang, H. J. Wang and D. P. Wilkinson, J. Power Sources, 2006, 155, 95–110 CAS.
- M. S. Saha, R. Y. Li, M. Cai and X. L. Sun, Electrochem. Solid State Lett., 2007, 10, B130–B133 CrossRef CAS.
- T. Matsui, T. Kanishi, K. Fujiwara, K. Tsutsui, R. Kikuchi, T. Takeguchi and K. Eguchi, Sci. Technol. Adv. Mater., 2006, 7, 524–530 CrossRef CAS.
- M. Nakada, A. Ishihara, S. Mitsushima, N. Kamiya and K. Ota, Electrochem. Solid State Lett., 2007, 10, F1–F4 CrossRef CAS.
- K. Ota, A. Ishihara, S. Mitsushima, K. Lee, Y. Suzuki, N. Horibe, T. Nakagawa and N. Kamiya, J. New Mat.Electrochem. Syst., 2005, 8, 25–35 Search PubMed.
- G. Chang, M. Oyama and K. Hirao, J. Phys. Chem. B, 2006, 110, 1860–1865 CrossRef CAS.
- G. Chang, M. Oyama and K. Hirao, J. Phys. Chem. B, 2006, 110, 20362–20368 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS.
- B. E. Hayden, D. V. Malevich and D. Pletcher, Electrochem. Commun., 2001, 3, 390–394 CrossRef CAS.
- J. R. Smith, F. C. Walsh and R. L. Clarke, J. Appl. Electrochem., 1998, 28, 1021–1033 CrossRef CAS.
- Rf. Bartholo and D. R. Frankl, Physical Review, 1969, 187, 828–832 Search PubMed.
- J. E. Graves, D. Pletcher, R. L. Clarke and F. C. Walsh, J. Appl. Electrochem., 1991, 21, 848–857 CrossRef CAS.
- F. P. Hu, F. W. Ding, S. Q. Song and P. K. Shen, J. Power Sources, 2006, 163, 415–419 CrossRef CAS.
- S. Shannnigarn and A. Gedanken, Small, 2007, 3, 1189–1193 CrossRef CAS.
- D. V. Bavykin, J. M. Friedrich and F. C. Walsh, Adv. Mater., 2006, 18, 2807–2824 CrossRef CAS.
- M. Gustavsson, H. Ekstrom, R. Hanarp, L. Eurenius, G. Lindbergh, E. Olsson and B. Kasemo, J. Power Sources, 2007, 163, 671–678 CrossRef CAS.
- H. Ekstrom, B. Wickman, M. Gustavsson, P. Hanarp, L. Eurenius, E. Olsson and G. Lindbergh, Electrochim. Acta, 2007, 52, 4239–4245 CrossRef.
- M. Hepel, I. Dela, T. Hepel, J. Luo and C. J. Zhong, Electrochim. Acta, 2007, 52, 5529–5547 CrossRef CAS.
- M. Wang, D. J. Guo and H. L. Li, J. Solid State Chem., 2005, 178, 1996–2000 CrossRef CAS.
- L. M. Vracar, S. L. Gojkovic, N. R. Elezovic, V. R. Radmilovic, M. M. Jaksic and N. V. Krstajic, J. New Mat.Electrochem. Syst., 2006, 9, 99–106 Search PubMed.
- K. W. Park, S. B. Han and J. M. Lee, Electrochem. Commun., 2007, 9, 1578–1581 CrossRef CAS.
- H. Yang, T. H. Lu, C. P. Liu, J. H. Liu and G. Q. Sun, Chem. J. Chin. Univ.-Chin., 2000, 21, 1283–1287 Search PubMed.
- G. Y. Chen, S. R. Bare and T. E. Mallouk, J. Electrochem. Soc., 2002, 149, A1092–A1099 CrossRef CAS.
- K. W. Park and K. S. Seol, Electrochem. Commun., 2007, 9, 2256–2260 CrossRef CAS.
- B. L. Garcia, R. Fuentes and J. W. Weidner, Electrochem. Solid State Lett., 2007, 10, B108–B110 CrossRef CAS.
- L. Xiong and A. Manthiram, Electrochim. Acta, 2004, 49, 4163–4170 CrossRef CAS.
- H. Q. Song, X. P. Qiu, F. S. Li, W. T. Zhu and L. Q. Chen, Electrochem. Commun., 2007, 9, 1416–1421 CrossRef CAS.
- J. M. Chen, L. S. Sarma, C. H. Chen, M. Y. Cheng, S. C. Shih, G. R. Wang, D. G. Liu, J. F. Lee, M. T. Tang and B. J. Hwang, J. Power Sources, 2006, 159, 29–33 CrossRef CAS.
- H. J. Kim, D. Y. Kim, H. Han and Y. G. Shul, J. Power Sources, 2006, 159, 484–490 CrossRef CAS.
- H. Q. Song, X. P. Qiu, X. X. Li, F. S. Li, W. T. Zhu and L. Q. Chen, J. Power Sources, 2007, 170, 50–54 CrossRef CAS.
- T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Adv. Mater., 1999, 11, 1307–1311 CrossRef CAS.
- M. Adachi, Y. Murata, M. Harada and S. Yoshikawa, Chem. Lett., 2000, 942–943 CrossRef CAS.
- S. Shanmugam, A. Gabashvili, D. S. Jacob, J. C. Yu and A. Gedanken, Chem. Mat., 2006, 18, 2275–2282 CrossRef CAS.
- T. Maiyalagan, B. Viswanathan and U. V. Varadaraju, J. Nanosci. Nanotechnol., 2006, 6, 2067–2071 CrossRef CAS.
- S. H. Lee, R. Deshpande, P. A. Parilla, K. M. Jones, B. To, A. H. Mahan and A. C. Dillon, Adv. Mater., 2006, 18, 763–766 CrossRef CAS.
- H. Chhina, S. Campbell and O. Kesler, J. Electrochem. Soc., 2007, 154, B533–B539 CrossRef CAS.
- S. Supothina, P. Seeharaj, S. Yoriya and M. Sriyudthsak, Ceram. Int., 2007, 33, 931–936 CrossRef CAS.
- F. Maillard, E. Peyrelade, Y. Soldo-Olivier, M. Chatenet, E. Chainet and R. Faure, Electrochim. Acta, 2007, 52, 1958–1967 CrossRef CAS.
- S. Jayaraman, T. F. Jaramillo, S. H. Baeck and E. W. McFarland, J. Phys. Chem. B, 2005, 109, 22958–22966 CrossRef CAS.
- K. W. Park, Y. E. Sung and M. F. Toney, Electrochem. Commun., 2006, 8, 359–363 CrossRef CAS.
- D. Y. Zhang, Z. F. Ma, G. X. Wang, K. Konstantinov, X. X. Yuan and H. K. Liu, Electrochem. Solid State Lett., 2006, 9, A423–A426 CrossRef CAS.
- L. F. Xiong and T. He, Electrochem. Commun., 2006, 8, 1671–1676 CrossRef CAS.
- H. Nakajima and I. Honma, Solid State Ion., 2002, 148, 607–610 CrossRef CAS.
- Y. M. Li, M. Hibino, M. Miyayania and T. Kudo, Solid State Ion., 2000, 134, 271–279 CrossRef CAS.
- K. W. Park, J. H. Choi, K. S. Ahn and Y. E. Sung, J. Phys. Chem. B, 2004, 108, 5989–5994 CrossRef CAS.
- P. J. Barczuk, H. Tsuchiya, J. M. Macak, P. Schmuki, D. Szymanska, O. Makowski, K. Miecznikowski and P. J. Kulesza, Electrochem. Solid State Lett., 2006, 9, E13–E16 CrossRef CAS.
- R. Ganesan and J. S. Lee, J. Power Sources, 2006, 157, 217–221 CrossRef CAS.
- J. B. Christian, S. P. E. Smith, M. S. Whittingham and H. D. Abruna, Electrochem. Commun., 2007, 9, 2128–2132 CrossRef CAS.
- T. Maiyalagan and B. Viswanathan, J. Power Sources, 2008, 175, 789–793 CrossRef CAS.
- P. J. Kulesza, K. Miecznikowski, B. Baranowska, M. Skunik, S. Fiechter, P. Bogdanoff and I. Dorbandt, Electrochem. Commun., 2006, 8, 904–908 CrossRef CAS.
- K. Y. Chen and A. C. C. Tseung, J. Electrochem. Soc., 1996, 143, 2703–2707 CrossRef CAS.
- V. Raghuveer and B. Viswanathan, J. Power Sources, 2005, 144, 1–10 CrossRef CAS.
- K. F. Zhang, D. J. Guo, X. Liu, J. Li, H. L. Li and Z. X. Su, J. Power Sources, 2006, 162, 1077–1081 CrossRef CAS.
- Y. X. Bai, J. J. Wu, X. P. Qiu, J. Y. Xi, J. S. Wang, J. F. Li, W. T. Zhu and L. Q. Chen, Appl. Catal. B-Environ., 2007, 73, 144–149 CrossRef CAS.
- J. Y. Xi, J. S. Wang, L. H. Yu, X. P. Qiu and L. Q. Chen, Chem. Commun., 2007, 1656–1658 RSC.
- H. B. Suffredini, G. R. Salazar-Banda and L. A. Avaca, J. Power Sources, 2007, 171, 355–362 CrossRef CAS.
- N. R. Elezovi'c, B. M. Babi'c, V. R. Radmilovi'c, S. L. Gojkovi'c, N. V. Krstaji'c and L. M. Vracar, J. Power Sources, 2008, 175, 250–255 CrossRef CAS.
- B. Liu, J. H. Chen, C. H. Xiao, K. Z. Cui, L. Yang, H. L. Pang and Y. F. Kuang, Energy Fuels, 2007, 21, 1365–1369 CrossRef CAS.
- S. Hara and M. Miyayama, Solid State Ion., 2004, 168, 111–116 CrossRef CAS.
- Y. Suzuki, A. Ishihara, S. Mitsushima, N. Kamiya and K. I. Ota, Electrochem. Solid State Lett., 2007, 10, B105–B107 CrossRef CAS.
- N. Park, T. Shiraishi, K. Kamisugi and S. Hayase, Chem. Lett., 2007, 36, 922–923 CrossRef CAS.
- S. D. Knights, K. M. Colbow, J. St-Pierre and D. P. Wilkinson, J. Power Sources, 2004, 127, 127–134 CrossRef CAS.
- N. Y. Jia, R. B. Martin, Z. G. Qi, M. C. Lefebvre and P. G. Pickup, Electrochim. Acta, 2001, 46, 2863–2869 CrossRef CAS.
- E. B. Easton, Z. G. Qi, A. Kaufman and P. G. Pickup, Electrochem. Solid State Lett., 2001, 4, A59–A61 CrossRef CAS.
- J. Li, S. B. Tang, L. Lu and H. C. Zeng, J. Am. Chem. Soc., 2007, 129, 9401–9409 CrossRef CAS.
- R. K. Selvan, I. Perelshtein, N. Perkas and A. Gedanken, J. Phys. Chem. C, 2008, 112, 1825–1830 CrossRef CAS.
- R. B. Levy and M. Boudart, Science, 1973, 181, 547–549 CAS.
- H. H. Hwu and J. G. G. Chen, J. Vac. Sci. Technol. A, 2003, 21, 1488–1493 CrossRef CAS.
- M. Nagai, M. Yoshida and H. Tominaga, Electrochim. Acta, 2007, 52, 5430–5436 CrossRef CAS.
- E. C. Weigert, A. L. Stottlemyer, M. B. Zellner and J. G. G. Chen, J. Phys. Chem. C, 2007, 111, 14617–14620 CrossRef CAS.
- M. Wu, P. K. Shen, Z. D. Wei, S. Q. Song and M. Nie, J. Power Sources, 2007, 166, 310–316 CrossRef CAS.
- H. X. Zhong, H. M. Zhang, Y. M. Liang, J. L. Zhang, M. R. Wang and X. L. Wang, J. Power Sources, 2007, 164, 572–577 CrossRef CAS.
- M. K. Jeon, H. Daimon, K. R. Lee, A. Nakahara and S. I. Woo, Electrochem. Commun., 2007, 9, 2692–2695 CrossRef CAS.
- R. Ganesan, D. J. Ham and J. S. Lee, Electrochem. Commun., 2007, 9, 2576–2579 CrossRef CAS.
- M. Nie, P. K. Shen and Z. D. Wei, J. Power Sources, 2007, 167, 69–73 CrossRef CAS.
- N. Keller, B. Pietruszka and V. Keller, Mater. Lett., 2006, 60, 1774–1777 CrossRef CAS.
- H. H. Hwu and J. G. Chen, J. Phys. Chem. B, 2003, 107, 2029–2039 CrossRef CAS.
- S. Shanmugam, D. S. Jacob and A. Gedanken, J. Phys. Chem. B, 2005, 109, 19056–19059 CrossRef CAS.
- T. C. Xiao, A. Hanif, A. P. E. York, J. Sloan and M. L. H. Green, Phys. Chem. Chem. Phys., 2002, 4, 3522–3529 RSC.
- X. G. Yang and C. Y. Wang, Appl. Phys. Lett., 2005, 86, 224104 CrossRef.
- H. H. Hwu, J. G. G. Chen, K. Kourtakis and J. G. Lavin, J. Phys. Chem. B, 2001, 105, 10037–10044 CrossRef CAS.
- H. Chhina, S. Campbell and O. Kesler, J. Power Sources, 2007, 164, 431–440 CrossRef CAS.
- G. T. Burstein, D. R. McIntyre and A. Vossen, Electrochem. Solid State Lett., 2002, 5, A80–A83 CrossRef CAS.
- X. G. Yang and C. Y. Wang, Appl. Phys. Lett., 2005, 86 Search PubMed.
- M. Rosenbaum, F. Zhao, U. Schroder and F. Scholz, Angew. Chem.-Int. Edit., 2006, 45, 6658–6661 CrossRef CAS.
- M. B. Zellner and J. G. G. Chen, J. Electrochem. Soc., 2005, 152, A1483–A1494 CrossRef CAS.
- M. Nie, P. K. Shen, M. Wu, Z. D. Wei and H. Meng, J. Power Sources, 2006, 162, 173–176 CrossRef CAS.
- H. Meng and P. K. Shen, Electrochem. Commun., 2006, 8, 588–594 CrossRef CAS.
- H. Meng, M. Wu, X. X. Hu, M. Nie, Z. D. Wei and P. K. Shen, Fuel Cells, 2006, 6, 447–450 CrossRef CAS.
- H. Meng and P. K. Shen, J. Phys. Chem. B, 2005, 109, 22705–22709 CrossRef CAS.
- R. Venkataraman, H. R. Kunz and J. M. Fenton, J. Electrochem. Soc., 2003, 150, A278–A284 CrossRef CAS.
- G. J. Lu, J. S. Cooper and P. J. McGinn, J. Power Sources, 2006, 161, 106–114 CrossRef CAS.
- M. B. Zellner and J. G. G. Chen, Catal. Today, 2005, 99, 299–307 CrossRef CAS.
- S. S. Zhang, H. Zhu, H. M. Yu, J. B. Hou, B. L. Yi and P. W. Ming, Chin. J. Catal., 2007, 28, 109–111 Search PubMed.
- K. C. Lee, A. Ishihara, S. Mitsushima, N. Kamiya and K. I. Ota, Electrochim. Acta, 2004, 49, 3479–3485 CrossRef CAS.
- A. Kongkanand, K. Vinodgopal, S. Kuwabata and P. V. Kamat, J. Phys. Chem. B, 2006, 110, 16185–16188 CrossRef CAS.
- M. Endo, Y. A. Kim, M. Ezaka, K. Osada, T. Yanagisawa, T. Hayashi, M. Terrones and M. S. Dresselhaus, Nano Lett., 2003, 3, 723–726 CrossRef CAS.
- J. Ding, K. Y. Chan, J. W. Ren and F. S. Xiao, Electrochim. Acta, 2005, 50, 3131–3141 CrossRef CAS.
- M. K. Debe, A. K. Schmoeckel, G. D. Vernstrorn and R. Atanasoski, J. Power Sources, 2006, 161, 1002–1011 CrossRef CAS.
- M. K. Debe, A. Schmoeckel, S. Hendricks, G. Vernstrom, G. Haugen and R. Atanasoski, ECS Transactions, 2006, 1, 51–66 Search PubMed.
- J. Zeng, J. Y. Lee, J. Chen, P. K. Shen and S. Song, Fuel Cells, 2007, 7, 285–290 CrossRef CAS.
- S. Takenaka, H. Matsumori, K. Nakagawa, H. Matsune, E. Tanabe and M. Kishida, J. Phys. Chem. C, 2007, 111, 15133–15136 CrossRef CAS.
- J. L. Zhang, Z. Xie, J. J. Zhang, Y. H. Tanga, C. J. Song, T. Navessin, Z. Q. Shi, D. T. Song, H. J. Wang, D. P. Wilkinson, Z. S. Liu and S. Holdcroft, J. Power Sources, 2006, 160, 872–891 CrossRef CAS.
- M. Mavrikakis, Nat. Mater., 2006, 5, 847–848 CrossRef CAS.
- C. M. Niu, Y. Z. Lu and C. M. Lieber, Science, 1993, 261, 334–337 CAS.
- M. Kim, S. Hwang and J. S. Yu, J. Mater. Chem., 2007, 17, 1656–1659 RSC.
- A. Maiti and A. Ricca, Chem. Phys. Lett., 2004, 395, 7–11 CrossRef.
- B. H. Morrow and A. Striolo, J. Phys. Chem. C, 2007, 111, 17905–17913 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
