Determination of inorganic and total mercury by flow injection vapor generation atomic absorption spectrometry using a W-coil atomizer
Orkun
Alp
and
Nusret
Ertaş
*
Gazi University, Faculty of Pharmacy, Analytical Chemistry Division, 06330, Ankara, Turkey. E-mail: nertas@gazi.edu.tr; Fax: +90 312 223 5018
First published on 30th October 2008
Abstract
A tungsten coil (150 W, 15 V) was used as the atomizer for the proposed Flow Injection Vapor Generation Atomic Absorption Spectrometry (FI-VGAAS) system to determine inorganic mercury and total mercury concentrations. In the proposed method inorganic mercury was measured when the W-coil is at room temperature and total mercury was measured when the W-coil temperature was set to 500 °C. The organic mercury concentration was calculated by subtracting the inorganic mercury concentration from the total mercury concentration. The calibration graphs were linear up to 100 ng ml−1. Limit of detection (LOD) values for inorganic mercury and methyl mercury were 0.60 ng ml−1 and 0.89 ng ml−1 respectively. The precision of the method in terms of RSD was between 2–3%, and the sampling frequency was 100 h−1. In order to validate the proposed method, known concentrations of methyl mercury and inorganic mercury were spiked into deionized water and tap water samples. Certified reference materials (CRM) IAEA-085 and NRC DOLT-3 were analyzed after microwave assisted extraction with 6.0 mol l−1HCl solutions. Both inorganic mercury and methyl mercury concentrations were in agreement with the certified values.
Introduction
The toxicity of mercury depends on its concentration and chemical form. Mercury can be found in three different chemical forms; elemental, inorganic and organic forms such as methyl mercury (CH3Hg) and dimethyl mercury (CH3HgCH3).1,2 Within these forms organic mercury species are more toxic than the other forms.3,4 Therefore, it is important to determine inorganic and organic mercury instead of total mercury.Cold Vapor Atomic Absorption Spectrometry (CVAAS) is a well known and widely used technique for mercury determination. Mercury speciation analysis has been done by CVAAS method without chromatographic separations. Stannous chloride (SnCl2) and sodium borohydride (NaBH4) are the most common reducing reagents for the determination of mercury.
Segade and Tyson5 used different concentrations of NaBH4 as reducing agent for determination of inorganic and total mercury. In their work, a low NaBH4 concentration (10−4% m v−1) was used to determine inorganic mercury. Both inorganic and methyl mercury were reduced to elemental mercury at higher NaBH4 concentrations (0.75% m v−1). Methyl mercury concentration was determined by taking the difference between total mercury and inorganic mercury.
Kaercher et al.6 determined inorganic and methyl mercury by Vapor Generation Atomic Absorption Spectrometry using different atomization temperatures. Inorganic mercury was determined by keeping the quartz cell furnace at room temperature and total mercury was determined by heating the quartz cell furnace to ≥ 650 °C. Similarly, Torres et al.7 determined inorganic and total mercury in biological samples by using different atom cell temperatures. Inorganic mercury was measured at room temperature and total mercury was measured by heating the quartz cell atomizer with an air-acetylene flame.
Recently, tungsten coils (W-coil) have been used as alternative atomizers in Atomic Absorption Spectrometry.8,9 W-coil can be heated up to 3000 °C, has a fast heating rate (30 K ms−1), requires a simple power supply and it is inexpensive compared to other atomizers.10
In this study, a simple and fast methodology for the determination of inorganic and total mercury is proposed. The method is based on the vapor generation of inorganic mercury and methyl mercury by using NaBH4 as reducing reagent at a low concentration. A 150 W tungsten coil, placed into a glass atom cell was used as the atomizer. Inorganic mercury was being reduced to elemental mercury using the low concentration of NaBH4, while the methyl mercury forms an intermediate volatile methyl mercury hydride (CH3HgH). When the W-coil atomizer is held at room temperature only inorganic mercury was measured. When the W-coil in the atomization cell was heated, in addition to inorganic mercury, volatile methyl mercury species decomposed and total mercury was measured. The difference equals to the methyl mercury concentration. Because the heating and cooling rate of W-coil is very fast, inorganic and total mercury in the same sample can be determined sequentially in a very short time.
Experimental
Instrumentation
Mercury measurements were performed using Unicam 939 Atomic Absorption Spectrometer with a deuterium background correction. A mercury hollow cathode lamp was operated at 6 mA. The wavelength was set to 253.7 nm and the spectral band pass to 0.5 nm. Flow injection manifold (Fig. 1) was constructed using 1.8 mm (i.d.) tygon peristaltic pump tubings, delivering the reducing and carrier solutions at required flow rate. The connecting tubings were made of 0.8 mm (i.d). PTFE. A Rheodyne model 5020 rotary valve with 500µl loop volume was used for sample introduction. Two peristaltic pumps were used; one (ALITEA VS 3 midi pump) for the carrier acid (HCl) and the reducing reagent (NaBH4), and the other (Gilson Minipuls 3) for draining gas-liquid separator (GLS).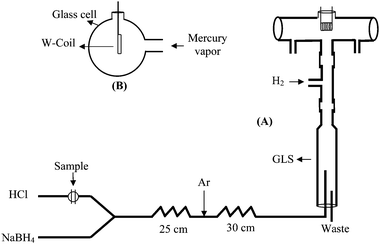 | ||
| Fig. 1 Scheme of FI-VGAAS system set up (A) and side view of W-coil atomizer (B). GLS: Gas liquid separator. | ||
The atomization cell with an optical path length 160 mm with 8 mm (i.d.) was made of glass with quartz windows attached to both sides. The inlet arm was 60 mm in length and 3.0 mm (i.d.). A 150W, 15 V W-coil (Osram), used for the atomization of methyl mercury, was placed in the middle of the atomization cell and sealed with play dough. A variable potential power supply and a transformer were used in order to apply various voltages to the W-coil atomizer. Peak height was measured throughout the experiments.
For the measurement of W-coil temperature, a Pt-Rh thermocouple (Tetcis, Ankara) which is capable of measuring temperatures up to 1800 °C was used. The temperature was measured by keeping the tip of the thermocouple 1 mm in front of the central part of the W-coil, at optimized gas composition and flow rates.
Reagents
All working solutions were prepared in deionized water (18.2 MΩ cm) which was obtained from Millipore, Simplicity, 185 water purification system. Carrier gases Ar and H2 were supplied from Habaş, Turkey.1000 mg l−1methyl mercury and inorganic mercury stock solutions were prepared by dissolving appropriate amount of solid CH3HgCl (Merck) and HgCl2 (Merck) in 0.24 mol l−1HCl. 0.01% (m v−1) NaBH4 (Merck) was prepared daily by dissolving solid reagent in 0.1% (m v−1) NaOH (Riedel de Haen) solution. Two certified reference materials, IAEA-085 human hair and NRC DOLT-3 dogfish liver, with different certified contents of methyl mercury and total mercury, were used to validate the proposed method.
Sample preparation
The certified reference materials (CRM) IAEA-085 and NRC DOLT-3 were treated by microwave assisted extraction in acid medium.5 In this procedure, 0.025 g IAEA-085 and 0.4 g of NRC DOLT-3 were weighed and 5.0 ml of 6.0 mol l−1HCl was added. This suspension was first shaken for 2 minutes, and then it was exposed to microwave radiation at 70 watt for 3 min. The sample was cooled down to room temperature and centrifuged at 5000 rpm for 5 minutes. Finally 1.0 ml of the supernatant solution was transferred to a calibrated flask and diluted to 10.0 ml with deionized water.Results and discussion
Optimization of flow injection parameters
The effects of the concentrations of NaBH4 and acid, the flow rates of reducing agent, carrier solution and the carrier gas, the lengths of the reaction coil and stripping coil and the sample volume on the absorbance signals of the inorganic and methyl mercury were studied.The effect of NaBH4 concentration was studied in the range of 10−4–1% (m v−1) for inorganic mercury and methyl mercury using aqueous standard solutions. As shown in Fig 2 when NaBH4 concentrations were between 10−4–0.05% (m v−1) only inorganic mercury was reduced to elemental mercury and atomic signal was not observed from methyl mercury. It has been reported that methyl mercury could generate an intermediate volatile product (CH3HgH)5,6 at low NaBH4 concentrations. The atomic absorbance signal from methyl mercury increased with NaBH4 concentrations between 0.1–0.4% (m v−1) and remained constant at higher concentrations. During all the above measurements, the atomization cell was at room temperature.
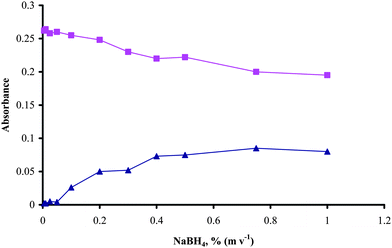 | ||
| Fig. 2 Effect of NaBH4 concentration, 40 ng ml−1 inorganic mercury (■) and methyl mercury (▲) in 0.6 mol l−1HCl, sample volume 500 µL, H2 flow rate 50 ml min−1, Ar flow rate 100 ml min−1, W-coil is at room temperature. | ||
NaBH4 concentration of 0.01% (m v−1), prepared in 0.1% (m v−1) NaOH, was selected for further experiments which allows the detection of only inorganic mercury when the W-coil in the atomization cell is at room temperature. In the next measurement, all the parameters were kept the same, except the power of the W-coil was on. In this case the recorded absorbance signal belongs to total mercury since the intermediate product (CH3HgH) from methyl mercury is atomized on the W-coil at 500 °C. The optimized parameters for the proposed method are shown in Table 1.
| Parameter | Hg +2 | CH3Hg+ |
|---|---|---|
| Sample HCl, mol l−1 | 0.6 | 0.6 |
| Carrier HCl, mol l−1 | 0.6 | 0.6 |
| NaBH4, m v−1 | 0.01 | 0.01 |
| Sample volume, µl | 500 | 500 |
| Ar flow rate, ml min−1 | 100 | 100 |
| H2 flow rate, ml min−1 | 50 | 50 |
| Atomization temperature, °C | 25 | 500 |
| Flow rates of NaBH4 and HCl, ml min−1 | 6.0 | 6.0 |
Once the vapor generation conditions were set, the atomization temperature suitable to decompose the volatile CH3HgH which is formed in the presence of methyl mercury was investigated. The temperatures between room temperature and 700 °C were tested by applying different voltages settings (Fig. 3). For the determination of total mercury, 500 °C was used throughout the experiments. The least squares regression analyses of the calibration curves for inorganic mercury are 0.0061[Hg2+] + 0.0029 at room temperature and 0.0058[Hg2+] + 0.0022 and at 500 °C and for methyl mercury 0.0032[CH3Hg+] + 0.0056 at 500 °C. According to t-test there is no significant difference between the slopes of inorganic mercury whether W-coil atomizer is heated up to 500 °C or kept at room temperature at 95% confidence level.
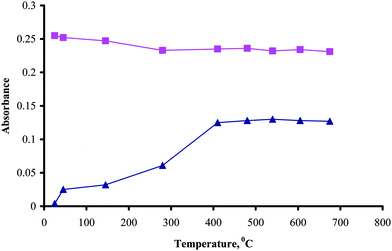 | ||
| Fig. 3 Effect of atomization temperature, 40 ng ml−1 inorganic (■) and methyl mercury (▲) in 0.6 mol l−1HCl, 0.01% NaBH4, sample volume 500 µL, H2 flow rate 50 ml min−1, Ar flow rate 100 ml min−1. | ||
The atomic signal from the methyl mercury species became constant between 400–700 °C but never reached the value of the inorganic mercury signal although they were same in mercury concentrations. Similar behavior was observed when the atomizer was changed to quartz-T tube and heated with air-acetylene flame. Different sensitivities for methyl mercury and inorganic Hg have also been reported by some authors.11
Analytical figures of merit
The analytical figures of merit of the proposed system for the determination of inorganic and total mercury are shown in Table 2. The limit of detection (LOD) was calculated by 11 consecutive measurements of blank solution. Fig. 4 demonstrates the performance of the method using continuous flow mode. The signal (A) belongs to inorganic mercury while the atom cell is at room temperature then the W-coil is heated up to 500 °C to obtain total mercury signal (B), the difference between A and B belongs to methyl mercury signal. The solution was a mixture of inorganic mercury (15 ng ml−1) and methyl mercury (30 ng ml−1). As it was shown in Fig. 4 inorganic mercury and methyl mercury determinations could be done consecutively in a fast way because of the heating and cooling rate of W-coil is fast and there is no need to wait between the measurements in contrast to externally heated quartz tube atomizer whether quartz tube heated by flame or heated resistively.| Parameter | Hg +2 | CH3Hg+ |
|---|---|---|
| Linear range, ng ml−1 | 5–100 | 5–100 |
| Relative standard deviation (n = 9), % | 3.1 | 2.6 |
| Limit of detection (LOD), ng ml−1 | 0.60 | 0.89 |
| Characteristic mass, ng | 0.31 | 0.69 |
| Sample frequency, h−1 | 100 | 100 |
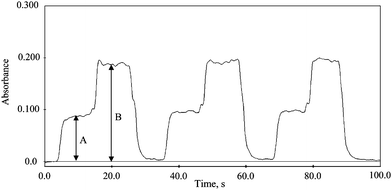 | ||
| Fig. 4 Consecutive measurement of 15 ng ml−1Hg+2 and 30 ng ml−1 CH3Hg+ in a mixture solution using continuous flow mode. (A) inorganic mercury signal W-coil is at room temperature, (B) total mercury signal W-coil is heated to 500 °C. | ||
Method validation
As an application of the proposed method various concentrations of (10–30 ng ml−1) methyl mercury and inorganic mercury were spiked into tap water and deionized water samples. The recoveries were between 95–108% for both mercury species as shown in Table 3. The proposed method was validated by analyzing two certified reference material with different certified contents of methyl mercury and total mercury. Mercury species were extracted by microwave assisted extraction in 6.0 mol l−1HCl and determination was performed using external calibration. As shown in Table 4 the concentrations found for methyl mercury and total mercury were in good agreement with certified values at 95% confidence level.| Mercury added, ng ml−1 | Hg +2 recovered, % | CH3Hg+ recovered, % |
|---|---|---|
| a Results are given as average ± standard deviation (n = 3). | ||
| Deionized water | ||
| 10 (Hg+2) + 30 (CH3Hg+) | 108 ± 2.1 | 104 ± 3.5 |
| 15 (Hg+2) + 15 (CH3Hg+) | 104 ± 3.2 | 97 ± 3.7 |
| 30 (Hg+2) + 10 (CH3Hg+) | 102 ± 3.0 | 99 ± 2.8 |
| Tap water | ||
| 10 (Hg+2) + 30 (CH3Hg+) | 96 ± 2.7 | 98 ± 3.3 |
| 15 (Hg+2) + 15 (CH3Hg+) | 95 ± 3.0 | 99 ± 4.1 |
| 30 (Hg+2) + 10 (CH3Hg+) | 100 ± 1.1 | 104 ± 1.2 |
| CRM | Certified, mg kg−1 | Measured, mg kg−1 | ||
|---|---|---|---|---|
| CH3Hg+ | Total | CH3Hg+ | Total | |
| a Results are given as average ± standard deviation (n = 3). | ||||
| IAEA-085 | 22.9 ± 1.0 | 23.2 ± 0.8 | 22.5 ± 0.7 | 23.1 ± 1.2 |
| DOLT-3 | 1.59 ± 0.12 | 3.37 ± 0.14 | 1.61 ± 0.1 | 3.38 ± 0.2 |
Conclusion
The proposed method provides an easy, fast and novel way for the determination of inorganic and total mercury. The heating and cooling rate of the W-coil is very fast and it is possible to determine inorganic and total mercury in a very fast sequential fashion (100 measurements h−1) using the same NaBH4 concentration. The obtained analytical figures show that the method is suitable for the determination of inorganic and total mercury in many biological and environmental samples in a simple and fast way.Acknowledgements
This work was financially supported by Gazi University Research Fund through grant BAP 02/2007-02References
- P. J. Craig, in:P. J. Craig (Ed.), Organometallic Compounds in the Environment, Principles, and Reactions, Longman, Leicester, UK, 1986 Search PubMed.
- M. Hempel, Y. K. Chau, B. J. Dutka, R. McInnis, K. K. Kwan and D. Liu, Analyst, 1995, 120, 721 RSC.
- M. Leermakers, W. Baeyens, P. Quevauviller and M. Horvat, Tr. AC, 2005, 24, 383 Search PubMed.
- J. L. Gomez-Ariza, F. Lorenzo, T. Garcia-Barrera and D. Sanchez-Rodas, Anal. Chim. Acta, 2004, 511, 165 CrossRef CAS.
- S. Rio Segade and J. F. Tyson, Spectrochim. Acta, Part B, 2003, 58, 797.
- L. E. Kaercher, F. Goldschmidt, J. N. Gottfried Paniz, É.M. de Moraes Flores and V. L. Dressler, Spectrochim. Acta, Part B, 2005, 60, 705 CrossRef.
- D. P. Torres, M. A. Vieira, A. S. Ribeiro and A. J. Curtius, J. Anal. At. Spectrom., 2005, 20, 289 RSC.
- J. A. Rust, J. A. Nobrega, C. P. Calloway and B. T. Jones, Spectrochim. Acta Part B, 2005, 60, 589 CrossRef.
- X. D. Hou, Z. Yang and B. T. Jones, Spectrochim. Acta Part B, 2001, 56, 203 CrossRef.
- M. F. Giné, F. J. Krug, V. A. Sass, B. F. Reis, J. A. Nóbrega and H. Berndt, J. Anal. At. Spectrom., 1993, 8, 243 RSC.
- C. C. Wan, C. S. Chen and S. J. Jiang, J. Anal. At. Spectrom., 1997, 12, 683 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
