Water-tolerant zeolite catalyst for the acetalisation of glycerol
Carolina X. A.
da Silva
,
Valter L. C.
Gonçalves
and
Claudio J. A.
Mota
*
Universidade Federal do Rio de Janeiro, Instituto de Química, Av Athos da Silveira Ramos 149, CT Bloco A, 21941-909, Rio de Janeiro, Brazil
First published on 30th October 2008
Abstract
We studied the acid-catalysed reaction of glycerol with aqueous formaldehyde and acetone in absence of solvents and using heterogeneous catalysts. The reactivity of acetone was usually higher than formaldehyde and the glycerol conversion was over 90% within 40 min of reaction time for all the heterogeneous acid catalysts studied. With aqueous formaldehyde solution, the glycerol conversion was within 60 to 80%, depending upon the acid catalyst used (Amberlyst-15, K-10 montmorillonite, p-toluene-sulfonic acid), due to the high amount of water in the reaction medium, which shifts the equilibrium and weakens the acid sites. However, the use of zeolite Beta, with Si/Al ratio of 16, leads to a conversion of over 95% within 60 min of reaction time. The hydrophobic character of the zeolite, due to the high silicon content, prevents the diffusion of the water to the interior of the pore, preserving the strength of the acid sites. In addition, the water formed during the acetalisation is expelled off from the pore environment, impairing the reverse reaction, and avoiding the use of hazard solvents, commonly employed to distil off the water formed.
Glycerol is formed as a byproduct in the biodiesel production from vegetable oils and animal fat.1 The forecast for 2010 points out2 to a global production of 1.2 million tons of glycerol, mostly coming from biodiesel production. Therefore, it is imperative to develop new uses for glycerol to prevent environmental problems and to add value to the biodiesel production chain.
The chemistry of glycerol has attracted the interest of the scientific community and several reviews have appeared in the literature.2,3 Most of the studies are concentrated on etherification,4hydrogenation,5oxidation,6 and dehydration.7 On the other hand, glycerol acetals and ketals have received considerably less attention. They can find applications as fuel additives,8surfactants9 and flavours.10 The glycerol formal, produced upon the reaction of glycerol with formaldehyde, finds applications as a disinfectant and solvent for cosmetics and medical usage.11 A drawback in the glycerol acetalisation is the formation of water, which weakens the acid strength of the catalyst and favors the reversibility of the reaction. The use of solvents to distil off the water formed has been employed12 to increase the yield of the glycerol ketals and acetals, but this practice is not environmentally recommended, since most of these solvents, such as benzene, toluene, chloroform and dichloromethane, are hazards to humans. In this communication, we wish to present the results of glycerol acetalisation with acetone and aqueous formaldehyde solution, under acid catalysis conditions, showing that the proper choice of a heterogeneous acid catalyst might prevent the use of hazard solvents.
The reactions were carried out under batch conditions. Typically, 5 g (54.3 mmol) of glycerol was stirred with 4.8 ml (65.5 mmol) of acetone or 5.3 ml (65.5 mmol) of aqueous formaldehyde solution (37%) and a specific mass of the catalyst, at 70 °C (Fig. 1,2). The weight of the catalyst varied to maintain 1.5 mmol of acid sites in every reaction. Table 1 shows some characterization data, as well as the pre-treatment temperature used to activate the catalysts. The molar ratio of glycerol to acetone or formaldehyde was 1 : 1.2. The kinetics of glycerol conversion was estimated by withdrawing samples at specific time intervals, followed by gas chromatography coupled with mass spectrometry analysis. In all experiments, 1,4-dioxane was used as an internal standard.
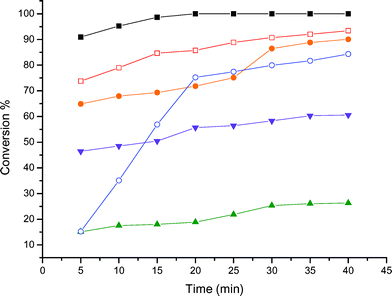 | ||
| Fig. 1 Kinetics of the glycerol reaction with acetone at 70 °C over various acid catalysts. (■) Amberlyst-15, (□) zeolite Beta, (●) K-10, (○) PTSA, (▼) HUSY, (▲) zeolite ZSM-5. | ||
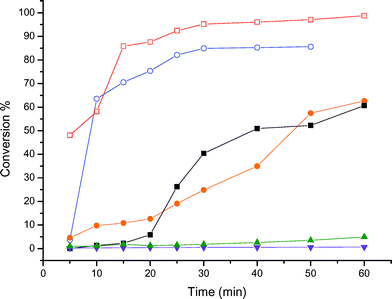 | ||
| Fig. 2 Kinetics of the glycerol reaction with aqueous formaldehyde at 70 °C over various acid catalysts. (■) Amberlyst-15, (□) zeolite Beta, (●) K-10, (○) PTSA, (▼) HUSY, (▲) zeolite ZSM-5. | ||
| Catalyst | Pre-treatment temperature (°C)a | Acidity (mmol/g)b | Area (m2/g) |
|---|---|---|---|
| a Rate = 10 °C/min. Time in activation temperature = 2 h. b Measured by n-butylamine adsorption at 150 °C.15 c Informed by the producer (Rohm and Haas). d Brackets stand for the Si/Al ratio. e Total Si/Al ratio = 2.8; framework Si/Al ratio = 4.5. | |||
| Amberlyst-15 | 120 | 4.2c | 50 |
| K-10 Montmorillonite | 150 | 0.5 | 240 |
| Zeolite Beta (16)d | 300 | 1.6 | 633 |
| Zeolite ZSM-5 (14) | 300 | 1.2 | 374 |
| Zeolite USY (2.8)e | 300 | 1.9 | 566 |
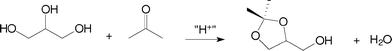
The reactions with acetone afforded only one ketal (Scheme 1), with a five-membered ring, as already reported in the literature.2 Amberlyst-15 acid resin showed the best performance among the catalysts tested, achieving over 95% of glycerol conversion within 15 min of reaction time. Zeolite Beta and K-10 montmorillonite behaved similarly in this reaction, showing a glycerol conversion of about 90% after 40 min. All these heterogeneous catalysts showed a better performance than p-toluene-sulfonic acid (PTSA), a model for homogeneous catalysis. The ZSM-5 zeolite showed a glycerol conversion of about 20% after 40 min of reaction time, whereas USY converted approximately 60% of the glycerol within the same period of time. The poor catalytic activity of the ZSM-5 zeolite can be explained in terms of the narrow pore structure, which probably does not permit the acetalisation to occur inside the pores. Thus, the reaction takes place at the external surface or at the pore entrance, leading to a poor yield of the ketal. USY is an aluminium-rich zeolite, having a hydrophilic character. Thus, the water formed during the reaction might remain inside the pores, weakening the acid sites and explaining its worse performance, compared with zeolite Beta, Amberlyst-15 and K-10 Montmorillonite.
The reactions with formaldehyde solution afforded two acetals (Scheme 2), one with a five-membered ring and another with a six-membered ring. The reaction proceeded slower than with acetone, when a same catalytic system was considered. For instance, in the reaction with acetone catalysed by Amberlyst-15, the glycerol conversion was over 95% after 15 min. Nevertheless, after the same period of time, the glycerol conversion was about 5% in the reaction with formaldehyde solution, using the same catalyst and conditions.
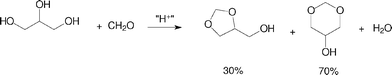 | ||
| Scheme 2 Acid-catalysed acetalisation of glycerol with aqueous formaldehyde. | ||
Contrarily to what was found in the reactions with acetone, Amberlyst-15 acid resin and K-10 Montmorillonite showed a worse performance than PTSA. They showed a glycerol conversion around 60% after 60 min of reaction time, whereas PTSA converted about 80% of the glycerol in 50 min of reaction. The best catalyst studied for the reaction of glycerol with formaldehyde solution was zeolite Beta, affording over 95% conversion after 60 min. This result might be explained in terms of the water-tolerance of this zeolite. Due to its high siliceous composition, the zeolite environment is hydrophobic and prevents the diffusion of the water molecules to the interior of the pores, where most of the acid sites are located. In the same way, the water formed upon the acetalisation is expelled off from the pores, thus preserving the integrity and strength of the acid sites and minimizing the reverse reaction. This behavior is typical of high-silica zeolites and has already been observed in other water-sensitive reactions, such as esterification and olefin hydration.13 In opposition to this behavior, USY showed almost no conversion after 60 min of reaction. Although the pore aperture of USY is as wide as zeolite Beta, its Si/Al ratio is much lower, leading to a hydrophilic character for this zeolite. Hence, the water molecules might diffuse and remain adsorbed inside the pores, weakening the acid sites and explaining the low conversion observed in the experiments. The behavior of ZSM-5 might be understood in terms of its pore structure. Although this zeolite has a high Si/Al ratio, and should be considered hydrophobic, its pore structure, with a diameter in the range of 5.6 Å, is too narrow to allow the formation of the glycerol acetals and ketals. This property is known as shape selectivity,14 and is typical of medium-pore zeolites. As observed in the reaction with acetone, ZSM-5 presented a low conversion for the acetalisation of glycerol with aqueous formaldehyde. This reinforces the hypothesis that the reaction takes place at the external surface of this zeolite, and therefore, the acid sites are weakened by the presence of the water molecules, explaining the low conversion observed.
We also tested the effect of catalyst loading. For the reactions with acetone, an increase of the catalyst loading did not significantly affect the performance, because the conversions were high enough, especially with Amberlyst-15. However, in reactions with formaldehyde solution the loading has an impact in the conversion. After 60 min, the conversion was 65% and 72% for 100% and 150% increase in the Amberlyst-15 loading, respectively, whereas with the standard loading the conversion was 61%. Notwithstanding, this increase in the number of active sites was not sufficient to level the catalytic activity of Amberlyst-15 and zeolite Beta. The conversion, using about one third of zeolite Beta loading, was significantly higher than that observed with Amberlyst-15. This is another additional proof of the importance of choosing a proper zeolite catalyst, with hydrophobic character, to perform this reaction.
The distribution of the two acetals formed in the reactions of glycerol with aqueous formaldehyde did not change very much with the catalyst used, being around 70% for the six-membered ring and 30% for the five-membered ring acetal. We did not find in the literature a more systematic study to account for the difference in selectivity between acetone and formaldehyde in the acetalisation of glycerol. A possible explanation is shown in Scheme 3. Upon the formation of the hemiacetal or hemiketal, there could be two different pathways for the two systems. For acetone/glycerol hemiketal, dehydration yields a tertiary carbenium ion, also stabilized by resonance with the non-bonded electron pairs of the adjacent oxygen atom. Then, there occurs a rapid nucleophilic attack of the secondary hydroxyl group to form the five-membered ring ketal. As the lifetime of the carbenium ion in the reaction medium is supposed to be short compared with the lifetime of the hemiketal, the product distribution is governed by kinetics, which favors the formation of the less thermodynamically stable five-membered ring transition state, as already observed in other cyclisation reactions.16 For the formaldehyde/glycerol hemiacetal, dehydration occurs simultaneously with the nucleophilic attack of the hydroxyl groups, as formation of a primary carbenium ion is not expected due to the high energy barrier. Thus, the system follows an SN2-like mechanism that might proceed with the nucleophilic attack of the primary hydroxyl group, leading to the six-membered ring acetal, or attack of the secondary hydroxyl group, which yields the five-membered ring acetal. As the lifetime of the protonated hemiacetal might be long in the reaction medium, there is enough time for molecular reorientation, so both products, of thermodynamic (six-membered acetal) and kinetic (five-membered acetal) control could be formed. We are now investigating in further details the difference in reaction mechanism of the two systems, but we believe this difference is more related with the structure of the carbonyl compound (aldehyde or ketone) rather than with the structure of the catalyst, since other studies,12 using different aldehydes and ketones, reported the same typical distribution.
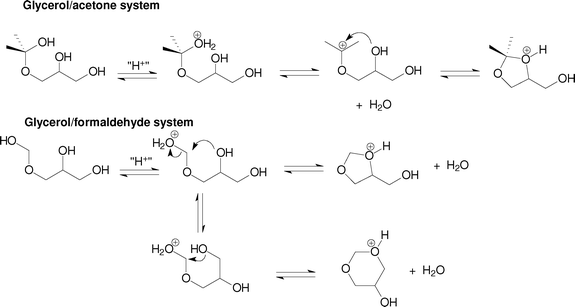 | ||
| Scheme 3 Possible mechanistic scheme to explain the product selectivity in the acetalisation of glycerol with formaldehyde and acetone. | ||
The results of glycerol acetalisation with acetone and formaldehyde solution shows that the choice of the proper heterogeneous catalyst might prevent the use of hazardous solvents, such as benzene and chloroform, normally used to distil off the water formed and shift the equilibrium. This procedure might be employed in other reactions, where water is present in the reaction medium or is formed during the reaction and affects the catalyst activity.
Acknowledgements
Authors thank Repsol/YPF, FINEP, CNPq, FAPERJ and PRH/ANP for financial support.References
- A. C. Pinto, L. N. Guarieiro, M. J. C. Resende, N. M. Ribeiro, E. A. Torres, W. A. Lopes, P. A. D. Pereira and J. B. Andrade, J. Braz. Chem. Soc., 2005, 16, 1313–1330 CAS.
- C. H. Zhou, J. N. Beltramini, Y. X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- (a) A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC; (b) M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. D. Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS.
- (a) R. S. Karinen and A. O. I. Krause, Appl. Catal. A, 2003, 306, 128–133; (b) K. Klepacova, D. Mravec and M. Bajus, Appl. Catal., A, 2005, 294, 141–147 CrossRef CAS; (c) Y. Gu, A. Azzouzi, Y. Pouilloux, F. Jerome and J. Barrault, Green Chem., 2008, 10, 164–167 RSC.
- (a) M. A. Dasari, P. P. Kiatsimkul, W. R. Sutterlin and G. J. Suppes, Appl. Catal., A, 2005, 281, 225–231 CrossRef CAS; (b) Y. Kusunoki, T. Miyazawa, K. Kunimori and K. Tomishige, Catal. Commun., 2005, 6, 645–649 CrossRef CAS; (c) T. Miyazawa, Y. Kusunoki, K. Kunimori and K. Tomishige, J. Catal., 2006, 240, 213–221 CrossRef CAS; (d) I. Furikado, T. Miyazawa, S. Koso, A. Shimao, K. Kunimori and K. Tomishige, Green Chem, 2007, 9, 582–588 RSC.
- (a) R. R. Soares, D. A. Simonetti and J. Dumesic, Angew. Chem., Int. Ed., 2006, 45, 3982–3985 CrossRef CAS; (b) P. McMorn, G. Roberts and G. J. Hutchings, Catal. Lett., 1999, 63, 193–197 CrossRef CAS.
- (a) S. H. Chai, H. P. Wang, Y. Liang and B. Q. Xu, J. Catal., 2007, 250, 342–349 CrossRef CAS; (b) E. Tsukuda, S. Sato, R. Takahashi and T. Sodesawa, Catal. Commun., 2007, 8, 1349–1353 CrossRef CAS.
- (a) Fr Pat, 2 833 607 A1, 2003; (b) Int Pat, 2005/093 015 A1, 2005.
- A. Piasecki, A. Sokolowski, B. Burczyk and U. Kotlewska, J. Am. Oil Chem. Soc., 1997, 74, 33–37 CrossRef CAS.
- (a) M. J. Climent, A. Veltry and A. Corma, Green Chem., 2002, 4, 565–569 RSC; (b) M. J. Climent, A. Corma and A. Veltry, Appl. Catal. A, 2004, 263, 155–161 CrossRef CAS.
- P. Sari, M. Razzak and I. G. Tucker, Pharm. Dev. Technol, 2004, 9, 97–106 CrossRef CAS.
- J. Deutsch, A. Martin and H. Lieske, J. Catal., 2007, 245, 428–435 CrossRef CAS.
- T. Okuhara, Chem. Rev, 2002, 102, 3641–3666 CrossRef CAS.
- B. Smit and T. L. M. Maessen, Nature, 2008, 451, 671–678 CrossRef CAS.
- V. L. C. Gonçalves, B. P. Pinto, J. C. da Silva and C. J. A. Mota, Catal. Today, 2008, 133–135, 673–677 CrossRef CAS.
- S. Chandrasekhar, Chem. Soc. Rev., 1987, 16, 313–338 RSC.
| This journal is © The Royal Society of Chemistry 2009 |