Chlorine borrowing: an efficient method for an easier use of alcohols as alkylation agents†
Philippe
Makowski
a,
Regina
Rothe
a,
Arne
Thomas
a,
Markus
Niederberger
b and
Frédéric
Goettmann
*ac
aMax-Planck Institute of Colloids and Interfaces, Scientific Campus Golm, 14476, Potsdam, Germany
bDepartment of Materials, ETH Zürich, Wolfgang-Pauli-Str. 10, 8093, Zürich, Switzerland. Fax: +41 44 632 11 01; Tel: +41 44 633 63 90
cInstitut de Chimie Séparative de Marcoule, UMR 5257, Site de Marcoule, BP 17171, 30207, Bagnols sur Cèze, France. E-mail: frederic.goettmann@cea.fr; Fax: (+33) 466 797 611; Tel: (+33) 466 905 758
First published on 14th November 2008
Abstract
Chlorine functionalised tin dioxide nanoparticles proved able to partially convert alcohols into the corresponding chlorides, which act as alkylation agents with an increased electrophilicity, as evidenced on ether formation and Friedel–Crafts reactions.
In the first issue of this Journal, J. H. Clark exposed his vision of green chemistry in the article “Green chemistry: challenges and opportunities”.1 Therein, he selected Friedel–Crafts acylations as an archetypical example of industrially relevant, but immensely wasteful reactions. Indeed, the use of both an acyl chloride as electrophile and a homogeneous Lewis acid as promoter resulted in large amounts of, partly harmful, waste products. The same considerations are true for Friedel–Crafts alkylations. Nevertheless, due to their importance in synthetic chemistry, a lot of work has been devoted to finding true heterogenous catalysts for these reactions, such as zeolites, polyoxometalates or mesoporous oxides.2–6 Another goal of these works was to enable the use of electrophiles greener than halogenoalkanes. In that respect, alcohols, which have already been largely used as alkylating agents for ketones, malonates or nitriles,7–10 were thought to be ideal candidates. Indeed, they are easily accessible from biomass or hydroformylation products and produce only water as a by product. Many successes were recorded in the use of alcohols for Friedel–Crafts alkylations. Yadav et al., for example, reported on the use of super acids to alkylate functional aromatics with alcohols.11,12 Alternatively, Roy and co-workers described the use of a bifunctionnal Ir-Sn catalyst for this reaction. In this approach the tin centre was reported to act as a Lewis acid to activate the alcohol, while the iridium centre activated the electrophile.13,14 The use of mesoporous carbon nitride as a catalyst for such reactions was also recently described.15 Most of these approaches, however, suffered either from a lack of versatility or too harsh reaction conditions.
In order to allow for a more general use of alcohols as alkylating agents for, for example, Friedel–Crafts reactions or other C–C bond forming reactions, but also for ether or amine syntheses, new activation paths are required. In that respect, recent progresses in understanding nonaqueous sol-gel chemistry are a great source of inspiration.16 Indeed, this chemistry deals with the controlled synthesis of metal oxides under water free conditions and rely on the organic chemistry of alcohols, carboxylic acids or ketones as an oxygen source. The reactions providing the needed oxygen were mainly found to be based on the formation of alkyl chlorides, esters, ethers or C–C coupling products and were recently reviewed.17 Our attention was attracted by the fact that during the nonaqueous synthesis of SnO2nanoparticles (SnO2NPs), starting with SnCl4, in benzyl alcohol as a solvent, almost all the alcohol was converted into the corresponding ether.18 This pointed to a possible catalytic activity of these particles, which will be investigated herein.
The employed SnO2nanoparticles were synthesised as previously described, by heating SnCl4 in benzyl alcohol at 100 °C for 24 h.18 The resulting particles are crystalline and feature a mean size of about 4 nm as described in the initial publication.† For reference experiments, chlorine free SnO2nanoparticles were also synthesised by using tin tert-butoxide as a precursor.†19 A preliminary test to convert benzyl alcohol (300 mg) in dibenzyl ether in the presence of a catalytic amount of SnO2NPs (25 mg) under mild conditions (100 °C for 20 h), indeed showed the formation of ether but also of a small amount of benzyl chloride.† The needed chlorine atoms had to be provided by the nanoparticle itself, as it was previously evidenced that these SnO2NPs were decorated with chloride ligands.18 We thus assumed that the formed chloro-alkanes were not only by-products of the reaction, but also acted as intermediates in the formation of the ether, as depicted in Scheme 1. Interestingly, the exchange of halogeno- and hydroxy-moieties between metal halides and alcohols to yield the corresponding metal oxide and halogeno-alkanes has early been identified as a major process in non-hydrolytic sol-gel chemistry.20
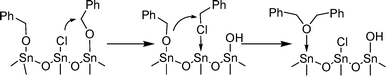 | ||
| Scheme 1 Proposed mechanism for the SnO2NPs catalysed condensation of benzyl alcohol. | ||
These observations are of great synthetic importance. Indeed, as halogenoalkanes are much better electrophiles than alcohols they open the possibility of a generalised use of alcohols as alkylating agentvia a kind of chlorine borrowing mechanism, as depicted in Scheme 2.
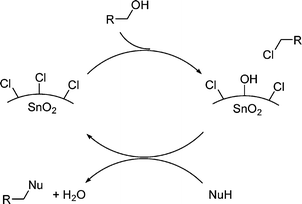 | ||
| Scheme 2 Proposed mechanism for a general SnO2NPs catalysed enhancement of the electrophilicity of alcohols. | ||
In order to test this hypothesis, we attempted to catalyse the conversion of alcohols into ethers and their further use in Friedel–Crafts alkylations with SnO2NPs. In a first set of experiments pure alcohols or mixtures of alcohols were heated between 120 and 150 °C with an oil bath in screw capped glass tubes in the presence of SnO2NPs. During the reaction, a liquid condensed on the upper cool glass tube surface, which was attributed to water formation. Due to the temperature of the reaction mixtures, no formation of a second phase was observed. Table 1 displays some of the results we obtained. As can be seen, the wanted ethers were obtained in good yields with high selectivities as soon as benzyl alcohol derivatives were used (Table 1, entries 1, 3, 4), while other alcohols proved to be more difficult to convert. However, this result is particularly advantageous for the preparation of dissymmetric ethers, as evidenced by the condensation of benzyl alcohol with hexanol and cyclohexanol. These results are consistent with those recently obtained by Corma and Renz with tin supported on micro- and mesoporous oxides, although we had to employ harsher reaction conditions.21 But it is, to the best of our knowledge, the first time a system containing mainly tin oxide is used for this purpose. Interestingly, particles prepared using Sn(tBuO)4 as a tin source did not exhibit the same catalytic activity (Table 1, entry 2), thus supporting our assumption that chlorine was playing a major role in this reaction.
| Entry | Substratea | Temp./°C | Time/h | Conv. (%)b | Productsc |
|---|---|---|---|---|---|
| a In a typical procedure 300 mg of the pure alcohol were heated in presence of 25 mg of NPs. b Conversion rates determined by GC on the basis of the initial substrate amount. c The indicated percentages correspond to the amount of the corresponding product relative to the overall amount of formed products. d Reference test with chlorine free nanoparticles. e For these tests 200 mg of benzyl alcohol were heated in 2 g of the other alcohol as a solvent with 25 mg of particles. | |||||
| 1 | Benzyl alcohol | 150 | 80 | 100 | Dibenzyl ether 95% |
| 2 | Benzyl alcohol d | 150 | 80 | 1 | Dibenzyl ether 100% |
| 3 | 4-Methylbenzyl alcohol | 120 | 48 | 44 | Di(4-methyl)benzyl ether 100% |
| 4 | 4-Chlorobenzyl alcohol | 130 | 20 | 64 | Di(4-chloro)benzyl ether 100% |
| 5 | Hexanol | 150 | 120 | 3 | Dihexyl ether 100% |
| 6 | Cyclohexanol | 120 | 120 | 2 | Dicyclohexylether 100% |
| 7 | Benzyl alcohol | 150 | 96 | 11 | Dibenzyl ether 10% |
| Hexanol e | Hexyl benzyl ether 90% | ||||
| 8 | Benzyl alcohol | 150 | 96 | 20 | Dibenzyl ether 10% |
| Cyclohexanol e | Cyclohexyl benzyl ether 90% |
In order to be able to determine an accurate turnover number, we further investigated the chemical composition of the employed SnO2nanoparticles. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) was used to quantify the tin content and ion chromatography to quantify the chlorine in solutions resulting from the dissolution of SnO2NPs in nitric acid. This enabled us to evaluate that 25 mg of catalyst corresponded to 0.14 mmol of tin and 0.06 mmol of chlorine. As a consequence, the turnover number (TON, determined as the number of mol of substrate converted at the end of the reaction per mol of catalyst) in the case of benzyl acohol (Table 1, entry 1) would be 20 calculated on the basis of tin and 46 calculated on the basis of chlorine. These are relatively modest TONs but they, at least, evidence that the process is really catalytic.
A series of tests was done on benzyl alcohol, in order to test the recyclability of our catalyst. For this purpose, the nanoparticles were separated by centrifugation after each reaction and reused with new batches of fresh benzyl alcohol at 150 °C for 80 h. As can be seen in Fig. 1, the first recyclings proved effective even if moderate activity and selectivity losses are observed. This indicates that the surface of our catalyst is still covered with active chlorinated sites after a few recyclings. The fourth test however evidenced strong activity and selectivity losses. The composition of the resulting reaction mixture could not be completely analysed, but GC-MS analysis showed the formation of benzaldehyde, benzoic acid benzyl ester and some higher mass compounds.
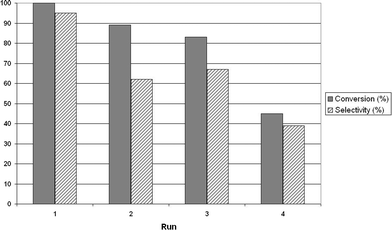 | ||
| Fig. 1 Recycling of the SnO2NPs. The test reaction was the conversion of benzyl alcohol into dibenzylether, at 150 °C for 80 h. The conversion was determined by GC-MS as the benzyl alcohol consumption. The selectivity is expressed as the ratio between formed dibenzyl ether and the benzyl alcohol consumption. | ||
In a second study, we investigated whether the intermediate chloride species could also act as electrophiles for Friedel–Crafts reactions, as depicted in Scheme 3. Even if pure tin oxide does not seem to be a common catalyst for Friedel–Crafts reactions, the fact that tin complexes proved Lewis acidic enough to promote such reactions22 encouraged us to proceed to our tests without an additional Friedel–Crafts catalyst. In a typical catalytic experiment 25 mg of catalyst, 100 mg of alcohol and 5 ml of aromatic compound were heated to 150 °C in 23 ml acid digestion bombs (Parr Instrument) for 120 h. Table 2 displays some of the obtained results.
| Entry | Aromatic compound | Benzene | Toluene | Anisole | ||
|---|---|---|---|---|---|---|
| Electrophile | Yield (%)b | Yield (%) | p/oc | Yield (%) | p/o | |
| a In a typical reaction 25 mg of catalyst, 100 mg of alcohol and 5 ml of aromatic compound were heated to 150 °C for 120 h. b The yields were determined by GC-FID with mesitylene as a reference and correspond to the molar ratio between the obtained alkylation product and the initial amount of alcohol. c p/o corresponds to the molar ratio between para and ortho substituted alkylation products. d Reference test with chlorine free SnO2NPs. e Reference test with chlorine free SnO2NPs but in presence of 20 mg of benzyl chloride, this test was only undertaken with anisole. | ||||||
| 1 | Benzyl alcohol | 93 | 100 | 0.9:1 | 97 | 1:1 |
| 2 | Benzyl alcohol d | 0 | 0 | — | 0 | — |
| 3 | Benzyl alcohol e | n.a. | n.a. | — | 50 | 1:1 |
| 4 | 4-Methylbenzyl alcohol | 90 | 100 | 0.5:1 | 100 | 1.4:1 |
| 5 | Cinnamyl alcohol | 100 | 100 | 1:0.55 | 100 | 1:0.26 |
| 6 | Isopropanol | 0 | 5 | 1:1 | 9 | 0.7:1 |
| 7 | Cyclohexanol | 0 | 0 | — | 10 | 1:1 |
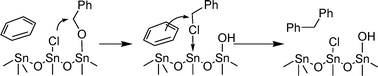 | ||
| Scheme 3 Proposed mechanism for the SnO2NPs catalysed benzylation of bezene with benzyl alcohol. | ||
Here again, the Friedel–Crafts alkylation products are obtained in high yields as long as very active alcohols are used (Table 2, entries 1, 4, 5). This observation is consistent with other reports on the use of benzyl alcohol derivatives.23–25 In the case of the reaction of benzyl alcohol with benzene the TON was calculated to be 6 on the basis of tin and 14 on the basis of chlorine. It is also worth noticing here, that, contrary to previous studies, the use of an electron rich aromatic derivative is not required. Indeed, benzene reacts nearly as well as toluene or anisole with active alcohols. In addition, toluene and anisole also give small amounts of alkylation products with less active secondary alcohols, which seems to be a novelty in the field.
As for the ether formation reaction, chlorine free SnO2NPs show no catalytic activity. On the contrary the same SnO2NPs yielded the desired benzyl alcohol alkylation product on anisole when 20 mg of benzyl chloride were added to the reaction mixture (Table 2, entry 3). Interestingly, the amount of obtained alkylation product is three time higher than the amount of added benzyl chloride, which clearly proves that chlorine transfer is possible and further supports our chlorine borrowing mechanism.
In conclusion, we have shown here that tin oxide nanoparticles with surface-adsorbed chloride ions could promote the electrophilicity of alcohols via a new chlorine borrowing mechanism. As compared to other approaches to use alcohols as an alkylating agent, the reaction conditions we had to employ remained harsh, but we are confident in that this inconvenience can be overcome. Indeed, tin dioxide is not known to be a good Friedel–Crafts catalyst and we think that our results can be enhanced by designing bifunctional systems featuring both chlorinated tin sites and stronger Lewis acidic sites. In addition, the possibility of avoiding the use of noble metals13,14 can possibly balance the requirement of harsher reaction conditions. Moreover, we are convinced that this mechanism could be much more general and allow alcohols to react more easily with a large variety of nucleophiles, such as ketones, nitriles or amines.
The Max-Planck Society is gratefully acknowledged for financial support within the framework of the enerchem project house, so is also the CEA (French Atomic Energy Commission). The authors also thank Agnès Grandjean, Véronique Dubois and Didier Maurel for the chemical analysis of the SnO2NPs
Notes and references
- J. H. Clark, Green Chem., 1999, 1, 1 RSC
.
- C. Anand, P. Srinivasu, S. Alam, V. V. Balasubramanian, D. P. Sawant, M. Palanichamy, V. Murugesan and A. Vinu, Microporous Mesoporous Mater., 2008, 111, 72 CrossRef CAS
.
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS
.
- H. Firouzabadi and A. A. Jafari, Curr. Org. Chem., 2008, 12, 233 CrossRef CAS
.
- G. Sartori and R. Maggi, Chem. Rev., 2006, 106, 1077 CrossRef CAS
.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS
.
- A. Fischer, P. Makowski, J. O. Mueller, M. Antonietti, A. Thomas and F. Goettmann, ChemSusChem, 2008, 1, 444 CrossRef CAS
.
- M. S. Kwon, N. Khn, S. H. Seo, I. S. Park, R. K. Cheedrala and J. Park, Angew. Chem., Int. Ed., 2005, 44, 6913 CrossRef CAS
.
- P. A. Slatford, M. K. Whittlesey and J. M. J. Williams, Tetrahedron Lett., 2006, 47, 6787 CrossRef CAS
.
- M. Yasuda, T. Somyo and A. Baba, Angew. Chem., Int. Ed., 2006, 45, 793 CrossRef CAS
.
- G. D. Yadav and G. S. Pathre, Microporous Mesoporous Mater., 2006, 89, 16 CrossRef CAS
.
- G. D. Yadav and G. S. Pathre, Appl. Catal., A, 2006, 297, 237 CrossRef CAS
.
- J. Choudhury and S. Roy, J. Mol. Catal. A: Chem., 2008, 279, 37 CrossRef CAS
.
- S. Podder, J. Choudhury and S. Roy, J. Org. Chem., 2007, 72, 3129 CrossRef CAS
.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Chem. Commun., 2006, 4530 RSC
.
- A. Vioux, Chem. Mater., 1997, 9, 2292 CrossRef
.
- M. Niederberger and G. Garnweitner, Chem.–Eur. J., 2006, 12, 7282 CrossRef CAS
.
- J. H. Ba, J. Polleux, M. Antonietti and M. Niederberger, Adv. Mater., 2005, 17, 2509 CrossRef CAS
.
- N. Pinna, G. Neri, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2004, 43, 4345 CrossRef CAS
.
- V. Lafond, P. H. Mutin and A. Vioux, J. Mol. Catal. A: Chem., 2002, 182–183, 81 CrossRef CAS
.
- A. Corma and M. Renz, Angew. Chem., Int. Ed., 2007, 46, 298 CrossRef CAS
.
- R. P. Singh, R. M. Kamble, K. L. Chandra, P. Saravanan and V. K. Singh, Tetrahedron, 2001, 57, 241 CrossRef CAS
.
- M. H. C. de la Cruz, M. A. Abdel-Rehim, A. S. Rocha, J. F. C. da Silva, A. da Costa Faro Jr and E. R. Lachter, Catal. Commun., 2007, 8, 1650 CrossRef CAS
.
- J. Le, Bras and J. Muzart, Tetrahedron, 2007, 63, 7942 CrossRef CAS
.
- J. S. Yadav, D. C. Bhunia, K. Vamshi, Krishna and P. Srihari, Tetrahedron Lett., 2007, 48, 8306 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedure for the used nanoparticles. See DOI: 10.1039/b807230b |
| This journal is © The Royal Society of Chemistry 2009 |
