The mechanism of the oxidation of benzyl alcohol by iron(III)nitrate: conventional versus microwave heating
Mark H. C. L.
Dressen
a,
Jelle E.
Stumpel
a,
Bastiaan H. P.
van de Kruijs
a,
Jan
Meuldijk
b,
Jef A. J. M.
Vekemans
a and
Lumbertus A.
Hulshof
*ac
aLaboratory of Macromolecular and Organic Chemistry, Applied Organic Chemistry, Eindhoven University of Technology, Den Dolech 2, 5612 AZ, Eindhoven, The Netherlands. E-mail: L.A.Hulshof@tue.nl.
bProcess Development Group, Eindhoven University of Technology, Den Dolech 2, 5612 AZ, Eindhoven, The Netherlands
cDSM Research, PO Box 18, 6160 MD, Geleen, The Netherlands
First published on 11th November 2008
Abstract
The mechanism of the oxidation of benzyl alcohol with iron(III)nitrate nonahydrate under conventional and under microwave heating conditions has been investigated and the reaction conditions have been optimized. A series of redox reactions leads to the formation of benzaldehyde and other products. Direct comparison between conventional and microwave heating revealed identical conversions profiles. Mastering the microwave induced heat, absence of a real microwave effect and byproduct formation are the major factors to advise a traditional batch-wise way of process development to a larger scale.
Introduction
In recent years much effort has been expended in an increasingly competitive world to meet the requirements of the fine chemical and pharmaceutical industry focusing on “first-time-right” performance, a short-time-to-market and avoiding surprises during process scale-up. A demand for larger quantities is not just rewriting a recipe by replacing “mg” with “kg” during chemical process research, which is largely based on changes of physical dimensions and other economic, technological and ecological factors including quality and safety aspects. Intensifications in process architecture, such as microreactor technology and microwave enhanced processing, as relatively novel tools in the field of organic chemistry, are actively pursued to achieve a better position in the industrial scene. The emersion of these promising techniques in the field of organic chemistry expands the toolbox of today's chemists.The increase of reaction rates and improved selectivity, combined with the automation of repetitive procedures, demonstrate the advantageous application of these enabling techniques and are considered to be suitable for the job. One of the major drawbacks of microwave-assisted chemistry is the difficulty associated with process scale-up. Since the penetration depth of microwaves is limited, the best opportunities for increased production volumes lie in continuous-flow processing equipment, albeit that this is somewhat uncommon in the fine chemical industry.1,2
Although this combined technological concept of flow chemistry and microwave heating has demonstrated positive results,2 the fundamental aspects of the rate enhancements remain unclear, and thermal as well as non-thermal effects are invoked to rationalize it.3–6 Limited insight into the so called microwave effects has been gained. These effects are defined as a rate enhancement or change of selectivity of chemical processes under microwave heating that cannot be reproduced under conventional heating. A thorough investigation of all techniques separately by accurate temperature measurements from inside either by fiber optics or by a gas-pressure sensor will strongly contribute to the reliability of the claims regarding microwave effects and will provide a real understanding of these rate enhancements.
The main focus of our research is aimed at a systematic investigation of the influence of microwave heating on chemical processes and assessing the technique for application on an industrial scale. We have demonstrated positive effects in terms of reaction rate enhancements of microwave heating compared to conventional heating in three cases of heterogeneous reactions.7 These effects vanished completely or partly when either the heterogeneity of the system was diminished or when the metal surface was cleaned with an initiator in the case of metal mediated reactions. The observations suggested that these favorable microwave-induced rate enhancements might not appear when homogeneous systems are carefully scrutinized under entirely comparable conditions for microwave and conventional heating. The absence of microwave effects under homogeneous conditions in combination with the low energy (∼10−5 eV) of a microwave photon make a direct influence of microwaves on the reaction mechanism highly unlikely.
The oxidation of benzyl alcohol with iron(III)nitrate nonahydrate has been amply reported. Good selectivity under mild reaction conditions and convenient isolation of the products were achieved employing various inorganic supports, such as silica gel,8 K10-clay,9HZSM-5 zeolite,10 kieselguhr11 and graphite.12 The K10 clay-supported version gave reaction rate enhancements under microwave irradiation conditions in the absence of solvents.13 The oxidation rate of benzyl alcohol has been reported to be positively influenced by a combination of microwave heating and microreactor technology (Scheme 1).4
 | ||
| Scheme 1 Oxidation of benzyl alcohol with iron(III)nitrate nonahydrate. | ||
To our surprise these rate enhancements were reported for a homogeneous system. The paper prompted us to gain more insight in the microwave effect in this particular case. We investigated in detail the mechanism of this oxidation under conventional heating conditions. Subsequently, microwave heating has been applied to this homogeneous reaction aimed at witnessing any beneficial effect in a batch-wise setup with accurate temperature control. Furthermore, the process scalability has been assessed predominantly based on chemical aspects.
Results of conventional heating
In contrast to the reactions in a flow-reactor described by Jachuck et al.,14 the oxidation of benzyl alcohol with Fe(NO3)3·9H2O has been performed neat under aerobic conditions in an open vessel at various elevated temperatures reached by conventional heating.Overview of operational reactions
In the first place it was found that the oxidation of benzyl alcohol with Fe(NO3)3·9H2O includes a series of possible redox reactions. These reactions may occur with the oxidant and benzyl alcohol in the presence of water from the nonahydrate. This water is released upon dissolution of the iron(III) salt in benzyl alcohol. The rather complex reaction network may give rise to uncertainty in the exact stoichiometry. In general this oxidation is based on the hydrolysis of iron(III)nitrate,15 see Scheme 2. | ||
| Scheme 2 The oxidation is based on the hydrolysis of iron(III)nitrate. | ||
During the reaction also brown fumes were observed referring to the presence of NO2, which led to a volume expansion of 42 mL gas per mL benzyl alcohol (molar ratio of benzyl alcohol : Fe(NO3)3·9H2O is 4 : 1). The evolution of brown fumes was not observed when the reaction was performed in an argon or nitrogen atmosphere, indicating a direct oxidation of NO to NO2 under aerobic conditions. However, working under anaerobic conditions lowered the conversion of benzyl alcohol by 15% as compared to aerobic conditions for the optimized reactions (Fig. 3). This result demonstrates that the absence or presence of oxygen has a profound effect on the stoichiometry of the reaction and also that a flow system leads to inferior conversions.
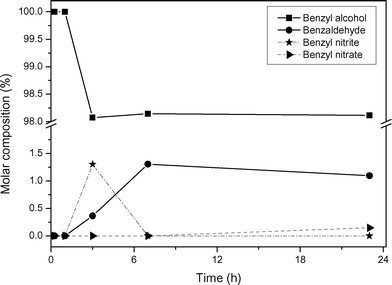 | ||
| Fig. 1 Time–molar composition plot of the oxidation of benzyl alcohol at a molar ratio of benzyl alcohol and Fe(NO3)3·9H2O of 100 : 1 at 50 °C under conventional heating and aerobic conditions. | ||
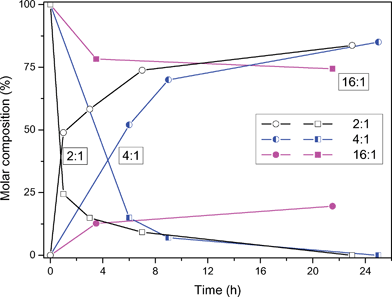 | ||
| Fig. 2 Conversion of benzyl alcohol (■) and formation of benzaldehyde (●) as a function of the initial molar ratio of benzyl alcohol and Fe(NO3)3·9H2O at 50 °C under conventional heating and aerobic conditions. | ||
 | ||
| Fig. 3 Time–molar composition plot of the oxidation of benzyl alcohol at a molar ratio of benzyl alcohol and Fe(NO3)3·9H2O of 4 : 1 at 100 °C under comparable conventional heating (CH) and microwave (MW) heating (aerobic conditions). | ||
It was expected that iron(III) either acts as a catalyst for the oxidation under aerobic conditions or is consumed by the oxidation. Therefore, the amount of iron(III) was determined by a titration with EDTA before and after the reaction.16 After the reaction was completed the amount of iron(III) in the reaction mixture was found to be unchanged within experimental error. However, by autodecomposition of iron(II)nitrate as an in-situ process, iron(II) may be formed, but this does not remain in this oxidative state in the reaction mixture by a redox reaction (see Scheme 3).17
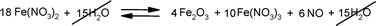 | ||
| Scheme 3 (Auto)decomposition of iron(II)nitrate reproduces iron(III). | ||
The role of iron(III) in the oxidation of benzyl alcohol is purely catalytic by coordinating the oxidant to the alcohol. Even though there is a combination of nitrate and iron(III) in one salt, a molar ratio of benzyl alcohol and Fe(NO3)3·9H2O of 100 : 1 at 50 °C is insufficient to bring the reaction to completion. The conversion completely flattens off after a few hours, see Fig. 1.
Although overall iron(III) remains in its original oxidative state, the counter-ion in its original salt form has changed into the oxide-salt thus lowering the iron(III) concentration in solution by precipitation during the reaction.
Formation of (by-)products
Throughout the reaction at various temperatures, the formation of two byproducts has been observed, namely benzyl nitrite18 and benzyl nitrate.19 The proposed sequence of reactions during the oxidation of benzyl alcohol to benzaldehyde are depicted in Schemes 4 and 5.In Scheme 4 the postulated reactions lead to a 2 : 1 molar ratio of benzyl alcohol and Fe(NO3)3·9H2O. To rationalize the observation that a 4 : 1 molar ratio nearly leads to completion, the reactions as proposed in Scheme 5 should also be integrated. Water and oxygen act as an oxidant in this process by reactivating NO.
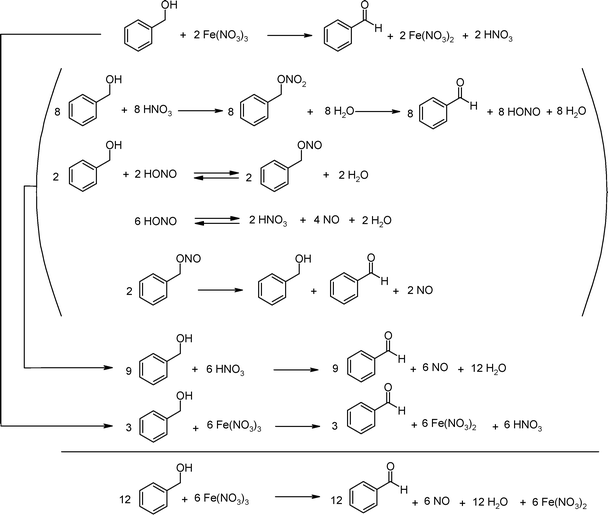 | ||
| Scheme 4 Reactions rationalizing a 2 : 1 ratio in stoichiometry of benzyl alcohol and Fe(NO3)3·9H2O. | ||
 | ||
| Scheme 5 Reactions rationalizing a higher than 2 : 1 ratio in stoichiometry of benzyl alcohol and Fe(NO3)3·9H2O. | ||
Molar ratio benzyl alcohol and Fe(NO3)3·9H2O
An increase in the amount of iron(III)nitrate, with respect to benzyl alcohol, leads to an increase of the reaction rate at 50 °C. See Fig. 2 for the time–conversion plots.At a molar ratio of 16 : 1 (benzyl alcohol : Fe(NO3)3·9H2O) at room temperature, benzyl alcohol was found to be saturated with Fe(NO3)3·9H2O affording a homogeneous solution.20 Larger amounts of Fe(NO3)3·9H2O resulted in two liquid phases. In that case, the bottom layer primarily consists of inorganic salt, melting point 47 °C, while the top layer is rich in benzyl alcohol. The heterogeneous nature of such reaction systems hampers a simple kinetic evaluation of the oxidation process.
Each reaction mixture with varying amounts of Fe(NO3)3·9H2O eventually forms a substantial amount of red-brown sediment on the reactor wall. An XPS analysis of the sediment points to a mixture of FeOx and FeNOx. The formation of sediment is explained by Schemes 2 and 3.
Almost full conversion within one hour was achieved at 100 °C when a 4 : 1 molar ratio of benzyl alcohol and Fe(NO3)3·9H2O was applied giving a selectivity of 75% to benzaldehyde, see Fig. 3. Three important issues are noteworthy. Firstly, under saturated conditions (16 : 1) the reactions appear to level off at 25% conversion, which is in line with the results obtained in Fig. 1. Secondly, over-oxidation leading to benzoic acid has been observed in all cases, which implicates the presence of an optimum for the conversion to benzaldehyde. And in the third place, high temperatures should be avoided due to instability of the byproducts during this oxidation.21
Results of microwave heating
In a next step, the influence of microwave heating on the course of the oxidation of benzyl alcohol was assessed at temperatures of 50 °C and 100 °C. These two temperature levels were primarily used to gain insight into the potential of microwave heating (MW) compared to conventional heating (CH), as claimed by Jachuck et al.4 The molar compositions of benzaldehyde and benzyl alcohol in the reaction mixture are shown in Fig. 3 and 4.Experiments with conventional heating and microwave heating at 50 °C and 100 °C can be directly compared due to similar reaction vessels, stirring speeds and heating profiles. Surprisingly, no significantly higher reaction-rate with microwave heating was observed at 100 °C for the experimental conditions (4 : 1 molar ratio of benzyl alcohol and Fe(NO3)3·9H2O). Subsequently, for the same initial reaction conditions at 50 °C for a 16 : 1 molar ratio of benzyl alcohol and Fe(NO3)3·9H2O again no beneficial effect was observed (Fig. 4).
 | ||
| Fig. 4 Time–molar composition plot of the oxidation of benzyl alcohol at a molar ratio of benzyl alcohol and Fe(NO3)3·9H2O of 16 : 1 at 50 °C under comparable conventional heating (CH) and microwave (MW) heating (aerobic conditions). | ||
Conclusions
Direct comparison (i.e. open vessel, same stirrer speed, same heating-up rate and internal temperature measurements) of the oxidation of benzyl alcohol with iron(III)nitrate nonahydrate under atmospheric conditions for homogeneous (initially) and heterogeneous reaction mixtures did not reveal any positive microwave effect. Under anaerobic conditions an increased amount of iron(III)nitrate nonahydrate is necessary to reach full conversion of benzyl alcohol.Based on the results obtained for both conventional and microwave heating it is concluded that the conventional batch heating procedure should be recommended as the method of choice for a scalable process. This conclusion is supported by the observation that any microwave-induced reaction-rate enhancement is lacking when the heating conditions are systematically compared. In our group, the transfer of batch processes to a flow reactor has demonstrated to occur with preservation of kinetics and reaction rates.22 Processing in a continuous mode is not preferable due to the necessity of aerobic conditions for completion of the reaction with the minimal economical amount of oxidant, the formation of a sediment during the reaction and the evolution of nitric fumes. The tremendously high heating rate as a result of a strong absorption of microwave energy by the reaction mixture leads to the conclusion that microwave heating is not the preferred method for safe operation on larger scales. These high heating rates lead to a rapid increment of temperature (and pressure) when the reaction is performed in a closed system with indirect temperature control. Accurate temperature control is a prerequisite for assignment of microwave effects on the rates of chemical reactions.
Experimental
In our setup, a commercially available, automated multimode microwave oven, MicroSynth from Milestone s.r.l. (Italy), was used. This oven operates at 2.45 GHz and is temperature-controlled by a fiber-optic sensor. During our microwave experiments the maximum power was set at 100 W. The average power input was 18 W (50 °C) and 76 W (100 °C). The reaction mixture was analysed by 1H NMR, GC-MS and GC-FID and conversion was determined by 1H NMR. 1H NMR spectra were recorded in CDCl3 with a Varian Mercury 200 MHz with the aid of 1,3,5-tri-tert-butylbenzene as the internal standard. The proton chemical shifts were calibrated to tetramethylsilane (TMS). GC analyses were performed using a CP-Chiralsil-Dex CB (25 m × 0.25 mm) column with a FID detector with the aid of tetradecane as the internal standard or Zebron ZB-35 (30 m × 0.25 mm) column with a MS detector. The XPS (X-ray photoelectron spectroscopy) measurements were carried out with a VG Escalab MKII spectrometer, equipped with a dual Al/Mg Kα X-ray source and a hemispherical analyzer with a five-channeltron detector. Spectra were obtained using the aluminium anode (Al Kα = 1486.6 eV) operating at 300 W and a constant pass energy of 20 eV with a background pressure of 2 × 10−9 mbar.Typical procedure for the oxidation of benzyl alcohol with iron(III)nonahydrate
Benzyl alcohol (2.0 mL, 19.4 mmol) and 1,3,5-tri-tert-butylbenzene (20 mg, 0.082 mmol) as internal standard were introduced in a 10 mL two neck round-bottomed flask, and heated in an oil bath or microwave oven. When the mixture reached the selected temperature, Fe(NO3)3·9H2O (0.48 g, 1.2 mmol) was added, and the reaction was started. When the desired reaction time was reached, the reaction mixture was eluted over a short silica column with a 5 μm-filter attached to the end, with an excess of diethyl ether. The diethyl ether phase was dried with MgSO4, filtered, and evaporated to yield the product(s). 1H NMR (CDCl3, 200 MHz) typical signals δ (ppm) 1.35 (s, 27H, 1,3,5-tri-tert-butylbenzene), 4.70 (s, 2H, benzyl alcohol), 5.43 (s, 2H, benzyl nitrate), 5.71 (s, 2H, benzyl nitrite), 8.12 (d, 2H, benzoic acid), 10.03 (s, 1H, benzaldehyde).Acknowledgements
The authors are grateful to SenterNovem for funding this research. We thank DSM Pharma Chemicals, Geleen, The Netherlands and Syncom B.V., Groningen, The Netherlands for their support.References
- I. R. Baxendale and M. R. Pitts, Chem. Today, 2006, 24, 41 Search PubMed.
- T. N. Glasnov and C. O. Kappe, Macromol. Rapid Commun., 2007, 28, 395 CrossRef CAS.
- A. De la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- R. J. J. Jachuck, D. K. Selvaraj and R. S. Varma, Green Chem., 2006, 8, 29 RSC.
- A. Cornélis and P. Laszlo, Synthesis, 1985, 10, 909 CrossRef.
- A. Loupy, Microwaves in Organic Synthesis, WILEY-VCH, Weinheim, 2006 Search PubMed.
- M. H. C. L. Dressen, B. H. P. Van de Kruijs, J. Meuldijk, J. A. J. M. Vekemans and L. A. Hulshof, Org. Process Res. Dev., 2007, 11, 865 Search PubMed.
- T. Nishiguchi and M. J. Bougauchi, Org. Chem., 1989, 54, 3001 CrossRef CAS.
- (a) A. Cornélis and P. Laszlo, Synthesis, 1980, 849 CrossRef; (b) S. E. Martin and D. F. Suárez, Tetrahedron Lett., 2002, 43, 4475 CrossRef CAS.
- M. M. Heravi, D. Ajami and M. M. Mojtahedi, Chem. Commun., 1999, 833 RSC.
- J.-D. Lou, L.-H. Zhu, Y.-C. Ma and L. Li, Synth. Commun., 2006, 36, 3061 CrossRef CAS.
- J.-D. Lou, X.-X. Cai, F. Li, L. Li and C.-L. Gao, Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2006, 36, 599 Search PubMed.
- R. S. Varma and R. Dahiya, Tetrahedron Lett., 1997, 38, 2043 CrossRef CAS.
- Experimental of ref. 4: The reactant was prepared at room temperature by dissolving solid Fe(NO3)3.9H2O in the benzyl alcohol solution. The mixture was stirred thoroughly and filtered using a Fisherbrand1 filter paper. Experiments were performed using the continuous isothermal reactor under the influence of microwave irradiation for a range of residence times (3–17 s) corresponding to different flow rates (1–5 mL min−1) and different microwave intensities (0 W to 39 W). The feed was pumped through the reactor using an HPLC pump and the heat transfer fluid (water) was circulated through the heat transfer zone of the reactor at 120 mL min−1 by using a peristaltic pump.
- Y. Shang and G. Van Weert, Hydrometallurgy, 1993, 33, 273 CrossRef CAS.
- A. I. Vogel, Vogel's textbook of quantitative chemical analysis, 5th edn, Burnt Mill, Longman Scientific & Technical, 1989, 326 Search PubMed.
- Y. Shang and G. Van Weert, Hydrometallurgy, 1993, 33, 255 CrossRef.
- A. Cornélis, P. Y. Herzé and P. Laszlo, Tetrahedron Lett., 1982, 23, 5035 CrossRef CAS.
- A. Baruah, B. Kalita and N. C. Baruam, Synlett, 2000, 7, 1064.
- The molar ratio of 16 : 1 (benzyl alcohol : Fe(NO3)3.9H2O) at room temperature leads to saturation of Fe(NO3)3·9H2O in benzyl alcohol which is the reaction condition of the article of ref. 4.
- P. Gray and A. Williams, Chem. Rev., 1959, 59, 239 CrossRef CAS.
- Unpublished results of transfer from batch-wise to flow conditions (FlowSynth of Milestone srl).
| This journal is © The Royal Society of Chemistry 2009 |
