Soybean oil extraction and separation using switchable or expanded solvents
Lam
Phan
a,
Heather
Brown
b,
James
White
b,
Allan
Hodgson
c and
Philip G.
Jessop
*a
aDepartment of Chemistry, Queen's University, Kingston, Ontario, Canada. E-mail: jessop@chem.queensu.ca; Fax: +1 613-533-6669; Tel: +1 613-5333-3212
bBattelle PNWD, 902 Battelle Blvd., PO Box 999, Mail Code: MSIN: P8-60, Richland, WA, USA 99352. E-mail: jim.white@pnl.gov; Fax: +1 509-372-4732
cBunge, 725 North Kinzie Avenue, Bradley, Illinois 60915, USA. E-mail: allan.hodgson@bunge.com; Fax: +1 815 523 8114; Tel: +1 815 523 8083
First published on 28th October 2008
Abstract
The extraction of soy oil from soybean flakes in industry requires large amounts of hexane solvent and results in significant losses and energy consumption during the distillative removal of the solvent. Hexanes and related hydrocarbon extractants are also becoming an environmental and health concern. A new method for extraction of the oil is sought, that would require neither hexane nor distillative removal of solvent. This article presents a preliminary assessment of several new methods for soy oil extraction and subsequent solvent removal from the oil. The most promising are (a) extraction by an amidine switchable solvent that can then be removed from the soy oil by carbonated water and (b) extraction by a moderately hydrophilic solvent that can then be removed from the oil by water.
Introduction
A new method for extraction of soy oil from crushed soybeans is needed because of environmental considerations and legislative pressures against the current method of extraction with hexane. The existing approach, introduced in the United States in 1930,1 involves a counter-current flow of hexane and soybean flakes, giving a saturated solution of oil in hexane. The best continuous soybean extraction has a solvent:bean mass ratio of 1, which is equivalent to a solvent:oil mass ratio of 5. Hexane losses are 1 kg per ton of beans processed.1Replacement of hexane with a solvent less prone to losses is becoming a priority for the industry. Supercritical CO2 (scCO2) could be used but requires 500 bar for the most energy-efficient extraction of soy oil from soybeans (i.e. minimum energy cost per kg of extracted oil).2,3 Soybean oil is miscible with many other low polarity organic solvents, especially those having a Hildebrand solubility parameter less than or equal to 22 MPa1/2 (Table 1). The problem with the use of low polarity solvents is that miscibility with oil is only desired during the extraction of the oil from the flakes; after extraction is complete, the separation of the oil from the solvent is desired, and thus the miscibility becomes a hindrance. We therefore suggest three alternative strategies for surmounting this problem:
| Solvent | δ,aMPa1/2 | εb | Misciblec |
|---|---|---|---|
| a Hildebrand parameter, from Allen.12 b Dielectric constant, from Smallwood13 except where noted. c “yes” indicates a 2:1 v/v solvent and oil mixture forms a single liquid phase at room temperature. “no” indicates two liquid phases are present. d Miscibility or immiscibility also reported by Erickson et al.14 | |||
| DBU | — | — | yes |
| Triethylamine | 15.1 | 2.410 | yes |
| Tripropylamine | 18.0 | 2.410 | yes |
| THF | 18.6 | 7.511 | yes |
| 2-Propylamine | — | 5.1 | yes |
| Acetone | 20.2 | 20.7 | yesd |
| Dioxane | 20.5 | 2.211 | yes |
| Acetic acid | 20.7 | 6.2 | no |
| Pyridine | 21.9 | 12.9 | yes |
| 2-Propanol | 23.5 | 18.3 | nod |
| 1-Propanol | 24.3 | 20.1 | yesd |
| Acetonitrile | 24.3 | 37.5 | no |
| DMSO | 24.5 | 46.6 | no |
| DMF | 24.8 | 36.7 | no |
| Ethanol | 26.0 | 22.4 | nod |
| Methanol | 29.6 | 32.6 | nod |
| Water | 47.9 | 79.7 | nod |
Strategy 1: Use a switchable-polarity solvent in its low polarity form to extract the soybean oil from the flakes, and then switch the solvent to its high polarity form to induce immiscibility with the oil, so that the solvent can be decanted from the oil. A switchable-polarity solvent (SPS) is a solvent that can switch back and forth between low polarity and high polarity forms.4–8
Strategy 2: Use a switchable-hydrophilicity solvent9 in its hydrophobic/lipophilic form to extract the soybean oil from the flakes, and then switch the solvent to its hydrophilic form and extract it from the oil with water. Remove the solvent from the water by switching the solvent back to its hydrophobic form. A switchable-hydrophilicity solvent (SHS) is a solvent that can switch back and forth between hydrophilic and hydrophobic forms.
Strategy 3: Use a normal (non-switchable) solvent of moderate polarity to extract the soybean oil from the flakes, and then extract the solvent from the oil with water. Remove the solvent from the water by dissolving CO2 in the solvent/water mixture to induce a phase split.
Experimental methods
Soy oil and flaked soybeans were used as received from Bunge. DBU (Aldrich, 98% grade) was dried viadistillation. Supercritical grade CO2 (99.999%, H2O < 0.5 ppm), nitrogen (99.998%, H2O < 3 ppm) and argon (99.998%, H2O < 5 ppm) were used as received from Praxair. NMR spectra were acquired at 400 MHz and referenced to TMS at 0 ppm unless specified otherwise. All other solvents were used as received from Aldrich or Fisher.Miscibility tests
A vial containing 4.0 ml of solvent and 2.0 ml of soybean oil was stirred for 10 minutes and left to settle for 20 min, then the miscibility of the soy oil and solvent was visually observed.Quantitative analysis of solvent/soy oil mixtures by 1H NMR spectroscopy
A calibration curve was prepared in the following manner. Different masses of oil and solvents were charged into sealed vials. The contents of each vial were stirred and then allowed to settle. If required, the contents were heated to an appropriate temperature to achieve miscibility. The 1H NMR spectrum of a portion of the mixture in CDCl3 was acquired and selected peaks from 4.1 to 4.2 ppm associated with the oil were compared to selected peaks of the solvent that did not have any overlap. The integration ratio was plotted against the mass% of solvent in the solvent/oil mixture (mass percentages ranged from 1 to 10%). Such calibration curves were prepared for mixtures of soy oil with: n-PrOH, i-PrOH, EtOH, DBU, 1,4-dioxane and benzylmethylamine.The scan time for 1H NMR analysis was 4 s, with a delay time of 1 s. The detection limit is typically 0.1 wt.% of the solvent. When an unknown mixture of soy oil and solvent is analyzed, its peak integration ratio can be used to calculate the amount of solvent in the soy oil.
Extraction of oil from soybean flakes
Flaked soybeans (4 g) were added to a 50 mL ace tube, equipped with a stir-bar, fritted dip-tube, pressure gauge, pressure release valve (set to 100 psi), and vent (Fig. 1). The system was heated to the desired extraction temperature via oil bath. A separate 25 mL Swagelok sample vessel, equipped with needle values at both ends, was charged with extraction solvent (20 g). The valves were closed and the sample vessel weighed. The entire vessel was then immersed in a hot water bath set to the desired extraction temperature. Once both vessels were at the desired temperature, the sample vessel was removed from the hot water bath and connected to the outlet of the fritted dip tube. The contents were then forced into the ace tube with sweeping nitrogen. The ace tube was then sealed and the sample vessel removed. The actual weight of solvent added was determined by subtracting the starting weight from the final weight of the sample vessel. This technique was employed to minimize error due to heat-up times. | ||
| Fig. 1 Apparatus for the evaluation of solvents for extraction of oil from soybean flakes. | ||
During the extraction, aliquots were removed for HPLC analysis using a luer-lock syringe to pull solvent through the fritted dip-tube. The dip tube was rinsed several times with the bulk solution to ensure that representative samples were taken.
A Shimadzu 10Avp system, equipped with an Alltech Altima HP C18 HL 3 μm 150 mm × 3.0 mm column and an Alltech 2000 ELSD, was used to analyze samples from each extraction trial. The method utilized a gradient of acetonitrile and dichloromethane shown in Table 2. All sample dilutions were performed using dichloromethane.
| Time (min) | Acetonitrile (vol%) | Dichloromethane (vol%) |
|---|---|---|
| 0 | 77 | 23 |
| 5 | 77 | 23 |
| 25 | 70 | 30 |
| 30 | 77 | 23 |
The HPLC method was an external standard method. To obtain an accurate calibration curve, five dilutions (1, 0.5, 0.2, 0.1, and 0.05 wt%) of soy oil were prepared. Each of the dilutions was injected once. The calibration obtained from this five point curve had an R2 value that fell between 0.995 and 0.999. External standards at high and low total oil concentrations were analyzed as spot checks, while running samples. The column was recalibrated when necessary as indicated by the performance of the external standards.
Separation of soy oil from switchable polarity solvents
For experiments with DBU/ROH SPS: CO2 was bubbled through a mixture of 3.0 ml of DBU, 1.6 ml of EtOH, and 4 ml of soy oil in a septum-capped vial for 1 h with stirring. The contents were left to settle over night. The oil layer was analyzed as described above. Similar procedures were done using different amounts of EtOH, MeOH, and water.For experiments with secondary amine SPS : CO2 was bubbled through a mixture of 4.0 ml of benzylmethylamine and 2.0 ml soy oil in a stirred and septum-capped vial for at least 1 h or until the heat from the reaction subsided. The contents were left to settle over night. The oil layer was analyzed as described above.
Extraction of dioxane from soy oil
To a septum-sealed vial containing 4 mL dioxane and 2 mL soy oil, 4, 6, 8, or 10 mL of distilled water was added. The contents were shaken and then left standing for 1 week at room temperature. The contents separated into two-phases, with the top being the soy oil, and the bottom as the aqueous-dioxane mixture. The oil phase was analyzed by 1H NMR spectroscopy and the dioxane content of that phase was determined with the use of a calibration curve (see supplementary material).Results and discussion
Strategy 1: Extraction of oil with switchable-polarity solvents
A switchable solvent is a solvent that can be reversibly “switched” from one form to another, where the two forms differ significantly in one or more properties. Most important for the present discussion is a switch in polarity. The first switchable polarity solvent (SPS) was reported in 2005 by the Jessop group4,15 and related designs have been published since.5–7 That first SPS consisted of an equimolar mixture of an alcohol and an amidine such as DBU (1,8-diazabicyclo-[5.4.0]-undec-7-ene). The mixture has a dramatically greater polarity in the presence of an atmosphere of CO2 than it has with no CO2, because CO2 reacts with the liquid mixture to create an ionic liquid (equation 1). Because the reaction is reversible, any action taken on the ionic liquid that removes the CO2, such as heating the liquid or bubbling N2 through it, causes the polar ionic liquid to revert to the original low-polarity non-ionic liquid. Secondary amines constitute another type of SPS; liquid secondary amines such as N-butyl-N-ethylamine and N-benzyl-N-methylamine are converted by CO2 into more polar liquids (mixtures of carbamate salt and carbamic acid, equation 2).5 | (1) |
 | (2) |
A switchable-polarity solvent (SPS) could be used in its low-polarity form as a solvent for the extraction of soy oil from soy flakes and then could be switched into its high-polarity form which would be immiscible with the soy oil (Fig. 2). Preferably, the high-polarity form would be so immiscible with the oil that the level of contamination of the oil with solvent would be extremely low. Further purification of the oil might be necessary to remove traces of the solvent. This strategy was evaluated with two kinds of switchable solvents, secondary amines and mixtures of DBU and alcohol.
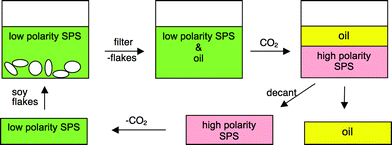 | ||
| Fig. 2 Schematic for oil separation using switchable-polarity solvents. Pink represents the solvent in its high-polarity form, while green represents its low-polarity form. | ||
The DBU/alcohol mixtures are miscible with soy oil as long as CO2 is absent, but their ionic liquid forms are immiscible with soy oil. Although the ionic liquids that form from DBU and short chain alcohols (methanol and ethanol) have melting points above room temperature,6 these ionic liquids are in fact liquid at room temperature when they contain small amounts of soy oil. They can therefore be used at room temperature.
In each separation trial, soy oil (4 ml), DBU (3 ml) and water or an alcohol (at an amount equimolar to DBU) were mixed together. CO2 was bubbled through the liquid for 1 h, after which the liquid mixture was allowed to settle overnight. The soy oil rose while the ionic liquid formed the lower layer. The soy oil layer was then analyzed for contamination with DBU. The separation of the ionic liquid layer from the oil layer was quite poor with methanol, so work with methanol was abandoned. The data (Table 3) showed that better results were obtained with ethanol than water. Because the oil still contained small amounts of both solvent components (ethanol and DBU), we speculated that additional CO2 treatment could precipitate more ionic liquid. Bubbling extra CO2 for 30 min at room temperature did indeed cause the precipitation of more ionic liquid and halved the level of contamination.
Using a higher than equimolar amount of ethanol only slightly decreases the amount of DBU contaminating the oil, but significantly increases the amount of ethanol contaminating the oil (using 5 ml ethanol: 2 ml DBU: 2 ml oil gave, after CO2 treatment, oil containing 1.3 wt% DBU and 7.1 wt% ethanol).
If needed, any trace amounts of remaining DBU could be easily removed by treatment of the oil with acidic or carbonated water or by passing the oil through silica.
These promising results showed that investigation of the extraction of oil from soybean flakes by DBU/ethanol SPS and related solvents was warranted. Extractions with an equimolar mixture of DBU and anhydrous ethanol were run at both 25 °C and 70 °C (Figs. 3 and 4). Flaked soybeans (4 g) obtained from the industrial process were added to a sealed reaction vessel. Pre-heated solvent (20 g) was then added to the vessel. The vessel contents were only stirred slowly to simulate the industrial process, in which there is no stirring but rather flow of liquid over the flakes. Samples were taken by drawing up small aliquots through a 0.45 μm filter and analyzing for total oil by HPLC. The performance of hexane at 60 °C (as currently used in the industry) is shown for comparison. Given the gentle agitation, it is likely that mass transfer limitations are, at least in part, influencing the rate of extraction.
 | ||
| Fig. 3 Extraction of soybean oil from flakes at 70 °C using DBU, DBU/ethanol, and ethanol. Percent oil recovery based upon 23.35% total oil in the flakes. Hexane control experiment was performed at 60 °C. | ||
At 25 °C, DBU/ethanol extracted oil more slowly than ethanol or DBU alone, and much more slowly than the hexane control (at 60 °C). Only 45% of the oil was recovered after 60 min using the DBU/ethanol solution. In fact, the addition of DBU to anhydrous ethanol reduces ethanol's extracting power by nearly half. Increasing the extraction temperature to 70 °C gave marked improvement of 20% in DBU's extracting ability and no change in ethanol's extracting ability. Unfortunately, the soy oil proved highly unstable in the DBU/ethanol solution at 70°C. Qualitative HPLC analysis showed that transesterification occurred under these conditions, resulting in the formation of mainly ethyl linoleate. For this reason, plus the possibility that water in the soybean flakes may interfere with the DBU/ethanol switchable solvent, this strategy has been rejected. A modification of the strategy shown in Fig. 2, using DBU as the sole extraction solvent and only adding the alcohol after the extraction is complete, would eliminate this transesterification problem for the first use of the solvent, but any attempt to recycle the DBU/ROH mixture for another extraction would result in trans-esterification.
A process for separating soy oil from the extraction solvent using secondary amine switchable solvents would be similar to one using amidine/alcohol switchable solvents except for the greater operational simplicity of using a single liquid component solvent rather than a mixture. Also, the transesterification observed with the DBU/ethanol system will not occur with the secondary amines.
Morpholine was chosen as a representative solvent to determine if secondary amines in general are capable of extracting soy oil from flakes. In the experiment, flaked soybeans (4 g) and solvent (20 g) were added to a sealed reaction vessel. Samples were taken by drawing up small aliquots through a 0.45 μm filter and analyzing for total oil by HPLC. An extraction of soybean flakes with hexane at 60 °C was used as a control. Morpholine at 25 °C is comparable to hexane at 60 °C for extracting soybean oil from flaked soybeans (Fig. 3). The extraction occurs slightly more slowly when using morpholine, but this could easily be due to the difference in extraction temperatures.
Dialkylamine/soy oil mixtures behave in different ways upon exposure to CO2. NHBuEt is miscible with soy oil before and after CO2 treatment. Morpholine precipitates as a white paste from soy oil upon treatment with CO2, but the paste can not be easily filtered from the oil. Benzylmethylamine has, in its ionic form, sufficient polarity to be immiscible with soybean oil. The nonionic form of benzylmethylamine is miscible with soy oil at room temperature, but after the amine/oil mixture has been exposed to CO2 and allowed to settle, two liquid phases slowly separate. Analysis of the oil layer showed that it contains 12 wt.% of the amine. Although removal of the remaining amine should presumably be possible by washing the oil in acidic water, the high level of contamination after the CO2 treatment makes this strategy undesirable.
Another concern with benzylmethylamine is the cancer risk. Although benzylmethylamine occurs naturally in carrots and green salad, tests in rats show that the amine can be a precursor for N-nitroso-N-benzylmethylamine, a cause of esophageal cancer.16
Because of the high level of contamination of the oil and the health concerns, work with benzylmethylamine was discontinued.
Strategy 2: Extraction of oil with switchable-hydrophilicity solvents followed by water wash
The second strategy to be evaluated employs a solvent that can switch from hydrophobic/lipophilic to hydrophilic and back again. In this strategy (Fig. 5), a switchable solvent would be identified that is sufficiently lipophilic to extract the oil from the soybean flakes. After filtration to remove the flakes, the oil/solvent mixture would be exposed to water and the solvent would be switched by CO2 to a state that is so hydrophilic that it partitions preferentially into the aqueous phase from the oil. If the hydrophilicity is sufficient, the amount of solvent remaining in the oil will be very low. The solvent would then have to be recovered from the water, either by distillation or by switching the solvent back to its hydrophobic form. | ||
| Fig. 4 Extraction of soybean oil from flakes at 25 °C using morpholine, DBU, DBU/ethanol, and ethanol. Percent oil recovery based upon 23.35% total oil in the flakes. Hexane control experiment was performed at 60 °C. | ||
![A process utilizing a switchable-hydrophilicity solvent (hydrophobic state labelled “B”) to extract soybean oil followed by switching of the solvent to a hydrophilic state ([BH][O2COH]) so that it is easily extracted by water.](/image/article/2009/GC/b810423a/b810423a-f5.gif) | ||
| Fig. 5 A process utilizing a switchable-hydrophilicity solvent (hydrophobic state labelled “B”) to extract soybean oil followed by switching of the solvent to a hydrophilic state ([BH][O2COH]) so that it is easily extracted by water. | ||
DBU was chosen as the first test solvent for this process for several reasons. Extraction data on actual flaked soybeans (detailed above, and shown in Fig. 3) showed 80% oil recovery after 60 minutes of extraction with pure DBU at 70°C. Also, we have found, during our work on switchable solvents,4,17 that the hydrophilicity of DBU is switchable, in the sense that the partitioning of DBU into an organic solvent from water is greater in the presence of CO2 than in its absence.9 However, it was not clear how well DBU can be removed from the oil by water. While the 1.8% remaining contamination reported in Table 3 is promising, it was hoped that using a significant excess of water might reduce the level of DBU found in the separated oil.
In each of five identical trials, soy oil, DBU, and water (2 ml of each) were mixed together, CO2 was bubbled through the mixture for 1 h, and the liquid was allowed to settle overnight. The soy oil layer was found to have an average of 0.5% DBU, with the highest being 1.2% and two of the five trials showing no detectable DBU (i.e. below the 0.1% detection limit of the analytical method). Thus, the use of excess water does decrease the amount of DBU in the soy oil compared to the 6.2% that was found when only an equimolar amount of water was used (as in Table 3).
The hardest part of the process in Fig. 5 is the separation of the solvent from the water. Removal of the CO2 causes DBU to become much less hydrophilic, but not to the extent that it separates from water. The only successful method found in our tests was to distill all of the water from the DBU. The addition of salt to the water does not force the DBU to separate. We are currently searching for amidines that are sufficiently hydrophobic that removal of CO2 from the water/amidine mixture would cause a phase separation. Such an amidine, were it to be found, would have the greatest potential for solving the soybean oil problem.
Strategy 3: Extraction of oil with moderate-polarity solvent followed by water wash
Extraction of oil from soybean flakes using a moderate-polarity solvent would give a mixture of soybean oil and the solvent, from which the solvent would be extracted by water (Fig. 6). Recycling of the solvent and the water would only be possible if the solvent could be separated from the water. Distillation is possible, but we are restricting ourselves to processes at or near ambient temperature. An alternative method for separating organic solvents from water is solvent expansion by CO2. Dissolution of pressurized CO2 gas into an organic liquid causes the liquid to expand volumetrically and to become less polar.18 The polarity drop can cause the liquid to become immiscible with water. Thus dissolution of CO2 into the solvent/water mixture could be the basis for a non-distillative method of separating the solvent from the water and allowing the recycling of both. Note that this strategy differs from the first two strategies in requiring the use of elevated pressures of CO2, likely between 50 and 75 bar.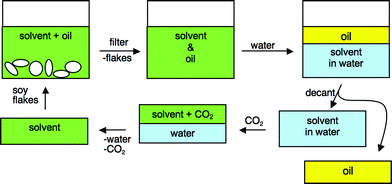 | ||
| Fig. 6 A process utilizing a moderate polarity solvent to extract soybean oil followed by extraction of the solvent by water and CO2 expansion to separate the solvent from water. | ||
In order for this process to work, the solvent must be a) miscible with soy oil, b) extractable from oil by water, and c) separable from water by CO2 pressure. A comparison of several potential solvents, all of which are miscible with soy oil, is shown in Table 4. Extractability of a solvent by water from a nonpolar organic liquid is usually quantified as the logP of the solvent, where P is the partition coefficient for the solvent molecule partitioning between 1-octanol and water. A positive logP value indicates a molecule that partitions preferentially into octanol rather than water. Those solvents with positive logP values can be rejected because they will be too difficult to extract from the oil with water. Finally, there is some limited data on the CO2 pressure required for the separation of solvents from water,19–23 summarized in a recent review paper.18 The remaining two solvents in Table 4, acetone and 1,4-dioxane, are the most likely candidates.
Three solvents were tested in a preliminary experiment: THF, dioxane and acetone. Mixtures of soy oil (2 ml), water (1 ml) and solvent (4 ml) were prepared in glass vials. After thorough mixing, the contents of the vials were allowed to settle. In the case of THF, the water formed a thin layer at the bottom (Fig. 7, left), showing that the THF did not partition significantly into the water, as expected given the fact that the logP of THF is greater than zero. In the case of dioxane, the water formed a thick layer at the bottom (Fig. 7, centre), indicating that the dioxane partitioned largely into the water rather than the oil. This is the desired situation for the proposed process. In the case of acetone, the separation was not clear (Fig. 7, right) until many hours had passed. This preliminary experiment shows that dioxane is a viable candidate.
 | ||
| Fig. 7 Mixtures of soy oil (2 ml), water (1 ml) and solvent (4 ml), where the solvent is THF (left), dioxane (centre), or acetone (right). | ||
To determine the amount of dioxane remaining in the soy oil after extraction of the bulk of the dioxane by water, the experiment was repeated with different amounts of water. After shaking and then a week of settling time (to ensure that equilibrium conditions were obtained), the amount of dioxane remaining in the soy oil was determined by 1H NMR spectroscopy (Fig. 8). Even using 15 mL of water was insufficient to bring the dioxane content of the oil down to a few percent. Using several smaller amounts of water or using continuous countercurrent washing with water would greatly decrease the amount of dioxane remaining in the oil and decrease the amount of water required to remove the dioxane. For example, two washes of 2 ml each results in the oil containing only 7.7 wt% dioxane, compared to 28% remaining after one wash of 4 ml (Fig. 8). Two washes, one of 10 ml and a second of 5 ml, brought the dioxane content of the oil to 2.7 wt%, which is 2.5 times lower than the amount after a single wash of 15 ml water.
 | ||
| Fig. 8 The amount of dioxane remaining in soy oil after extraction of most of the dioxane with a single wash with water. The hollow circle represents the result after two washes with water totalling 4 ml. | ||
It is known from the literature that application of CO2 pressure to a water/dioxane mixture can cause the mixture to separate into two phases: a water-rich phase and a dioxane-rich phase. Application of a minimum of 28 bar of CO2 at 40 °C causes water and dioxane to split in this manner,23 but application of higher pressures gives much better separation. From Fig. 9, one can determine the minimum pressure required to induce a phase split; draw a horizontal line at the composition of the mixture - the pressure at which that line crosses a curve is the minimum pressure required. Mixtures having compositions below 2 wt% or above 80 wt% water will not phase-split. For example, a 70/30 water/dioxane mixture would start to split into two liquid phases at 44 bar. Increasing the pressure to 57 bar would improve the separation, giving an aqueous phase containing 19.7% dioxane and a dioxane phase containing 2.2% water (neglecting the CO2 content of the liquid phases).
 | ||
| Fig. 9 The composition of the two liquid phases present in a dioxane/water mixture under CO2 pressure, neglecting the CO2 content of the phases, as a function of the CO2 pressure at 40 °C. Dioxane/water mixtures having an overall composition between the two curves would split into two liquid phases when subjected to the indicated pressure of CO2. The upper curve gives the composition of the water-rich phase while the lower curve represents the dioxane-rich phase. Calculated from the data of Lazzaroni et al.23 | ||
The water/dioxane mixture resulting from the washing of dioxane from the soy oil could be separated into a water-rich phase and a dioxane-rich phase by the application of gaseous CO2 pressure. For this to be effective, the amount of water used to wash the dioxane from the oil must be kept at a minimum, because CO2 pressure will not induce the phase separation of small amounts of dioxane from water. Counter-current extraction of dioxane from the oil should help minimize the amount of water needed. It is reasonable to anticipate that the wash water from an optimized process would contain 30% dioxane by volume. As shown in Fig. 9, the application of a CO2 pressure of 57 bar to such a mixture would create a phase split. The dioxane phase, which would contain very little water, could be re-used immediately for the extraction of more soy oil from flakes. The aqueous phase would still contain a significant amount of dioxane (about 20%). It seems unlikely that this aqueous phase could be re-used without removal of more of the dioxane. Further experimentation is necessary to determine methods for improving the separation of the dioxane from water; potential methods might include using different temperatures or pressures, the use of liquid or supercritical CO2 instead of gaseous CO2, additives in the water, or replacing dioxane with a different solvent.
Conclusions
Several hexane-free alternatives for the extraction of soybean oil from flakes have been evaluated.a) Strategy 1, using an amidine/alcohol switchable solvent, gave excellent separation but was rejected because transesterification occurs during the extraction and because water from the soybean flakes may interfere with the DBU/ethanol solvent switching process.
b) Strategy 1, using a secondary amine switchable solvent, was rejected because the only secondary amine that worked successfully has serious health implications, because the contamination levels were too high, and because the separation was too slow.
c) Strategy 2, the combination of an amidine and excess water gave superior solvent/oil separation, adequate oil extraction, and was insensitive to adventitious water. The contamination levels of residual amidine in the soy oil are very low. This method takes advantage of the fact that amidines can be made to switch their hydrophilicity by application or removal of CO2 in the system. While the separation of the amidine from water remains difficult, the results show that the identification of a switchable hydrophilicity solvent, especially one that in the absence of CO2 is sufficiently hydrophobic to readily separate from water, is greatly needed. Work in that direction is underway.
d) Strategy 3, the use of dioxane to extract soy oil from flakes, followed by removal of the dioxane from the oil by water, results in soy oil that contains 2 to 8% dioxane after batch extraction. Use of counter-current extraction should give much better results. The water phase contains up to 30 vol% dioxane. Partial separation of the dioxane from the water can be achieved by CO2 pressurization, giving a fairly pure dioxane phase and an aqueous phase that still contains a significant amount of dioxane. Extraction of dioxane from water using higher pressures of CO2 or especially liquid CO2 would require slightly higher pressure but give much more complete separation of dioxane from water.
Acknowledgements
The authors gratefully acknowledge samples and funding from Bunge Limited and funding from Battelle Pacific Northwest Division. PGJ acknowledges the support of the Canada Research Chairs program.Notes and references
- D. R. Erikson, Practical Handbook of Soybean Processing and Utilization, AOCS Press and United Soybean Board, St. Louis, 1995 Search PubMed.
- U. Sievers, Chem. Eng. Process., 1998, 37, 451–460 CrossRef CAS.
- N. T. Dunford, in Nutritionally Enhanced Edible Oil and Oilseed Processing, eds. N. T. Dunford and H. B. Dunford, AOCS Press, Editon edn., 2004, pp. 100-116 Search PubMed.
- P. G. Jessop, D. J. Heldebrant, L. Xiaowang, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- L. Phan, J. R. Andreatta, L. K. Horvey, C. F. Edie, A.-L. Luco, A. Mirchandi, D. J. Darensbourg and P. G. Jessop, J. Org. Chem., 2008, 73, 127–132 CrossRef CAS.
- L. Phan, X. Li, D. J. Heldebrant, R. Wang, D. Chiu, E. John, H. Huttenhower, P. Pollet, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539–545 CrossRef CAS.
- T. Yamada, P. J. Lukac, M. George and R. G. Weiss, Chem. Mater., 2007, 19, 967–969 CrossRef CAS.
- T. Yamada, P. J. Lukac, T. Yu and R. G. Weiss, Chem. Mater., 2007, 19, 4761–4768 CrossRef CAS.
- P. G. Jessop and L. Phan, 2008, manuscript in preparation.
- F. Ratkovics and L. Domonkos, Acta Chim. Acad. Sci. Hung., 1976, 89, 325–330 CAS.
- D. R. Lide, ed., CRC Handbook of Chemistry and Physics, 84th edn., CRC Press, Boca Raton, Florida, 2003 Search PubMed.
- A. F. M. Barton, Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd edn., CRC Press, Boca Raton, 1991 Search PubMed.
- I. M. Smallwood, Handbook of organic solvent properties, Halsted, New York, 1996 Search PubMed.
- D. R. Erickson, E. H. Pryde, O. L. Brekke, T. L. Mounts and R. A. Falb, Handbook of Soy Oil Processing and Utilization, American Soybean Association, St. Louis, 1980 Search PubMed.
- P. G. Jessop, C. A. Eckert, C. L. Liotta, “Switchable Solvents and Methods of Use Thereof,” U.S. Patent, Application 60/781,336, filed 13 March 2006. Canadian Patent Application No. 2,539,418, filed 13 March 2006.
- C. C. Hsia, T. T. Sun, Y. Y. Wang, L. M. Anderson, D. Armstrong and R. A. Good, Proc. Nat. Acad. Sci. USA, 1981, 78, 1878–1881 CrossRef CAS.
- D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335–5338 CrossRef CAS.
- P. G. Jessop and B. Subramaniam, Chem. Rev., 2007, 107, 2666–2694 CrossRef CAS.
- M. Wendland, H. Hasse and G. Maurer, J. Supercrit. Fluids, 1993, 6, 211–222 CrossRef CAS.
- M. Wendland, H. Hasse and G. Maurer, J. Supercrit. Fluids, 1994, 7, 245–250 CrossRef CAS.
- T. Adrian and G. Maurer, in High Pressure Chemical Engineering:Proceedings of the 3rd International Symposium on High Pressure Chemical Engineering, Zurich, Switzerland, 7–9 October, 1996, eds. P. R. von Rohr and C. Trepp, Elsevier, Amsterdam, Editon edn., 1996, pp. 241-246 Search PubMed.
- J. Lu, M. J. Lazzaroni, J. P. Hallett, A. S. Bommarius, C. L. Liotta and C. A. Eckert, Ind. Eng. Chem. Res., 2004, 43, 1586–1590 CrossRef.
- M. J. Lazzaroni, D. Bush, R. Jones, J. P. Hallett, C. L. Liotta and C. A. Eckert, Fluid Phase Equilib., 2004, 224, 143–154 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
