DOI:
10.1039/B812318G
(Paper)
Green Chem., 2009,
11, 48-52
Enhancement of cyclic ether formation from polyalcohol compounds in high temperature liquid water by high pressure carbon dioxide
Received
24th July 2008
, Accepted 10th October 2008
First published on 7th November 2008
Introduction
Biomass has a large amount of oxygen atoms because plants combine carbon dioxide with water using solar energy to store oxygen as sugar building blocks, (CH2O)n. The selective removal of oxygen atoms from biomass-derived carbohydrates, which are polyalcohol compounds like fructose, sorbitol, and glycerol in most cases, by dehydration or hydrogenolysis is important to obtain valuable products with desired boiling points, water solubilities, octane numbers and viscosities.1 The chemistry of intramolecular dehydration of polyalcohol compounds provides a key technology for developing an efficient conversion process of biomass derivatives to useful materials;2 however, biomass-derived carbohydrates, such as fructose and sorbitol, have five or six hydroxyl groups in a molecule and their intramolecular dehydration mechanisms are complicated.
High temperature liquid water has attracted much attention as an alternative to harmful organic solvents because of its high proton concentration, which enhances the rates of acid-catalyzed reactions, such as dehydration in water, without adding any hazardous acid.3,4Cyclic ethers are essential materials for the chemical industry and they are produced from diols by intramolecular dehydration over strong mineral acids, aluminium silicates, and ion-exchange resins.5 Savage's group reported that the addition of carbon dioxide to water (473–623 K) enhanced the production of tetrahydrofuran (THF) from 1,4-butanediol (1,4-BDO).6–8 The added carbon dioxide was dissolved in water to form carbonic acid, accelerating the acid catalysis of high temperature water. An acid solvent composed of water and carbon dioxide is environmentally-benign not only because both water and carbon dioxide are non-toxic, but also separation and recycling of these two components are easily performed by depressurization after reaction. In this manuscript, we investigated the dehydration mechanism of polyalcohol compounds having two or three hydroxyl groups, as simple model compounds of biomass-derived carbohydrates, to corresponding five- or six-membered cyclic ethers in water under high pressure carbon dioxide at 573 K.
Experimental
1,2,4-Butanetriol (1,2,4-BTO, Wako Pure Chemical Industries), 1,2,5-pentanetriol (1,2,5-PTO, Tokyo Chemical Industry), 1,4-butanediol (1,4-BDO, Wako Pure Chemical Industries), 1,4-pentanediol (1,4-PDO, Aldrich) and 1,5-pentanediol (1,5-PDO, Wako Pure Chemical Industries) were purchased and used without any further purification.
The dehydration of polyalcohol compounds was carried out in a batch reactor (inner volume: 6 cm3) made of a SUS316 tube.9 After 3 cm3 of polyalcohol aqueous solution (1.0 and 0.3 mol dm−3) was loaded in the reactor, the gas phase was purged with argon gas to remove air. Carbon dioxide (10 and 15 MPa) was then loaded in the reactor at 323 K.10 The reactor was submerged into a molten-salt bath at 573 K for a given reaction time and then submerged into a water bath for cooling to ambient temperature quickly after the reaction. The water in the reactor maintained vapor-liquid equilibrium at 573 K under 8.6 MPa of partial pressure. The partial pressure of carbon dioxide at 573 K was estimated to be 17.7 and 26.6 MPa, based on the equation of Charles's law, corresponding to the initial pressure of 10 and 15 MPa at 323 K, respectively. A mixture of a reactant and liquid products was taken out from the reactor with distilled water.
The quantitative analysis of liquid products was conducted by gas chromatography with a flame ionization detector (GC-FID) equipped with a DB-WAX capillary column (Agilent Technologies) using 1-propanol (Wako Pure Chemical Industries) as an internal standard material. The products were identified by their retention times of the GC-FID analysis, compared with those for known materials; 3-hydroxytetrahydrofuran (3-HTHF, Wako Pure Chemical Industries), tetrahydrofurfuryl alcohol (THFA, Sigma-Aldrich), tetrahydrofuran (THF, Wako Pure Chemical Industries), 2-methyltetrahydrofuran (2-MTHF, Sigma-Aldrich) and tetrahydropyran (THP, Wako Pure Chemical Industries). 4-hydroxytetrahydropyran (Aldrich) was used for the quantitative analysis for 3-hydroxytetrahydropyran (3-HTHP). The material balance of all reactions was more than 95%. Initial formation rates of cyclic ethers were estimated from initial slopes of the fitting curves of their profiles.
Results and discussion
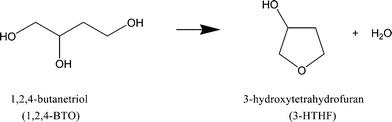
Dehydration of 1.0 mol dm−3 of 1,2,4-butanetriol (1,2,4-BTO) proceeded in water at 573 K (Fig. 1). 3-Hydroxytetrahydrofuran (3-HTHF) was the only product of this reaction (Scheme 1). The 3-HTHF yield for 10 minutes in liquid water at 573 K was 2.9% (as 1,2,4-BTO conversion) and increased to 10.7 and 22.4% by the addition of 17.7 and 26.6 MPa of carbon dioxide, respectively. On the other hand, the final yield of 3-HTHF after 3 hours of reaction was almost the same (70%) for the three conditions (under 0, 17.7 and 26.6 MPa of carbon dioxide), indicating that the added carbon dioxide enhanced the 3-HTHF formation rates to reach the equilibrium yield (70%).
 |
| | Fig. 1 Yields of 3-hydroxytetrahydrofuran (3-HTHF, closed symbols) and 1,2,4-butanetriol (1,2,4-BTO, open symbols) as a function of elapsed time for the 1,2,4-BTO dehydration reaction at 573 K in water (initial 1,2,4-BTO concentration: 1.0 mol dm−3, carbon dioxide partial pressure: 0 (circles), 17.7 (triangles) and 26.6 MPa (squares)). | |
The dehydration of 1.0 mol dm−3 of 1,2,5-pentanetriol (1,2,5-PTO) also proceeded in water at 573 K and provided two products; tetrahydrofurfuryl alcohol (THFA) and 3-hydroxytetrahydropyran (3-HTHP) (Scheme 2, Fig. 2). The yields of THFA (25.6, 35.7, and 45.6%) were about 6 times larger than those of 3-HTHP (3.9, 5.2 and 7.6%) for 10 minutes of reaction (under 0, 17.7, and 26.6 MPa of carbon dioxide, respectively). The final yields of THFA and 3-HTHP for the dehydration of 1,2,5-PTO were 70% and 10%, respectively, and here again the pressure of carbon dioxide did not affect the equilibrium yields of the products.
 |
| | Fig. 2 Yields of tetrahydrofurfuryl alcohol (THFA, closed symbols), 3-hydroxytetrahydropyran (3-HTHP, checked symbols), and 1,2,5-pentanetriol (1,2,5-PTO, open symbols) as a function of elapsed time for the 1,2,5-PTO dehydration reaction at 573 K in water (initial 1,2,5-PTO concentration: 1.0 mol dm−3, carbon dioxide partial pressure: 0 (circles), 17.7 (triangles) and 26.6 MPa (squares)). | |
Dehydration of 0.3 mol dm−31,2,4-BTO and 0.3 mol dm−31,2,5-PTO also proceeded in water at 573 K (Fig. 3). 3-HTHF was produced from 1,2,4-BTO and the 3-HTHF yield increased by the addition of 17.7 MPa of carbon dioxide (Fig. 3 (a)). THFA and 3-HTHP were produced from 1,2,5-PTO and the yield of THFA and 3-HTHP increased by the addition of carbon dioxide (Fig. 3 (b)). The product yields from different initial reactant concentrations (0.3 and 1.0 mol dm−3) were quite similar to each other in both cases of 1,2,4-BTO and 1,2,5-PTO. The initial formation rates of cyclic ethers are summarized in Table 1. Formation rates of 3-HTHF, THFA and 3-HTHP increased with increasing carbon dioxide pressure or the initial concentrations of 1,2,4-BTO and 1,2,5-PTO. On the other hand, the formation rates divided by the initial reactant concentrations were unaffected by the initial reactant concentrations between 0.3 and 1.0 mol dm−3 (Table 1). This result indicates that the dehydration of 1,2,4-BTO and 1,2,5-PTO to the cyclic ether compounds has a first-order dependence on polyalcohol concentrations.
| Reactant |
Product |
CO2 pressure/MPa |
C
i
a/mol dm−3 |
r
i
b
,
c/10−4 mol dm−3 s−1 |
r
i/Cic/10−4 s−1 |
|
Initial reactant concentration.
Initial formation rate.
The experimental errors were within 5%.
|
|
1,2,4-BTO
|
3-HTHF
|
0 |
0.3 |
0.21 |
0.69 |
| 1.0 |
0.61 |
0.61 |
| 17.7 |
0.3 |
0.61 |
2.0 |
| 1.0 |
1.7 |
1.7 |
|
1,2,5-PTO
|
THFA
|
0 |
0.3 |
1.4 |
4.7 |
| 1.0 |
4.3 |
4.3 |
| 17.7 |
0.3 |
1.9 |
6.3 |
| 1.0 |
5.9 |
5.9 |
|
3-HTHP
|
0 |
0.3 |
0.12 |
0.41 |
| 1.0 |
0.47 |
0.47 |
| 17.7 |
0.3 |
0.24 |
0.81 |
| 1.0 |
0.87 |
0.87 |
 |
| | Fig. 3 (a) Yields of 3-hydroxytetrahydrofuran (3-HTHF, closed symbols) and 1,2,4-butanetriol (1,2,4-BTO, open symbols) as a function of elapsed time for the 1,2,4-BTO dehydration reaction at 573 K in water (initial 1,2,4-BTO concentration: 0.3 mol dm−3, carbon dioxide partial pressure: 0 (circles) and 17.7 MPa (triangles)). (b) Yields of tetrahydrofurfuryl alcohol (THFA, closed symbols), 3-hydroxytetrahydropyran (3-HTHP, checked symbols), and 1,2,5-pentanetriol (1,2,5-PTO, open symbols) as a function of elapsed time for the 1,2,5-PTO dehydration reaction at 573 K in water (initial 1,2,5-PTO concentration: 0.3 mol dm−3, carbon dioxide partial pressure: 0 (circles) and 17.7 MPa (triangles)). | |
Effect of carbon dioxide on cyclic ethers formation from triol compounds in high temperature liquid water
The intramolecular dehydration reaction of 1,4-butanediol (1,4-BDO) to tetrahydrofuran (THF) is reported to be catalyzed by acid agents, such as ion exchange resins,11 zeolites12,13 and heteropolyacids.14,15 The intramolecular dehydration reactions of triols to the cyclic ether compounds would be an acid-catalyzed reaction.
The increase of the proton concentration by the addition of carbon dioxide could be explained by the dissolution of carbon dioxide in high temperature liquid water, as shown below.
| | | H2O + CO2⇄ H2CO3⇄ H+ + HCO3−⇄ 2H+ + CO32− | (1) |
Hunter and Savage estimated the proton concentration of high temperature liquid water with an addition of carbon dioxide (Equation (2)).6–8
| |  | (2) |
where [H
+], [CO
2(aq)],
KW, and
Ka1 represent the concentrations of
proton and dissolved carbon dioxide, ionization constant of
water, and the first ionization constant of
carbonic acid, respectively. They claimed that
proton concentration depends approximately on the first dissociation of
carbonic acid essentially because its second dissociation is negligible.
Equation (2) can be written as,
| |  | (3) |
where
KH and
PCO2 represent the Henry's law constant of carbon dioxide dissolved in
water and the pressure of carbon dioxide, respectively.
The formation of cyclic ethers has a first-order dependence on polyalcohol concentrations and is an acid-catalyzed reaction. The formation rates could be represented as follows,
where
r,
k, [
triol], [H
+],
α represent a formation rate of the
cyclic ether, an apparent reaction constant, a concentration of
triol and
proton, and an apparent reaction order of
proton, respectively. We fitted
Equation (4) to the formation rates of the
cyclic ethers from 1,2,4-BTO and 1,2,5-PTO
versusproton concentrations at 573 K with 0, 17.7 and 26.6 MPa carbon dioxide. The apparent reaction orders of
proton (
α) are 0.6, 0.2 and 0.3 for 3-HTHF from 1,2,4-BTO and THFA and 3-HTHP from 1,2,5-PTO, respectively. The dehydration of 1,2,4-BTO was enhanced strongly by
proton concentration, compared with the dehydration of 1,2,5-PTO because
α of 1,2,4-BTO dehydration (0.6) was larger than those of 1,2,5-PTO dehydration (0.2 and 0.3). It should be noted that the apparent reaction orders of
proton for the dehydration reactions are not unity, indicating that the dehydration of 1,2,4-BTO and 1,2,5-PTO to the
cyclic ether compounds does not have a first-order dependence on
proton concentration and the reaction is not simply initiated by a collision between the
triol molecule and
proton. We will discuss the promotion effect by carbon dioxide in terms of basicities of the hydroxyl groups and intramolecular hydrogen bond in
triol compounds along with the dehydration results of various polyalcohol compounds in the following section.
Formation rates of cyclic ethers from various polyalcohol compounds
We investigated initial dehydration rates concerning three more polyalcohol compounds to understand the dehydration mechanism of polyalcohol compounds to five- or six-membered cyclic ethers, and the results are summarized in Table 2. The dehydration of 1,4-pentanediol (1,4-PDO) and 1,5-pentanediol (1,5-PDO) proceeded to produce a five-membered ether, 2-methyltetrahydrofuran (2-MTHF) and a six-membered ether, tetrahydropyran (THP), respectively (Scheme 3).
Table 2 Initial formation rates of cyclic ethers for dehydration reactions in water at 573 K
| Reactanta |
Product |
CO2 pressure/MPa |
r
i
b
,
c/10−4 mol dm−3 s−1 |
|
Initial reactant concentration: 1.0 mol dm−3.
Initial formation rate.
The experimental errors were within 5%.
The same data as shown in Table 1.
|
|
1,4-BDO
|
THF
|
0 |
0.68 |
| 17.7 |
4.7 |
|
1,2,4-BTO
d |
3-HTHF
|
0 |
0.61 |
| 17.7 |
1.7 |
|
1,4-PDO
|
2-MTHF
|
0 |
0.86 |
| 17.7 |
10.0 |
|
1,5-PDO
|
THP
|
0 |
0.063 |
| 17.7 |
0.42 |
|
1,2,5-PTO
c |
THFA
|
0 |
4.3 |
| 17.7 |
5.9 |
|
3-HTHP
|
0 |
0.47 |
| 17.7 |
0.87 |
The formation rate of 2-MTHF in 1,4-PDO dehydration was higher than that of THP in 1,5-PDO dehydration at 573 K (Table 2). Both formation rates were also accelerated by the addition of 17.7 MPa of carbon dioxide. These results could explain the higher THFA formation rate in 1,2,5-PTO dehydration than the 3-HTHP formation rate (Table 2). The difference between the dehydration rates of 1,4-PDO and 1,5-PDO indicates, (1) the formation rates of five-membered cyclic ethers are higher than six-membered cyclic ethers, as revealed by the results that the dehydration rate of 1,4-BDO to THF (0.68 × 10−4 mol dm−3 s−1) was larger than that of 1,5-PDO to THP (0.063 × 10−4 mol dm−3 s−1); and, (2) the dehydration of 1,4-PDO proceeds through the formation of a secondary carbocation, and/or the basicity of the secondary hydroxyl group in the 4-position in 1,4-PDO is higher than that of the primary hydroxyl group, as shown by the result that the dehydration rate of 1,4-PDO to 2-MTHF (0.86 × 10−4 mol dm−3 s−1) was higher than that of 1,4-BDO to THF (0.68 × 10−4 mol dm−3 s−1). Therefore, in the case of the dehydration of 1,2,5-PTO, the formation rate of THFA, which is a five membered heterocyclic ether and formed by dehydration between primary and secondary hydroxyl groups, is much faster than that of 3-HTHP (a six membered heterocyclic ether and formed by dehydration between two primary hydroxyl groups). Table 2 also shows that the promotion effect by carbon dioxide on the dehydration rates of alkanediols (1,4-BDO and 1,5-PDO) was larger than that of corresponding alkanetriols (1,2,4-BTO and 1,2,5-PTO). These results may be explained by the intramolecular hydrogen bond between the two hydroxyl groups in the 1- and 2-position in triols, which was investigated by density functional theory (DFT) methods.16–18 The DFT studies show that the hydroxyl group in the 2-position was preferentially protonated by the intramolecular hydrogen bond. The intramolecular interaction between the two hydroxyl groups in the 1- and 2-position of triols would make the effect of protons from external molecules smaller, which results in less promotion effect of carbon dioxide for triols.
Conclusions
Cyclic ethers were produced by the dehydration reactions of polyalcohol compounds in high temperature liquid water, which was accelerated by the presence of carbon dioxide dissolved in the water. Five-membered cyclic ethers were formed faster than six-membered cyclic ethers and the formation rates of the cyclic ethers depended strongly on the structure of the polyalcohol compounds. The position of the hydroxyl groups is crucial for the efficient intramolecular dehydration, which leads to a new technology for conversion of biomass derivatives to useful materials.
Notes and references
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS
 .
.
-
Pacific Northwest National Laboratory (PNNL) and National Renewable Energy Laboratory (NREL), Top value added chemicals from biomass, Report, 2004 Search PubMed
 .
.
- P. E. Savage, Chem. Rev., 1999, 99, 603–622 CrossRef CAS
 .
.
- N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef CAS
 .
.
- U. Limbeck, C. Altwicker, U. Kunz and U. Hoffmann, Chem. Eng. Sci., 2001, 56, 2171–2178 CrossRef CAS
 .
.
- S. E. Hunter and P. E. Savage, AIChE Journal, 2008, 54, 516–528 CrossRef CAS
 .
.
- S. E. Hunter, C. E. Ehrenberger and P. E. Savage, J. Org. Chem., 2006, 71, 6229–6239 CrossRef CAS
 .
.
- S. E. Hunter and P. E. Savage, Ind. Eng. Chem. Res., 2003, 42, 290–294 CrossRef CAS
 .
.
- A. Yamaguchi, N. Hiyoshi, O. Sato, M. Osada and M. Shirai, Catal. Lett., 2008, 122, 188–195 CrossRef CAS
 .
.
- A. Yamaguchi, N. Hiyoshi, O. Sato, C. V. Rode and M. Shirai, Chem. Lett., 2008, 37, 926–927 CrossRef CAS
 .
.
- S. H. Vaidya, V. M. Bhandari and R. V. Chaudhari, Appl. Catal., A, 2003, 242, 321–328 CrossRef CAS
 .
.
- Y. V. S. Rao, S. J. Kulkarni, M. Subrahmanyam and A. V. R. Rao, J. Org. Chem., 1994, 59, 3998–4000 CrossRef CAS
 .
.
- M. Aghaziarati, M. Kazemeini, M. Soltanieh and S. Sahebdelfar, Ind. Eng. Chem. Res., 2007, 46, 726–733 CrossRef CAS
 .
.
- T. Baba and Y. Ono, J. Mol. Catal., 1986, 37, 317–326 CAS
 .
.
- H. Li, H. Yin, T. Jiang, T. Hu, J. Wu and Y. Wada, Catal. Commun., 2006, 7, 778–782 CrossRef CAS
 .
.
- R. A. Klein, J. Comput. Chem., 2002, 23, 585–599 CrossRef CAS
 .
.
- R. A. Klein, J. Comput. Chem., 2003, 24, 1120–1131 CrossRef CAS
 .
.
- M. M. Deshmukh, N. V. Sastry and S. R. Gadre, J. Chem. Phys., 2004, 121, 12402–12410 CrossRef CAS
 .
.
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here. 






.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
