Pretreatment of lignocellulosic biomass associated with the autoxidation of ethanol to acetal
Yoshiyuki
Sasaki
,
Takashi
Endo
,
Noriko
Tanaka
and
Hiroyuki
Inoue
*
Biomass Technology Research Center, National Institute of Advanced Industrial Science and Technology (AIST), 2-2-2, Hiro-Suehiro, Kure, Hiroshima 737-0197, Japan. E-mail: inoue-h@aist.go.jp; Tel: +81 823 72 1935; Fax: +81 823 73 3284
First published on 22nd October 2008
Abstract
Lignocellulosic biomass was effectively delignified by aerobic oxidation in ethanol. During the process, ethanol was partially oxidized to acetal, a promising fuel additive, and other oxygenates. The resulting pulp could then be enzymatically converted into sugars in good yields for ethanol production.
The production of value-added chemicals from lignocellulosic biomass, which is the most abundant organic resource on the earth, can be effectively achieved by fractionating the biomass into its two main ingredients: carbohydrates and lignin. One of the most promising methods for separating these ingredients is the oxidative delignification of lignocellulosic biomass followed by the enzymatic saccharification of the resulting pulp. In particular, aerobic oxidation has the advantage of using a cheap oxidant such as oxygen or air. Wet oxidation is the most extensively investigated process; in this process, oxygen is used as the oxidant and water as the solvent. However, the saccharification of the residue obtained by this treatment gave a relatively low sugar yield (257 mg/g softwood), probably because of the poor solubility of oxygen in water (0.028 mL L−1), resulting in insufficient delignification; the lignin content is still as high as 24–30% in the residue.1 This problem may be solved to some extent by using oxidation catalysts such as polyoxometalates, as in the case of a pulp bleaching process.2 However, the use of a metal-containing catalyst may lead to high cost and the possible contamination of the dissolved metals when the enzymatic saccharification of the resulting pulp is carried out.
During our investigations on the effective pretreatment methods for lignocellulosic biomass, we found that pulverized woody biomass could be effectively delignified by aerobic oxidation in ethanol, and the resulting pulp could be enzymatically hydrolyzed to monomeric sugars in good yields. During the pretreatment, ethanol was found to be partially oxidized to acetal (acetaldehyde diethyl acetal) and other oxygenates. On the other hand, the pretreatment under the same reaction conditions but in water resulted in the carbonization of the biomass.
Acetal is a stable and nontoxic oxygenate that has many uses, e.g., as a solvent, fragrance producing liquor additive, an antioxidant for perfumes, and a fuel additive for maintaining or improving the cetane number of diesel oil and reducing particulate matter (PM, imperfect combustion products).3 It can be produced either by the dehydrative condensation of acetaldehyde and ethanol,4 or more preferably, by direct oxidation and acetalization of ethanol. A Wacker-type catalytic system has been employed for the latter reaction; in 1967, Lloyd first reported this type of catalytic system,5 and more recently, Bueno et al. improved its catalytic performance by adding p-toluenesulfonic acid. The aerobic oxidation of ethanol was performed at 70 °C for 6 h to afford acetal in 28% yield.6
To our surprise, the aerobic oxidation of ethanol proceeded without any catalyst at 150 °C, but a significant amount of by-products such as acetic acid and methane was formed; it is usually difficult to control the rate and the course of radical reactions such as the abovementioned oxidation reaction. However, it has now been found that when lignin or lignocellulosic biomass is used as a kind of antioxidant in the aerobic oxidation of ethanol, acetal and acetaldehyde are predominantly formed from ethanol. Moreover, when lignocellulosic biomass is used in this reaction, its lignin fraction, except those partly oxidized to CO and CO2, is effectively dissolved in the solution and its carbohydrate fraction can be separated as a cellulose-rich pulp (Scheme 1). These observations are a result of the carbohydrates contained in biomass being more resistant to oxidation than lignin.7 The resulting pulp can be enzymatically hydrolyzed to monomeric sugars.
 | ||
| Scheme 1 | ||
Isolated lignin called organosolv lignin purchased from Sigma-Aldrich and two kinds of wood tips purchased from a paper mill were used as lignocellulosic biomass. The contents of cellulose, hemicellulose, and lignin in the tips of Douglas fir were determined to be 47%, 28%, and 25% and those in the tips of eucalyptus to be 43%, 34%, and 28% respectively. The wood tips were pulverized, sieved, dried in vacuo, and stored in a desiccator before use. Ethanol (99.5%) and all the other chemical reagents were used as purchased.
Aerobic oxidation was performed in a 50 mL autoclave made of stainless steel. A typical experimental procedure is as follows: Douglas fir samples pulverized to a particle size of 0.42–1 mm (1,000 mg) and 99.5% ethanol (6,900 mg) were placed in the autoclave, and oxygen (714 mg or 500 mL at ambient pressure and temperature) was introduced at 1.3 MP. The autoclave was heated at 150 °C for 19 h. After the reaction, the gas products were collected in a sampling bag through a flowmeter and analyzed by GLC (GL Sciences GC 323 equipped with an MS-5A column for H2, O2, N2, CH4, and CO and Pora-Q column for CO2). The gas phase was composed of unreacted O2 (220 mL), CO2 (36 mL), CO (11 mL), and H2 (3 mL). The liquid products were analyzed by GLC (Shimadzu GC 14A equipped with a Restek Rtx-624 column) using 1,4-dioxane as the internal standard. The liquid phase was composed of unreacted EtOH (5,591 mg), acetal (536 mg), CH3CHO (146 mg), HCO2Et (137 mg), AcOEt (66 mg), and AcOH (49 mg). The lignin fraction dissolved in ethanol was isolated as a viscous liquid (422 mg) by evaporating the entire reaction solution in vacuo. Its number-average molecular weight (452) was determined using Gel Permeation Chromatography (GPC; Shimadzu), and it was found to be one-third of that of organosolv lignin (1,445). Since the weight of the dissolved lignin was greater than the lignin content in the original tip, it was considered that ethanol was contained in it. The solid residue was washed three times with diethyl ether and dried in vacuo to afford a pulp fraction (642 mg).
A part of the pulp (40 mg) was dispersed in 1 mL of 50 mM acetate buffer (pH 5.0) and the resulting buffer was hydrolyzed with an enzyme mixture consisting of 0.4 FPU (filter paper units) cellulase activity of Acremonium Cellulase (Meiji Seika), 0.05 mg of Cellulase Y-2NC (Yakult Pharmaceutical Industry), and 0.05 IU (international units) β-glucosidase activity of Novozyme 188 (Novozymes). Units of enzyme activities were determined according to the standard procedure recommended by the Commission on Biotechnology, IUPAC.8 For cellulase, 1 FPU was defined as the amount of enzyme that releases 1 μmol reducing sugars/min under the assay condition using filter paper as substrate. For β-glucosidase, 1 IU was defined as the amount of enzyme that releases 1 μmol p-nitrophenol/min under the assay condition using p-nitrophenyl-β-D-glucoside as substrate.
The reaction mixture was incubated at 45 °C for 120 h. After the resultant solution was centrifuged, the hydrolysate in the supernatant was analyzed with LC (Jasco LC system equipped with a Bio-rad Aminex HPX-87H column). Glucose and mannose were formed in yields of 27 mg and 4 mg, respectively; the total sugar yield determined on the basis of the carbohydrate content of the original tip was 58%. The lignin content of the resulting pulp was determined as Klason lignin, and a part of the pulp (0.3 g) was hydrolyzed with 72% sulfuric acid to give a residue (13.2 mg, 4.36%).
Fig. 1 shows the time course of the aerobic oxidations of ethanol with and without biomass in terms of changes in the reaction pressure. In the latter case, the pressure began to decrease shortly after the reaction temperature reached 150 °C, while in the former case, only a gradual decrease was observed in the pressure up to a certain time. However, beyond that time, the pressure decreased fairly rapidly.
 | ||
| Fig. 1 Time course of the reaction with and without biomass in terms of the pressure change. Reaction conditions at 150 °C: ethanol 6.9 g, Douglas fir (0.42–1 mm), 1 g; O2, 1.3 MPa (initial). | ||
In order to determine the details of the oxidation process in the two cases, the gas, liquid, and solid products were analyzed for the reactions stopped at the points indicated by the arrows in Fig. 1. The numbers in the arrows representing the reactions are used as abscissae in Figs. 2–4. Fig. 2 shows the change in the yields of the gas products. The amounts of CO2 and CO constantly increased up to point 4, while the formation of H2 and CH4 became significant after that point. Since only a small amount of CO2 was detected in the reaction without biomass (point 6), CO2 formed during this stage is considered to be derived mainly from the biomass. Fig. 3 shows the change in the yields of the liquid products. While acetal and acetaldehyde were predominantly formed up to the vicinity of point 3, the formation of ethyl formate, ethyl acetate, and acetic acid became significant after that point, probably due to the degradation of the biomass. Fig. 4 shows the change in the yield of the pulp and yields of the sugars (glucose and mannose) obtained by the enzymatic saccharification of the pulp. The sugar yields steadily increased with a decrease in the pulp yield up to the vicinity of point 3. Thereafter, it decreased even if the pulp yield decreased. This may be due to the degradation and partial acetylation of the pulp.
 | ||
| Fig. 2 Change in the yields of the gas products. | ||
 | ||
| Fig. 3 Change in the yields of the liquid products. | ||
 | ||
| Fig. 4 Change in the yields of the pulp and those of the sugars obtained by the enzymatic saccharification of the pulp. Saccharification conditions at 45 °C for 120 h: 0.4 FPU cellulase mixture and 4% pulp loading. | ||
Table 1 shows the effects of various reaction conditions on the aerobic oxidation of ethanol. The aerobic oxidation reaction of ethanol without lignin or lignocellulosic biomass rapidly proceeded at 150 °C, as shown in Fig. 1, and significant amounts of ethyl acetate and acetic acid were formed together with acetal and acetaldehyde (entry 1). In the presence of organosolv lignin, the formation of these acetyl compounds was effectively suppressed without the yields of acetal and acetaldehyde being considerably affected; the total yield of acetal and acetaldehyde was 14% (entry 2). When the lignocellulosic biomass (Douglas fir) was used as an antioxidant, the result obtained was almost identical to that for lignin in terms of the liquid products; the total yield of acetal and acetaldehyde was 11% (entry 3). Some parts of the other products are considered to be derived from the lignocellulosic biomass, especially from hemicellulose that is more vulnerable to the oxidation and/or solvolysis than cellulose.7
| Entry | Woody biomass | Size/mm | Loading/mg | Temp./°C | Time/h | Pulp/mg | Lignin b/% | Sugarc/mg | Sugar recovery/% | Acetal/%d | CH3CHO/%d | HCO2Et %d | AcOEt/%d | AcOH/%d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: Ethanol 6.9 g, O2 1.3 MPa (initial). b Klason lignin of the resulting pulp. c Saccharification conditions: cellulase mixture 0.4 FPU mL−1, pulp loading 4%, at 45 °C for 120 h. d Expressed as conversions from the original ethanol. e Air 6.5 MPa (initial). | ||||||||||||||
| 1 | none | — | — | 150 | 3 | — | — | — | — | 14.6 | 8.7 | 1.7 | 2.9 | 6.5 |
| 2 | Organosolv lignin | — | 250 | 150 | 8 | — | — | — | — | 10.9 | 3.3 | 0.2 | 0.5 | 0.2 |
| 3 | Douglas fir | 0.42–1 | 1,000 | 150 | 19 | 642 | 4.4 | 484 | 58 | 9.1 | 2.2 | 1.2 | 1.0 | 0.5 |
| 4 | Douglas fir | 0.42–1 | 1,000 | 160 | 10 | 639 | 6.0 | 472 | 57 | 7.5 | 3.2 | 0.9 | 0.6 | 0.4 |
| 5 | Douglas fir | 0.42–1 | 1,000 | 170 | 6 | 629 | 6.8 | 403 | 49 | 5.9 | 3.7 | 0.9 | 0.4 | 0.3 |
| 6 | Douglas fir | 0.42–1 | 1,000 | 180 | 5 | 634 | 10.7 | 321 | 39 | 4.5 | 2.8 | 0.6 | 0.3 | 0.1 |
| 7 | Douglas fir | 0.25–0.42 | 1,000 | 150 | 16 | 653 | 3.9 | 492 | 59 | 6.9 | 3.1 | 1.0 | 0.6 | 0.3 |
| 8 | Douglas fir | 0.42–1 | 1,000 | 150 | 17 | 681 | 2.8 | 479 | 58 | 8.2 | 1.5 | 0.7 | 0.5 | 0.2 |
| 9 | Douglas fir | 1–2 | 1,000 | 150 | 17 | 688 | 5.8 | 452 | 54 | 7.0 | 2.7 | 0.9 | 0.8 | 0.4 |
| 10 | Douglas fir | 2–4 | 1,000 | 150 | 16 | 690 | 5.8 | 456 | 55 | 7.2 | 3.7 | 2.0 | 4.3 | 1.9 |
| 11 | Douglas fir | 0.42–1 | 1,500 | 150 | 19 | 1,027 | 6.4 | 720 | 58 | 6.4 | 3.0 | 1.3 | 0.8 | 0.2 |
| 12 | Douglas fir | 0.42–1 | 2,000 | 150 | 23 | 1,390 | 10.4 | 821 | 49 | 6.0 | 3.5 | 1.6 | 1.2 | 0.2 |
| 13 | Eucalyptus | 0.25–0.42 | 1,000 | 150 | 16 | 672 | 21.0 | 495 | 61 | 4.7 | 1.8 | 0.6 | 0.3 | 0.1 |
| 14 | Douglas fire | 0.42–1 | 1,000 | 150 | 16 | 655 | 5.5 | 480 | 58 | 6.4 | 3.3 | 1.0 | 0.7 | 0.4 |
The effect of the reaction temperature was then examined (entries 3–6). We attempted to regulate the reaction time so that the yield of the pulp was between 620 and 690 mg; this yield range gave the maximum yields of the sugars in the enzymatic saccharification process, as shown in Fig. 4. As expected, high reaction temperatures required short reaction times. However, the pulps obtained at high reaction temperatures contained high amounts of lignin; subsequently, the saccharification of the pulps resulted in low sugar yields.
In entries 7–10, the total sugar amounts recovered from Douglas fir were estimated as 452–492 mg/g wood, and were not significantly affected by the size of the wood particles. The results indicate that these reaction conditions were comparable to other pretreatments to improve the enzyme hydrolysis of woody biomass.9 Significant amounts of ethyl acetate and acetic acid were formed with Douglas fir samples with the particle size of 2–4 mm (entry 10) which was also the case in the reaction without any lignin (entry 1). These results indicate that the lignin contained in the large-sized particles does not effectively function as an antioxidant, probably because of the limited diffusion rate of the oxidant; it is desirable to use biomass that is pulverized to a particle size less than 2 mm.
The effect of biomass loading was also examined. The yield of the sugars was constant up to 1,500 mg (entry 11), but the loading of 2,000 mg of biomass resulted in a relatively low yield of the sugars, even when a longer reaction time was employed (entry 12). Eucalyptus gave a slightly better yield of sugars (glucose and xylose) than the Douglas fir, despite the fact that its delignification rate was as low as 50% (entry 13); on the other hand, in the case of Douglas fir, the delignification rates were 70–90%. The pretreatment of biomass with air instead of oxygen (entry 14) gave almost the same result as the corresponding pretreatment with oxygen (entry 3), but a considerably higher reaction pressure was needed for a sufficient amount of oxygen to be present. This is because the reactor is a batch system and no additional supply of air is possible during the reaction; otherwise, such a high pressure would not be necessary because the solubility of oxygen in ethanol (unlike the solubility in water) is sufficiently high (0.222 mL L−1) so as to not cause any diffusion problem.
Fig. 5 shows the estimated overall reaction mechanism. The first step of the oxidation process is considered to be the attack of O2 on the C-1 atom of ethanol that gives a kind of peroxide intermediate; this intermediate may be unstable and readily decomposed to acetaldehyde, thereby releasing the active oxidizing species such as hydrogen peroxide. This active species is probably consumed either for the further oxidation of acetaldehyde to acetic acid or for the oxidation of lignin to CO and CO2 if it is available. Meanwhile, acetaldehyde may undergo decarbonylation to give CH4 and CO in the absence of the antioxidant.
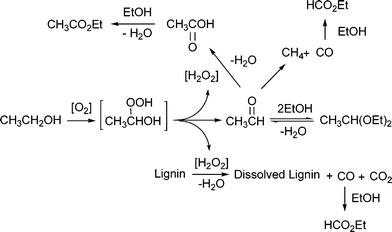 | ||
| Fig. 5 Estimated overall reaction mechanism. | ||
In conclusion, the pretreatment of lignocellulosic biomass for enzymatic saccharification and fermentation was successfully associated with the oxidation of ethanol to acetal, a promising fuel additive. We believe that these results will contribute to the realization of a “Biomass to Bioacetal” process.
References
- H. Palonen, A. B. Thomsen, M. Tenkanen, A. S. Schmidt and L. Viikari, Appl. Biochem. Biotechnol., 2004, 117, 1 CrossRef CAS.
- A. R. Gaspar, J. A. F Gamelas, D. V. Evtuguin and C. P. Neto, Green Chem., 2007, 9, 717 RSC.
- M. R. Capeletti, L. Balzano, G. de la Puente, M. Laborde and U. Sedran, Appl. Catal., 2000, 198, L1 CAS.
- V. M. T. M. Silva and E. Rodrigues, AIChE J., 2002, 48, 625 CrossRef CAS.
- W. G. Lloyd, J. Org. Chem., 1967, 32, 2817.
- A. C. Bueno, J. A. Gonçalves and E. V. Gusevskaya, Appl. Catal. A, 2007, 326, 1 CrossRef.
- H. B. Klinke, A. B. Thomsen and B. K. Ahring, Appl. Microbiol. Biotechnol., 2004, 66, 10 CrossRef CAS.
- T. M. Wood and K. M. Bhat, Methods Enzymol., 1988, 160, 87 Search PubMed.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
