An environmentally benign solvent-free Tishchenko reaction
Daniel C.
Waddell
and
James
Mack
*
University of Cincinnati, 301 Clifton Court, Cincinnati, OH, USA. E-mail: james.mack@uc.edu; Fax: +1 513 556 9239; Tel: +1 513 556 9249
First published on 6th November 2008
Abstract
Herein, we describe the solvent-free ball milling Tishchenko reaction. Using high speed ball milling and a sodium hydride catalyst, the Tishchenko reaction was performed for aryl aldehydes in high yields in 0.5 hours. The reaction is not affected by the type of ball bearing used and can be successful when conducted in a liquid nitrogen environment.
Introduction
Environmental concerns about solvent-based chemistry have stimulated a renewed interest in the study of chemical reactions under solvent-free conditions.1–3 Although most of the research conducted in this area has been performed by using a mortar and pestle, high speed ball milling (HSBM) is an attractive solvent-free method that has started to gain attention. In the HSBM method, a ball bearing is placed inside a vessel that is shaken at high speeds.4,5 The high speed attained by the ball-bearing has enough force to make an amorphous mixture of the reagents that subsequently facilitate a chemical reaction.The use of commericial ball mills have allowed these reactions to be scaled up to industrial levels, therefore understanding organic reactions using this methodology can signifigantly reduce solvent waste.4,6–9 We recently reported that the rate of the Baylis–Hillman reaction is increased under HSBM conditions and we developed a safe solvent-free method for the reduction of esters.10,11 In this work, we describe the Tishchenko reaction under these novel conditions.
The conversion of aldehydes to their dimeric esters, better known as the Tishchenko reaction (Scheme 1) has been known for more than a hundred years.12 This reaction is heavily used in industry,13 and it is inherently environmentally benign since it utilizes catalytic conditions and is 100% atom economic. Over the years, chemists have looked to develop new reagents that are more efficient than the aluminum based catalysts traditionally used. Metal catalysts such as alkali metals,14–17alkali metal oxides, lanthanides,18–22 and many others have been developed towards the improvement of Tishchenko chemistry. Unfortunately, many of these catalysts react sluggishly with aromatic aldehydes or provide the ester product in low yield.23–25 It was demonstrated that catalytic diisobutylaluminum hydride (DIBAL-H) reacts with aliphatic aldehydes to give the dimeric ester but it does not give the Tishchenko product with aryl aldehydes.24Catalysts such as lithium bromide and lanthanide catalysts undergo the Tishchenko reaction with aryl aldehydes in high yield, but long reaction times (2–3 days) are required.25 One of the simplest and cheapest catalysts that has been used for the generation of benzyl benzoate from benzaldehyde has been sodium hydride. Although sodium hydride is generally thought of as a non-nucleophilic base,26 Swamer and Hauser demonstrated in refluxing benzene that benzaldehyde can be converted to benzyl benzoate in moderate yield.27 Since benzaldehyde was the lone example in this report, the scope and limitations of the Tishchenko reaction using sodium hydride as the catalyst are still absent from the literature. Further, the use of benzene as the solvent under these conditions signifigantly increases the health risk, especially on large scale. We thought the use of high speed ball milling under solvent-free conditions would afford us the possibility of using sodium hydride as the catalyst, while avoiding the use of benzene. Further, we predicted that under HSBM conditions catalytic activity would be very high due to the high concentration of materials in the reaction vials.
 | ||
| Scheme 1 Tishchenko reaction. | ||
Results and discussion
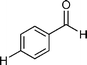
Our results are summarized in Table 1. We started our process by reacting various aryl aldehydes in the presence of a catalytic amount of sodium hydride. We attempted 1% and 2% mol catalyst but found the reaction was most effective with 10% sodium hydride. Typically, the reactions were conducted in a custom made 1/2″× 2″ inch screw-capped stainless steel vial and milled with a 1/8″ inch stainless steel ball-bearing in a Spex certiprep mixer/mill 8000M open to the atomosphere for 30 minutes (Scheme 2). At the conclusion of the reaction the products were recrystallized with 95% ethanol and dried over a Hirsch funnel. Liquid products were isolated from extraction with the minimal amount of methylene chloride.28 Upon isolation 1H NMR,13C NMR and GC-MS were compared to literature values to confirm product formation. We were able to convert benzaldehyde to benzyl benzoate in as little as 30 minutes under HSBM conditions compared to 5 hrs in solution.27 The turnover frequencies (TOF's) were determined from complete conversion.29| Entry | Substrate | Time (hrs) | % Conversion | % Yield | TOF |
|---|---|---|---|---|---|
| 1 |

|
2 | >99 | 91 | 2.5 |
| 2 |

|
0.5 | >99 | 92 | 10 |
| 3 |
|
0.5 | >99 | 98 | 10 |
| 4 |

|
0.5 | 86 | 80 | 10 |
| 5 |
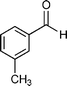
|
2 | 94 | 86 | 2.5 |
| 6 |
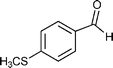
|
2 | >99 | 93 | 2.5 |
| 7 |
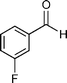
|
0.5 | >99 | 91 | 10 |
| 8 |
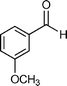
|
2 | >99 | 97 | 2.5 |
| 9 |

|
2 | 80 | 69 | 2.5 |
| 10 |

|
2 | 80 | 70 | 2.5 |
| 11 |

|
16 | 95 | 91 | 1 |
| 12 |
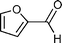
|
2 | 86 | 69 | 2.5 |
| 13 |

|
2 | >99 | 93 | 2.5 |
| 14 |

|
16 | >99 | 97 | 1 |
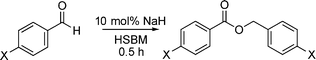 | ||
| Scheme 2 Conducting the Tishchenko reaction under solvent-free ball milling conditions. | ||
Our success with aryl aldehydes led us to investigate the Tishchenko reaction of aliphatic and α,β unsaturated aldehydes. Using sodium hydride as the catalyst we attempted the ball milled Tishchenko reaction with 3-phenylpropional, pentanal, and 1,2,3,6-tetrahydro-benzaldehyde. Our results showed that straight chain non-hindered aldehydes such as 3-phenylpropional and pentanal gives only a small amount of the dimeric ester with these substrates; with the majority of the products arising from aldol condensation chemistry. However, when sterically hindered aldehydes are used, such as 1,2,3,6-tetrahydro-benzaldehyde the dimeric ester is the major product and we observed no product resulting from the aldol condensation. α,β unsaturated systems such as cinnamaldehyde and 2-butenal gave a mixture of products which included the expected dimeric ester as well as products which arise from conjugate additon into the double bond. We also examined the reaction with acetaldehyde for the preparation of ethyl acetate but instead of obtaining ethyl acetate under these conditions the reaction resulted in the formation of a polymer.30
Since the Tishchenko reaction is catalyzed by various metals, we wanted to investigate the role of the ball material on this reaction. Using the dimerization of benzaldehyde to form benzyl benzoate as the benchmark, we explored the affect of ball material on the Tishchenko reaction under HSBM conditions. Benzaldehyde and 10 mol% sodium hydride was ball milled for fifteen minutes and the % conversion was analyzed by 1H NMR. The reactions were conducted in dupilcate and run with a 1/8″ stainless steel ball, 1/8″ brass ball and in the absence of a ball. In this particular reaction we saw similar % conversion irrespective of the ball material used, each giving ∼30% conversion. The most surprising result is that we observed the reaction was just as sucessful in the absence of a ball bearing. This suggests the high speed movement of the vial provides enough energy to cause this reaction. We thought since benzaldehyde is a liquid, a ball may not be needed in order create the proper mixing at the molecular level for this reaction to take place. To test this hypothesis, we ball milled p-chlorobenzaldehyde, a solid, to determine if the reaction procedes in the absence of a ball. To our surprise, after 30 minutes of milling the reaction proceded in similar yield to reactions conducted with brass and stainless steel balls (∼99% conversion). This suggests at least for this particular reaction that the shaking of the vial has enough energy to make an amorphous mixture of the reagents to provide a chemical reaction even without a ball!
On very large scale, ball milled reactions can generate a signifigant amount of heat, thus we wanted to conduct these reactions in the Spex Certiprep freezer mill in a liquid nitrogen environment to determine the feasibility of the reaction under low temperature conditions. Using our typical conditions, p-chlorobenzaldehyde and sodium hydride was placed inside a reaction vial and cooled to −196 °C. The reaction was milled for 30 minutes at which point it was allowed to warm to room temperature. 1H NMR and GC-MS both confirmed the presence of the expected dimeric ester of p-chlorobenzaldehyde (∼5% yield). This demonstrates the ability to conduct ball milling experiments in a low temperature environment which would allow this process to be implemented with highly exothermic reactions.
In an attempt to avoid handling sodium hydride, we wanted to generate our catalyst in situ. Sodium formate is known under certain condtions to break down to sodium hydride and carbon dioxide.26,31,32 We thought we could use sodium formate, which is safe and very easy to handle as a direct precusor to the sodium hydride catalyst. Unfortunately, after ball milling benzaldehyde and sodium formate for 16 hours we saw no evidence of the desired dimeric ester. It has been shown that various metal catalyst can facilitate the disproportation of sodium formate to sodium hydride and carbon dioxide.33–36 We tested whether a combination of catalytic sodium formate (10 mol%) and palladium tetrakistriphenylphospine (1 mol%) would lead to the generation of the sodium hydride catalyst. Using this mixture along with benzaldehyde we were able to generate trace amounts of benzyl benzoate after milling for 44 hours (Scheme 3).
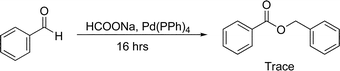 | ||
| Scheme 3 Generation of sodium hydride catalyst from sodium formate and palladium tetrakistriphenylphosphine. | ||
In addition to sodium hydride, we also investigated other benign catalysts that could give high yields of the Tishchenko reaction using ball milling conditions. Ball milling benzaldehyde with catalysts such as lithium bromide and calcium oxide did not provide the desired dimeric ester but rather gave unreacted starting material. We custom made vials out of nickel and molybdenum to investgate if these metals would lead to Tishchenko products, however, neither of these gave the dimeric ester product even under long milling times. It was reported that benzaldehyde can be converted to benzyl benzoate using magnesium metal as the catalyst in refluxing toluene.37 We ball milled p-chlorobenzaldehyde along with magnesium metal and observed >95% yield of expected dimeric ester. We are in the process of making magnesium vials to determine if the magnesium vial itself will be a sutiable catalyst under these conditions.
Conclusions
In conclusion, we report an environmentally benign method for the Tishchenko reaction using solvent-free ball milling conditions. We used sodium hydride as the catalyst instead of the more traditional aluminum catalyst which allowed the reaction to procede in high yield and short reaction times. We discovered in this particular reaction the type of ball used has little to no affect on the yield or rate of the reaction and the reaction proceeded equally well without the addition of a ball. We also observed the ball milled Tishchenko reaction can take place while the vial is immersed in a liquid nitrogen environment. Currently, we are in the process of creating a magnesium vial that is expected to act as the catalyst as well. Ball milling is a novel method for conducting organic reactions. With a better understanding of how these reactions procede we will be able to make significant strides in the development of various solvent-free reactions.Experimental
All NMR spectra were recorded on a Bruker Avance 400 spectrometer. Deuterated NMR solvents were obtained from Cambridge Isotope Laboratories, Inc., Andover MA, and used without further purification. p-bromobenzaldehyde, p-chlorobenzaldehyde, p-anisaldehyde, benzaldehyde, p-tolualdehyde, p-(methylthio)benzaldehyde, m-anisaldehyde, napthaldehyde, 2-thiophenecarboxaldehyde and sodium hydride were purchased from Acros Organics and used without further purification. m-Fluorobenzaldehyde, m-tolualdehyde and 1,2-phthalic carboxaldehyde were purchased from Sigma-Aldrich and used without further purification. Ball milling was carried out in a 8000M SpexCertiprep Mixer/Mill. Ball milling under a liquid nitrogen was carried out in a 6750 SpexCertiprep Freezer/Mill. Ball bearings were purchased from Small Parts incorporated. Custom made vials were made by the machine shop at the University of Cincinnati with metal rods purchased from ESPICorp Inc.Typical procedure
Benzaldehyde (0.22 g, 2.07 mmol), and sodium hydride (0.004 g, 0.2 mmol) were added to a custom-made 2″ by 1/2″ screw capped stainless steel vial along with a 1/8″ inch stainless steel ball bearing. The vial was placed in an 8000M Spex Certiprep mixer/mill and the contents were ball milled for 0.5 h. The resulting mixture was dissolved in methylene chloride28 (15 mL) and washed with 10% HCl (15 mL). The organic layer was dried over anhydrous MgSO4 and the solvent was evaporated under reduced pressure. This afforded benzyl benzoate in > 98% yield.Notes and references
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef CAS.
- K. Tanaka, Solvent-Free Organic Synthesis, Wiley-VCH, Cambridge, 2003 Search PubMed.
- G. Rothenberg, A. P. Downie, C. L. Raston and J. L. Scott, J. Am. Chem. Soc., 2001, 123, 8701–8708 CrossRef CAS.
- C. Suryanarayana, Prog. Mater Sci., 2000, 46, 1–184.
- L. Takacs, Prog. Mater Sci., 2002, 47, 355–414 CrossRef CAS.
- V. V. Boldyrev, J. Mater. Sci., 2004, 39, 5117–5120 CrossRef CAS.
- M. A. Mikhailenko, T. P. Shakhtshneider and V. V. Boldyrev, J. Mater. Sci., 2004, 39, 5435–5439 CrossRef CAS.
- G. Kaupp, CrystEngComm, 2003, 5, 117–133 RSC.
- G. Kaupp, CrystEngComm, 2006, 8, 794–804 RSC.
- J. Mack and M. Shumba, Green Chem., 2007, 9, 328–330 RSC.
- J. Mack, D. Fulmer, S. Stofel and N. Santos, Green Chem., 2007, 9, 1041–1043 RSC.
- W. Tischtschenko, Chem. Zentralbl, 1906, 1 Search PubMed.
- T. Seki, T. Nakajo and M. Onaka, Chem. Lett., 2006, 35, 824–829 CrossRef CAS.
- R. Crimmin Mark, G. M. Barrett Anthony, S. Hill Michael and A. Procopiou Panayiotis, Org. Lett., 2007, 9, 331–333 CrossRef CAS.
- M. A. Pasha and B. Ravindranath, Indian J. Chem. Sect B, 1985, 24B, 1068–1069 CAS.
- O. P. Tormakangas and A. M. P. Koskinen, Tetrahedron Lett., 2001, 42, 2743–2746 CrossRef CAS.
- F. J. Villani and F. F. Nord, J.Am.Chem.Soc., 1947, 69, 2605–2607 CrossRef CAS.
- K. Yokoo, N. Mine, H. Taniguchi and Y. Fujiwara, J. Organomet. Chem., 1985, 279, C19–C21 CrossRef CAS.
- H. Beberich and P. W. Roesky, Angew. Chem. Int. Ed., 1998, 37, 1569–1571 CrossRef.
- M. R. Burgstein, H. Berberich and P. W. Roesky, Chem. Eur. J., 2001, 7, 3078–3085 CrossRef CAS.
- G. B. Deacon, A. Gitlits, P. W. Roesky, M. R. Burgstein, K. C. Lim, B. W. Skelton and A. H. White, Chem. Eur. J., 2001, 7, 127–138 CrossRef CAS.
- J. Mlynarski, J. Jankowska and B. Rakiel, Tetrahedron: Asymmetry, 2005, 16, 1521–1526 CrossRef CAS.
- S.-y. Onozawa, T. Sakakura, M. Tanaka and M. Shiro, Tetrahedron, 1996, 52, 4291–4302 CrossRef CAS.
- Y.-S. Hon, Y.-C. Wong, C.-P. Chang and C.-H. Hsieh, Tetrahedron, 2007, 63, 11325–11340 CrossRef CAS.
- M. M. Mojtahedi, E. Akbarzadeh, R. Sharifi and M. S. Abaee, Org. Lett., 2007, 9, 2791–2793 CrossRef CAS.
- P. Caubere, Top. Curr. Chem., 1978, 73, 105–124 CAS.
- F. W. Swamer and C. R. Hauser, J.Am.Chem.Soc., 1946, 68, 2647–2649 CrossRef CAS.
- Using the minimal amount of ethyl acetate is just as effective in this procedure for isolating the desired product.
- J. Takehara, S. Hashiguchi, A. Fujii, S. -i. Inoue, T. Ikariya and R. Noyori, Chem. Commun., 1996, 233–234 RSC.
- , Conducting the same reaction in refluxing toluene gives the same polymeric material.
- R. A. W. Johnstone, A. H. Wilby and I. D. Entwistle, Chem. Rev., 1985, 85, 129–170 CrossRef CAS.
- T. A. Bryson, J. M. Jennings and J. M. Gibson, Tetrahedron Lett., 2000, 41, 3523–3526 CrossRef CAS.
- D. E. Linn, Jr., R. B. King and A. D. King, Jr., J. Organomet. Chem., 1988, 355, C1–C4 CrossRef.
- Y. Ben-David, M. Gozin, M. Portnoy and D. Milstein, J. Mol. Catal., 1992, 73, 173–180 CAS.
- X. Li, X. Wu, W. Chen, F. E. Hancock, F. King and J. Xiao, Org. Lett., 2004, 6, 3321–3324 CrossRef CAS.
- A. Schlatter, M. K. Kundu and W.-D. Woggon, Angew. Chem. Int. Ed., 2004, 43, 6731–6734 CrossRef CAS.
- P. I. Schorigin, W. Gussewa and A., Chem. Ber., 1933, 66, 1435 CrossRef.
| This journal is © The Royal Society of Chemistry 2009 |
