Aerobic oxidation of alcohols using various types of immobilized palladium catalyst: the synergistic role of functionalized ligands, morphology of support, and solvent in generating and stabilizing nanoparticles†
Babak
Karimi
*a,
Asghar
Zamani
a,
Sedigheh
Abedi
a and
James H.
Clark
b
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), PO. Box 45195–1159, Gava Zang, Zanjan, Iran. E-mail: karimi@iasbs.ac.ir; Fax: +98-241-4214949; Tel: +98-241-4153225
bClean Technology Centre, University of York, York, Yorkshire, UK YO10 5DD. E-mail: jhc1@york.ac.uk; Fax: +44 1904 432705; Tel: +44 1904 432559
First published on 14th November 2008
Abstract
Preparation and characterization of a variety of immobilized palladium catalyst, based on either ligand functionalized amorphous or ordered mesoporous silica, is described. The resulting Pd-loaded materials act as efficient catalyst for the oxidation of a variety of alcohols using molecular oxygen and air. Our studies show that in the case of supported palladium catalyst on hybrid amorphous silica, the nature of ligand and the solvent could effectively control the generation of nanoparticles. Furthermore, we have found that nanoparticles with smaller size and higher activity were generated from the anchored palladium precursor when the aerobic oxidation of alcohols was carried out in α,α,α-trifluorotoluene (TFT) instead of toluene. On the other hand, in the case of aerobic oxidation reactions by using supported palladium catalyst on hybrid SBA-15, the combination of organic ligand and ordered mesoporous channels resulted in an interesting synergistic effect that led to enhanced activity, prevention of Pd nanoparticles agglomeration, and finally generation of a durable catalyst.
Introduction
The selective oxidation of alcohols to form carbonyl compounds is one of the most fundamental transformation in both laboratory and industrial synthetic chemistry.1 Reagents that are traditionally used for these oxidations such as stoichiometric Cr(VI) salts,1a,2 DMSO-coupled reagents,3 or hypervalent iodines,4 are often toxic and show poor atom efficiency and their use thereby presents significant environmental issues which render them impractical. The recent Technology Vision 2020 report published by the Council for Chemical Research highlights selective oxidation of organic compounds as one of the critical challenges facing the chemical industry;5Dioxygen-coupled strategies are the most attractive means of attaining this goal.6 This has resulted in much attention being recently directed toward the development of new protocols for the aerobic oxidation of alcohols using transition-metal catalysts.7 Among them, palladium-based catalysts show promising catalytic activity, and different types of palladium-based homogeneous8 and heterogeneous catalysts9 in the form of metal complexes or nanoparticles have been reported.10 However, they often require high catalyst loading and the vast majority of them still use homogeneous palladium catalyst/ligand systems which are of limited practical application on an industrial scale owing to the difficulties in recovering and the reusing the expensive metals and ligands from the reaction mixture. One means to circumvent this problem is the design and synthesis of new improved heterogeneous catalysts with superior activity as well as high reusability. In this regard a few heterogeneous Pd catalysts including Pd/C,9aPd-hydrotalcite,9c–dPd/TiO29b,g have been reported. However, these heterogeneous Pd systems suffer from high catalyst loading and/or low catalytic activities or limited substrate scope. Recently, the application of palladium nanoparticles dispersed in an organic polymer has also been disclosed for the aerobic oxidation of alcohols.10 However, this heterogeneous system also suffers from high catalyst loading (up to 5 mol%) and the organic polymers in these protocols are very expensive and potentially susceptible to oxidative degradation under oxidation conditions. There are a few excellent catalysts including hydroxyapatite supported palladium (Pd-HAP),9f,11a Au-CeO2,11bAu-Pd/TiO2,11cPt/PS-PEG-NH2,11d and PI-Au,11e but most of them produce only high turnover frequencies at elevated (∼ 160 °C) temperatures. Furthermore, a major problem is that palladium agglomeration and the formation of palladium black can cause catalyst deactivation in many cases. To address these issues, we have recently reported in a preliminary communication that hybrid ordered mesoporous (SBA-15) silica is a very suitable support in preparing a highly recoverable palladium-based catalyst for the aerobic oxidation of alcohols.12 We have also demonstrated for the first time that the combination of an organic ligand and ordered mesoporous channels resulted in an interesting synergistic effect that led to enhanced activity, the prevention of the agglomeration of the Pd nanoparticles, and the generation of a durable catalyst for aerobic oxidation of alcohols. Herein, we present the preparation and characterizations of a variety of supported palladium catalyst based on both functionalized amorphous and SBA-15 silica and discuss more fully our findings concerning the synergistic effect of functionalized ligand, the structure of support, and the nature of solvent in generating and stabilizing Pd nanoparticles during the aerobic oxidation of alcohols. We also describe the effect of above-mentioned issues on aspects such as substrate scope and recycling behavior of catalysts.Results and discussion
Preparation and characterization of catalysts
In contrast to organic polymers, inorganic supports like silica show advantages such as mechanical stability and resistance against aging, solvent, chemical reagents, and high temperature.13 Therefore, an important recent strategy to convert a homogeneous process into a heterogeneous one is to covalently introduce the active site onto large surface area inorganic solids through an organic entity (flexible space) to create organic-inorganic hybrid (interphase) catalysts.14 An interphase is defined as a region within a material in which a stationary (organic-inorganic hybrid catalyst) and mobile compound (solvent and reagent) penetrate each other at a molecular level. According to the definition, an interphase catalyst (or pre-catalyst) is composed of three parts: an inert matrix (support), a flexible organic spacer, and an active center.14b Therefore, owing to the partial mobility of the reactive center, an interphase catalyst is able to simulate homogeneous reaction conditions, and at the same time it has the advantage of easy separation and recovery of the heterogeneous catalysts. In the present study, we use the interphase strategy to synthesize three different kinds of supported palladium pre-catalyst onto both amorphous and ordered mesoporous silica for application as heterogeneous catalyst for the aerobic oxidation of alcohols. The preparation procedure to obtain the catalyst (Cat 1, Pd@SiO2-L1) is demonstrated in Scheme 1.15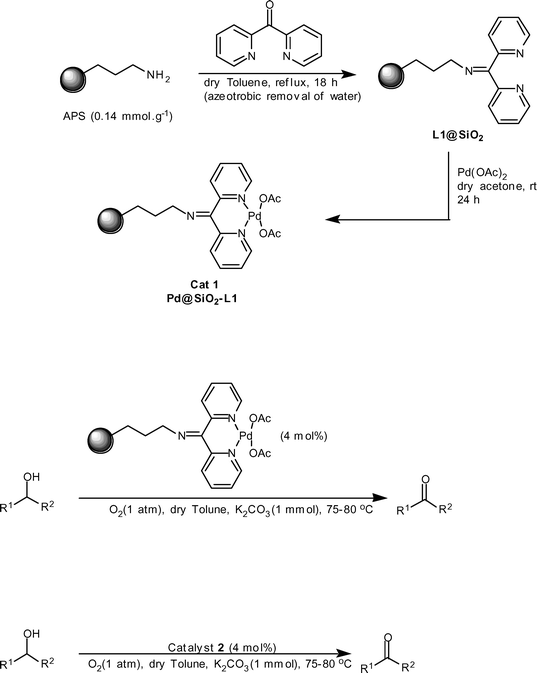 | ||
| Scheme 1 | ||
Catalyst 1 was characterized by atomic absorption spectroscopy (AA), thermogravimetric analysis (TGA), and DRIFT-IR spectroscopy.15 From the TGA analysis of L1@SiO2, it was calculated that the loading of the bipyridyl ligand bound to the silica surface was 0.13 mmol.g−1. The loading of palladium in 1 was determined using AA and shows a loading at 0.120 ± 0.001 mmol.g−1. This indicates that more than 90% of the surface-bound ligand were complexed with palladium.†15
The second catalyst (Cat 2, Pd@SiO2-L2) was synthesized by APS (0.33 mmol.g−1) precursor using a known procedure with slight modifications (Scheme 2).16
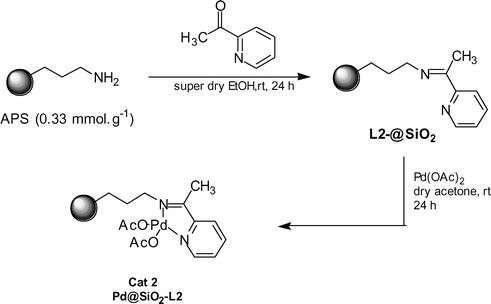 | ||
| Scheme 2 | ||
TGA analysis was used to determine the amount of ligand incorporated into APS.† Weight loss is mainly divided into three regions: below 100 °C, 150 °C–400 °C and 400–614 °C. Weight loss 30–151 °C was assigned to the loss of adsorbed water (1.8%). The large weight loss between 480–615 °C was owing to the decomposition of covalently bonded organic groups (4.6%). The amount of ligand anchored on the surface of L2@SiO2 was found to be 0.28 mmol.g−1. This data was further confirmed by elemental analysis. The catalyst 2 (Pd@SiO2-L2) was then prepared by stirring a mixture of L2@SiO2 (4 g) and Pd(OAc)2 (0.112 g, 0.5 mmol) in dry acetone (100 mL) at room temperature for 24h. After stirring, the resulting solid was filtered, and washed with acetone until the washings were colourless and dried at 95 °C overnight to afford Pd@SiO2-L2. The amount of palladium anchored on the surface of 2 was found to be 0.08 mmol.g−1 (corresponding to 28% of the ligands available) on the basis of atomic absorption spectroscopy. To determine the thermal stability, TGA analysis of 2 was also conducted in air from room temperature to 700 °C, and typical weight loss curves have been shown.† This sample shows a weight loss of a small amount of loosely bound water (less than 2%) below 200 °C. This is followed by a weight loss of about 9.1% between 350 and 500 °C due to the decomposition of organic ligand bound palladium complex. There after, an additional loss of 3.2% is observed, probably resulting principally decomposition of free anchored imine complex (see supporting information).
In order to prepare the third catalyst (Pd@SBA-15-L3, 3), we chose to employ the ordered mesoporous silicate SBA-1517 as a support for a bidentate ligand because it has regular porosity, and high surface area. In addition, it is well known that organic groups inside large pore mesoporous materials are more accessible than those on amorphous silica. Therefore we reasoned that SBA-15, owing to the above-mentioned property, might be better suited as a support for preparing heterogeneous Pd catalysts. SBA-15 was obtained from pluronic P123 (EO20PO70EO20, EO = ethylene oxide, PO = propylene oxide, MAV = 5800, Aldrich) as a lyotropic ligand that was liquid crystal templated, and (EtO)4Si under acidic conditions following the reported literature procedure.17b The resulting SBA-15 was then functionalized with a bipyridylamide ligand followed by complexation with Pd(OAc)2 to give the corresponding immobilized catalyst 3 (Scheme 3).†
 | ||
| Scheme 3 | ||
A typical nitrogen adsorption/desorption type IV profile with a sharp hysteresis loop, which is characteristic of the highly ordered mesoporous materials, was obtained for 3 (Fig. 1a).
 | ||
| Fig. 1 The isotherm plot of a sample of Catalyst 3: (a) before the first reaction cycle and; (2) after the first reaction cycle. | ||
A BET surface area of 455 m2.g−1 and a total pore volume of 0.76 cm3.g−1 were obtained for the material. Thus values are smaller than those for the starting SBA-15 (864 m2g−1). BJH calculations showed an average pore diameter of 7.6 nm for 3, a value which is in good agreement with the pore diameter estimated from the TEM image (Figs. 4a, b). On the basis of the AA analysis of a solution obtained by washing the catalyst with nitric acid, the amount of palladium loading on 3 was found to be 0.022 ± 0.001 mmol.g−1. We used such a low loading in order to minimize catalyst leaching.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst/support | x (mol%) | solvent | time (h) | yield (%)a,b | ||
| O2 | air | O2 | air | ||||
| a GC yield unless otherwise stated. b Yields in parentheses refer to isolated pure products. c TFT = α,α,α-trifluorotoluene. | |||||||
| 1 | 1/SiO215 | 4.0 | toluene | 8 | 12 | >99 (85) | >99 |
| 2 | 1/SiO2 | 4.0 | TFT c | 8 | 12 | >99 | >99 |
| 3 | 1/SiO2 | 4.0 | toluene | 2 | 2 | 7 | 5 |
| 4 | 1/SiO2 | 4.0 | TFT | 2 | 2 | 11 | 9 |
| 5 | 2/SiO2 | 3.2 | toluene | 1.25 | 1.25 | >99 | >99 |
| 6 | 2/SiO2 | 2.0 | toluene | 3 | 3 | >99 | >99 |
| 7 | 2/SiO2 | 1.0 | toluene | 7 | 7 | >99 | >99 |
| 8 | 2/SiO2 | 0.4 | TFT | 1.5 | 2 | >98 | >98 |
| 9 | 2/SiO2 | 0.2 | TFT | 4 | — | >98 | — |
| 10 | 2/SiO2 | 0.2 | Toluene | 2 | — | 35 | — |
| 11 | 2/SiO2 | 0.2 | TFT | 2 | — | 51 | — |
| 12 | 3/SBA-1512 | 0.4 | toluene | 3.5 | 5.5 | >99 (83) | >99 |
| 13 | 3/SBA-15 | 0.4 | TFT | 3.5 | 5 | >99 | >99 |
| 14 | 3/SBA-15 | 0.4 | toluene | 2 | — | 72 | — |
| 15 | 3/SBA-15 | 0.4 | TFT | 2 | — | 73 | — |
| 16 | NHC-Pd/IL@SiO223 | 5 | toluene | 12 | — | Nd | — |
| 17 | NHC-Pd/IL@SiO223 | 5 | TFT | 12 | — | Nd | — |
Although all catalysts were able to furnish the corresponding benzaldehyde in excellent yield within the indicated time obviously, catalyst 2 in TFT (entry 11) and catalyst 3 in both TFT and toluene (entries 14, 15) exhibited higher catalytic activities than that of catalyst 1 (entries 3, 4).
It is worth mentioning that catalyst 2 in TFT (equivalent to 0.4 mol% Pd) is even more effective than the same catalyst sample in toluene (entry 11 vs. 12). Moreover, our preliminary investigations showed that catalyst 2 in TFT and toluene gave consistent activity in 5 and 6 subsequent reactions and exhibited TONTFT = 2500 and TONTol = 187, respectively at 80 °C, with almost quantitative yield of the product. To clarify the above mentioned observations, we have studied the evolution of 2 by means of transmission electron microscopy (TEM) analysis. Interestingly, inspection of the TEM image of a sample of catalyst 2 after recovery from the aerobic oxidation of benzyl alcohol in TFT and toluene clearly indicates, the generation of Pd nanoparticles onto the surface of amorphous SiO2 (Fig. 2).
 | ||
| Fig. 2 TEM images of the recovered catalyst 2. (a) After the first reaction cycle in toluene (particle size in the range of 6–17 nm and an average particle size ≈10 nm); (b) After the first reaction cycle in TFT (particle size in the range 3–14 nm and an average particle size ≈6.7). | ||
Furthermore, the images show that the oxidation reaction using catalyst 2 in TFT clearly favors production of smaller metal nanoparticles than those obtained in toluene. It is well-known that the catalyst performance can be very sensitive to particle size because the surface structure and electronic properties can change greatly within the nano-size range.18 Moreover, it has been demonstrated that the catalytic activity of Pd nanoparticles increases with decreasing their average size.19 Therefore, it would be logical to speculate that the increase in the size of the nanoparticles onto the surface of 2 in toluene is the reason why the process is slower than in TFT.
This observation might be due to the fact that TFT more efficiently caps many of the free metallic surface sites of the nanoparticles and provides fewer sites for the Ostwald ripening process.20 On the other hand, the lower catalytic activity (higher catalyst loading) of 2 in toluene is probably due to generation of a smaller amount of nanoparticles of the bigger size throughout the silica surface due to larger nanoparticle aggregations. We may expect that in the case of 1 (Pd@SiO2-L1) and 3 (Pd@SBA-15-L3), TFT will result in rapid nucleation of a great number of smaller Pd nanoparticles compared to toluene and therefore cause a similar solvent effect. However, as can be seen from the data in Table 1 neither 1 nor 3 showed this effect (entries 3 vs. 4 and 14 vs. 15).
Therefore it seems that in the case of 1 and 3 other factors should also be taken into account. On the basis of our previous studies, we also found that catalyst 1 requires high catalyst concentration (up to 4 mol%, 80 °C, 8 h) and it also suffers from the disadvantage of prolonged reaction time and a significant reduction in its catalytic activity after three reaction cycles.15 At first glance, one might conclude that this catalytic deactivation is due to Pd leaching from the solid to the solution. However, both individual AA analysis of the solution of each three reaction cycles and hot-filtration test indicates no Pd species that leached into the solution.15 To gain better insight into the structural changes, the recovered sample of 1 has also been studied by means of TEM to try to find a reason for its rapid deactivation. Fig. 3 shows a typical TEM (in dark-field mode) image of 1 after the third cycle of aerobic oxidation of benzyl alcohol. It can be seen, this material shows extensive agglomeration of palladium with an irregular size distribution above 100 nm.
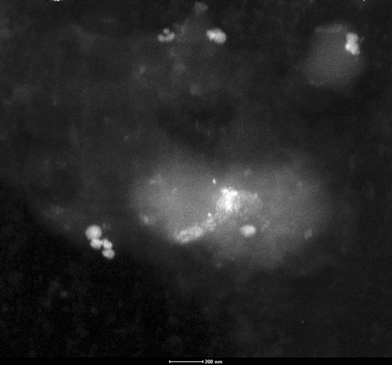 | ||
| Fig. 3 Dark-field TEM image of catalyst 1 after the third reaction cycle. | ||
This result clearly shows that Pd-agglomeration is the major reason for deactivation of catalyst 1 just after the third cycle. One reason the process is so much more prominent in the case of both 2 and 3 than in 1 is presumably owing to smooth hydrolysis of un-complexed imino group in 1 during the oxidation reaction using the water by-product. Unfortunately, we have not yet been able to obtain any evidence for the hydrolyzed bipyridyl ketone that leached from catalyst 1. However, TGA analysis of the recovered catalyst 1 showed a slight decrease in the weight loss as compared with the fresh catalyst, in support of the above proposal.
The lack of binding provides a means of rapid sintering of small palladium particles throughout the surface of silica and produce very large (much less reactive) palladium clusters. This may also explain why the catalyst 1 shows similar behaviour in both TFT and toluene, i.e. in the absence of a well-bonded ligand, Pd agglomeration would rapidly result in catalyst deactivation regardless to the small difference in the stabilizing ability of TFT compared to toluene. This observation also highlights the crucial role of anchored ligands in generating and stabilizing nanoparticles during a typical catalytic process.
With regard to material 3 we have reported in an earlier communication12 that at 0.4 mol% it is an efficient and durable heterogeneous catalyst for the aerobic oxidation of a wide range of alcohols including benzyl alcohol itself. In this study, we have found that the catalytic activity and durability of 3 was not significantly altered by changing the solvent from toluene to TFT (Table 1, entries 14 vs. 15). Moreover, after the first oxidation cycle using 3 (Table 1, entry 8) to afford benzaldehyde in 83% isolated yield (>99% conversion), the recovered catalyst exhibited consistent catalytic activity in 12 consecutive reactions (total TON ≅ 3000 at 80 °C),12 which is much higher than those observed in the case of both 1 and 2. To find a reason for this high reactivity and more importantly, high durability of 3 at a molecular level, TEM analysis has also been performed on a sample of 3 before and after catalysis. Fig. 4 shows representative TEM images of 3 before the first cycle of aerobic oxidation both perpendicular (Fig. 4a) and across (Fig. 4b) to the hexagonally uniformed channels of ≈7 nm in size. On the other hand, Fig. 4c shows a representative TEM image of the same sample of 3 after recovery from the first cycle of aerobic oxidation of benzyl alcohol. By comparing these two set of TEM images before and after the first reaction cycle, we can see that Pd nanoparticles with regular size (≤ 7 nm) were mostly generated inside the highly ordered channels and that the nanoarchitecture of the catalyst (SBA-15 channels) largely survived.21
 | ||
| Fig. 4 TEM images of fresh catalyst 3. (a) Perpendicular to the channels; (b) across the channels; (c) TEM image of the recovered catalyst 3 across the channels, well-dispersed palladium nanoparticles with a relatively regular size can clearly be seen inside the channels. | ||
Moreover, the N2adsorption-desorption analysis of the recovered catalyst (Fig. 1b) showed a very similar isotherm to those of the fresh catalyst 3 (Fig. 1a) with relatively sharp adsorption and desorption branches in the P/P0 range of 0.5–0.8. This strongly indicates a relatively narrow mesopore size distribution even in the recovered catalyst, although the total pore volume decreases from 0.76 to 0.57 cm3 g−1. This result also suggests that most of the nanometre-scaled void space and channels of the host SBA-15 remain open, although a small portion of the channels may be blocked by Pd nanoparticles (Fig. 2b). In order to better clarify the role of bipyridyl ligands in our protocol we set up two sets of controlled experiments. First, we prepared a new catalyst in which SBA-15 lacking organic ligands was loaded with Pd(OAc)2 with the same Pd loading as 3. The oxidation of benzyl alcohol was then conducted under the same reaction condition using this catalyst. Interestingly, we found that the corresponding benzaldehyde was produced in >99% conversion after 5 hours in the first experiment. However, when the oxidation of benzyl alcohol was repeated for two subsequent runs with the same catalyst sample, the catalyst activity was dramatically decreased. The significant deactivation of the material along with a color change to dark grayish is presumably due to the formation of large palladium clusters (palladium black) onto the outer surface of SBA-15.12 In the second experiment, the SBA-15 with 3-cyanopropyl group was loaded with Pd(OAc)2 and the resulting pale yellow solid was tested for catalytic activity in the same process. In this case, the solid catalyst showed a high degree of leaching and also the corresponding benzaldehyde was produced in low (less than 25%) yield after 5 h due to the rapid formation of Pd-black. Therefore, we believe that the bipyrydyl ligands in catalyst 3 provide a means of uniform distribution of mononuclear palladium center throughout the solid support, to ensure controlled nanoparticle formation mostly inside the ordered mesoporous channels of SBA-15.12 We can also explain why the catalyst 3 shows high and the same catalytic activity in both TFT and toluene. The cooperation of functionalized organic ligand (the bipyridine ligand) inside the ordered mesoporous channels and size restriction imposed by meso-channels of the parent SBA-15 resulted in an interesting synergistic effect enhancing the activity, preventing Pdnanoparticles agglomeration, and producing an inherently durable catalyst independent of the choice of TFT or toluene. Much to our surprise, our recently developed silica supported N-heterocyclic carbene palladium/ionic liquid catalysts that have been shown to be highly efficient and recyclable catalysts for the Heck reaction, were ineffective in oxidizing benzyl alcohol under similar reaction conditions to those of catalysts 1–3 (Table 1, entries 16, 17).22 The reasons for this latter observation are under investigation.
| Entry | Substrate | Product | Pd | time (h) | yield (%)a | ||
|---|---|---|---|---|---|---|---|
| (mol%) | O2 | air | O2 | air | |||
| a GC yield based on an internal standard method unless otherwise stated. b A trace amount of the corresponding esters (≈ 4%) and carboxylic acid (≈ 11%) were formed. | |||||||
| 1 |
|

|
0.4 | 1.5 | 2 | 98 | 98 |
| 2 | 0.2 | 4 | — | 97 | — | ||
| 3 |
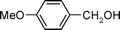
|
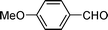
|
0.1 | 3 | 3 | 100 | 100 |
| 4 |
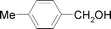
|

|
0.2 | 3 | 1.5 | 95 | 95 |
| 5 |

|
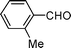
|
0.7 | 10 | — | 91 | — |
| 6 |

|
|
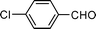
0.3 | 8 | 15 | 93 | 95 |
| 7 |
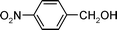
|

|
0.2 | 24 | — | 78 | — |
| 9 |

|
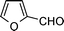
|
0.2 | 2 | — | 92 | — |
| 10 |
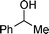
|

|
0.4 | 2.5 | 4 | >99 | 100 |
| 0.2 | 8.5 | — | >99 | — | |||
| 11 |

|

|
0.4 | 2.5 | 4 | 97 | 95 |
| 12 |

|
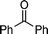
|
0.4 | 10 | — | 100 | — |
| 13 |

|
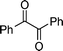
|
0.4 | 4 | 7 | 100 | 97 |
| 14 |
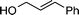
|
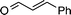
|
2 | 5 | 8 | 94 | 96 |
| 15 |

|
|
2 | 7 | 10 | 100 | 94 |
| 16 |

|

|
2 | 5 | 8 | 100 | 100 |
| 17 |

|

|
2 | 7 | 10 | 98 | 97 |
| 18 |

|

|
3 | 24 | — | 13b | — |
| 19 |
|

|
3 | 24 | — | 21 | — |
| 20 |

|

|
3 | 24 | — | 18 | — |
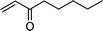
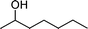
In general, many of the previously reported homogeneous transition metal complexes are unable to catalyze the oxidation of alcohols that can chelate Pd(II) catalyst, as a starting material or product, because the strong coordination to metal centers deactivates the catalyst. However, the successful oxidation of benzoin and furfuryl alcohol as model substrates shows a superior capability of 2 in oxidizing similar substrates (Table 2, entries 9, 13). Catalyst 2 in particular showed excellent reactivity for the selective oxidation of various types of allylic alcohols yielding the corresponding α,β-unsaturated carbonyl compounds in excellent yields (Table 2, entries 14–17). It is worth mentioning that in the oxidation of allylic alcohols, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds remained intact without an intramolecular hydrogen transfer. Secondary benzylic alcohols were also efficiently oxidized into the corresponding ketone (Table 2, entries 10–13). Even the reaction under air (balloon filled) instead of oxygen proceeded well, thus indicating that the reaction is not markedly retarded by the concentration of dissolved oxygen in the solvent. Unfortunately, using 2, aliphatic alcohols such as 1-octanol and 2-octanol and 4-phenyl cyclohexanol proved to be poor substrates, yielding the corresponding carbonyl compound in 13%, 21%, and 18% yields, respectively (Table 2, entries 18–20).
C double bonds remained intact without an intramolecular hydrogen transfer. Secondary benzylic alcohols were also efficiently oxidized into the corresponding ketone (Table 2, entries 10–13). Even the reaction under air (balloon filled) instead of oxygen proceeded well, thus indicating that the reaction is not markedly retarded by the concentration of dissolved oxygen in the solvent. Unfortunately, using 2, aliphatic alcohols such as 1-octanol and 2-octanol and 4-phenyl cyclohexanol proved to be poor substrates, yielding the corresponding carbonyl compound in 13%, 21%, and 18% yields, respectively (Table 2, entries 18–20).
To determine whether the catalyst 2 is functioning in a heterogeneous manner, or whether it is merely a reservoir for more active soluble forms of Pd, various heterogeneity tests were performed. First, the reaction of benzyl alcohol was conducted in the presence of catalyst 2 for 1 hour until a conversion of 55% was reached. Then the solid was hot-filtered and transferred to another Schlenk flask containing K2CO3 in TFT at 80 °C under O2 atmosphere. The catalyst free solution was then allowed to continue to react, but no further reaction took place even after 12 h. Furthermore, our primarily investigation using AA analysis indicates that no Pd species leached into solution within the detection limit. Nevertheless, it is difficult at this stage to exactly attribute the actual catalytic activity solely to the ligand-bound Pd or to Pd nanoparticles. It would not be also surprising if the supported Pd nanoparticles serve as a reservoir for a trace of non-detectable Pd particles which react via a homogeneous pathway.22 To better clarify this issue, we have also conducted a three-phase-test with a heterogeneous alcohol (Scheme 4).
 | ||
| Scheme 4 | ||
Unfortunately, this substrate was decomposed during the oxidation reaction so that we have detected the decomposed alcoholic part of the solid in the reaction solution. We are now searching to obtain a more suitable solid alcohol for this purpose and we will present the results in due course.
Experimental
Preparation of aminopropyl silica (APS)
Mesoporous silica gel (average pore diameter 60 Å) was activated by refluxing in concentrated hydrochloric acid (6 M) for 24 h and then washed thoroughly with the deionized water and dried before undergoing chemical surface modification. Refluxing the activated silica gel (10 g) with 3-aminopropyltrimethoxysilane (1.5 mmol) in dry toluene for 18 h. The solid materials were filtered off and washed with hot toluene for 12 h in a continuous extraction apparatus (Soxhelet) and then dried in oven at 110 °C overnight to give the surface bound amine (APS) group at a loading ca. 0.14 mmol g−1 (by elemental analysis and back titration).Preparation of surface bound bipyridyl ligand (L1@SiO2)
The resulting AMPS (0.14 mmol.g−1, 5 g) was allowed to react with 2,2′-dipyridyl ketone (1 mmol, 0.184 g, Aldrich) in refluxing toluene with continuous removal of water using a Dean–Stark trap. The solid was filtered off and was washed thoroughly with hot toluene and ethanol to remove unreacted ketone. It was dried in air at 110 °C overnight to furnish the corresponding surface bound bidentate ligand 1 at a loading ca. 0.13 mmol g−1 (Determined by TGA analysis).Preparation of Pd@SiO2-L1 (Cat 1)
The catalyst was prepared by stirring a mixture of surface bound ligand 1 (4 g) and palladium acetate (0.52 mmol, 0.117 g, Merck) in dry acetone (100 mL) at room temperature for 24 h. After stirring the resulting white brown solid was filtered, washed with large volume of acetone, ethanol and ether. It was then dried in an oven at 95 °C overnight to furnish the corresponding catalyst 1 at a loading ca. 0.12 ± 0.01 mmol g−1 (Determined by TGA analysis and atomic absorption spectroscopy (AA)).Preparation of (L2@SiO2)
2-acetylpyridine (0.605 g, 5 mmol) was added to a mixture of the oven dried AMPS (0.33 mmol.g−1, 5 g) in super dry ethanol (150 mL) in a 250 mL round bottomed flask. The reaction mixture was stirred at 60 °C for 24 h. The ligand-grafted silica was filtered at the reaction temperature and the resulting solid and was washed thoroughly with hot toluene and ethanol to remove un-reacted ketone. It was dried in air at 95 °C overnight to furnish the corresponding surface bound bidentate ligand L2@SiO2 at a loading ca. 0.28 mmol g−1 (Determined by TGA analysis).Preparation of Pd@SiO2-L2 (Cat 2)
The catalyst was prepared by stirring a mixture of surface bound ligand L2@SiO2 (4 g) and palladium acetate (0.50 mmol, 0.112 g, Merck) in dry acetone (100 mL) at room temperature for 24 h. After stirring the yellow solid was filtered, washed with large volume of acetone until washing were colourless. It was then dried in an oven at 95 °C overnight to furnish the corresponding catalyst 2 (Scheme S2, ESI†) at a loading ca. 0.080 ± 0.001 mmol g−1 (Determined by TGA analysis (Figure S5b) and atomic absorbtion spectroscopy (AA)).Preparation of SBA-15
The synthesis of SBA-15 has been achieved using known procedure described by Stucky and his co-workers (see ref. 17b). In a typical preparation procedure, 4.0 g of Pluronic P123 (Aldrich, average Mw ≅ 5800) was dissolved in 30 g of water and 120 g of 2 M HCl solution with stirring at 35 °C. Then 8.50 g of tetraethoxysilane (TEOS) was added into that solution with stirring at 35 °C for 20 h. The mixture was aged at 80 °C overnight without stirring. The solid was filtered off and washed thoroughly with hot ethanol/water using a Soxhelet apparatus for 18 h to remove the surfactant molecules. It was dried in air at 110 °C overnight.Preparation of SBA-15 surface bound carboxylic acid
The preparation of SBA-15 surface bound carboxylic acid was achieved according to the known procedure described by Clark et al.:24 The resulting SBA-15 (6 g) was allowed to react with 3-cyanopropyltriethoxysilan (2 mmol, 0.462 g, Fluka) in refluxing dry toluene (150 mL) under nitrogen for 24 h. The solid was filtered off and was washed thoroughly with hot toluene and ethanol. It was dried in air at 90 °C overnight to furnish the corresponding surface bound cyanopropyl group at a loading ca. 0.33 mmol g−1 (Determined by elemental analysis). The absorption band at 2256 cm−1 along with bands 2900–3000 cm−1 clearly indicates the attachment of cyanopropyl group onto the surface of SBA-15 (Fig. 1). The CN-SBA-15 was hydrolyzed by heating in 50% (v/v) aqueous sulfuric acid at 150 °C for 3h. After cooling to room temperature, the resulting solid was filtered off and filter cake was washed with an excess of deionized water. Drying in an oven at 110 °C overnight furnished the corresponding SBA-15-COOH with approximately the same loading.Preparation of Pd@SBA-15-L3 (Cat 3)
The ligand A was first prepared by stirring a mixture of surface-bound carboxylic acid (5 g), 2,2′-bipyridylamine (1.7 mmol, 0.291 g, Aldrich) and dicyclohexylcarbodiimide (1.7 mmol, 0.350 g, Merck) in dry THF (150 mL) at reflux temperature for 72 h. The solid was filtered off and washed thoroughly with hot ethanol using a Soxhelet apparatus for 18 h to remove both the urea by-products and unreacted starting materials. It was then dried in an oven at 110 °C overnight to furnish the corresponding surface-bound bipyridyl amideA at a loading ca. 0.2 mmol g−1 (Determined by TGA and elemental analysis, Fig. 2). The catalyst was then prepared by stirring a mixture of surface bound ligand A (4 g) and palladium acetate (0.11 mmol, 0.025 g, Merck) in dry acetone (100 mL) at room temperature for 24 h. After stirring, the white brown solid was filtered, washed with acetone, ethanol and ether in order to remove any adsorbed palladium on the surface. It was then dried in an oven at 95 °C overnight to furnish the corresponding catalyst 3 at a loading ca. 0.022 ± 0.001 mmol g−1 (atomic absorption spectroscopy (AA)).General experimental procedure for oxidation using molecular oxygen
A mixture of K2CO3 (1 mmol) and catalyst 1 (0.18 g, ∼0.4 mol% of Pd) in TFT (5 mL) was prepared in a two-necked flask. The flask was then evacuated (water aspirator) and refilled with pure oxygen for three times (balloon filled). To this solution the alcohol (1 mmol, in 1 mL TFT) was then injected and the resulting mixture was stirred at 80 °C under an oxygen or air atmosphere (for the indicated time in the Table 2). After completion of the reaction, the reaction mixture was filtered off and the catalyst rinsed twice with CH2Cl2 (5 mL) the excess of solvent was removed under reduced pressure to give the corresponding carbonyl compoundsConclusions
The preparation and characterization of a variety of supported palladium catalysts based on ligand functionalized amorphous and ordered mesoporous silicas (SBA-15) were described. The resulting Pd-loaded materials act as highly efficient catalysts for the oxidation of a variety of alcohols using molecular oxygen and air. The catalysts show high thermal stability and could be recovered and reused for several reaction cycles in a batch-wise system depending on their structures of the support, functionalized ligand, and the solvent. This study shows that in the case of supported palladium catalyst on hybrid amorphous silica (catalysts 1 and 2), the nature of the ligand could significantly affect the generation and the distribution of nanoparticles onto the solid surface. Furthermore, we have found that nanoparticles with smaller size were generated from the anchored palladium precursor when the aerobic oxidation of alcohols were carried out in TFT instead of toluene. Our studies using TEM analysis of the samples before and after catalysis, show that in the case of supported palladium catalyst on hybrid amorphous silica, the nature of the ligand could effect the generation of nanoparticles. Furthermore, we have found that nanoparticles with smaller size were generated from the anchored palladium precursor when the aerobic oxidation of alcohols were carried out in TFT instead toluene. On the other hand, in the case of aerobic oxidation reactions using supported palladium catalyst on hybrid SBA-15, the combination of organic ligand and ordered mesoporous channels resulted in an interesting synergistic effect enhancing catalytic activity, preventing Pd nanoparticles agglomeration, and enabling generation of a durable catalyst. Based on this study, a typical reactivity and durability sequence is: 3/toluene ≈3/TFT > 2/TFT > 2/toluene >> 1/TFT ≈1/toluene. Both catalysts 3 and 2 show high TOFs of approximately 18150 h−1 and 7230 h−1− in the oxidation of 1-phenylethanol at 150 °C under solvent-free conditions. These results may find wide potential applications in designing other types of transition metal nanocatalysts. Further applications of this approach with other transition metal based nanoparticles are currently underway in our laboratories.Acknowledgements
The authors acknowledge IASBS Research Councils and Iran National Science Foundation (INSF) for support of this work.Notes and references
- (a) M. Hudlicky, Oxidation in Organic Chemistry, ACS Monograph Series; American Chemical Scociety, Washington DC, 1990 Search PubMed; (b) R. A. Sheldon, and J. K. Kochi, Metal Catalyzed Oxidationd of Organic Compounds, Academic Press, New York, 1984 Search PubMed.
- S. V. Ley, and A. Madfin, Comprehensive Organic Synthesis, B. M. Trost, I. Fleming, S. V. Ley, Eds.; Pergamon: Oxford, U.K., 1991; vol.7, pp 251-289 Search PubMed.
- A. Mancuso and D. Swern, Synthesis, 1981, 165 CrossRef CAS.
- (a) D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155 CrossRef CAS; (b) D. B. Dess and J. C. Martin, J. Am. Chem. Soc., 1991, 113, 7277 CrossRef CAS.
- Vision 2020 Catalysis Report (www.ccrhq.org/vision/index/roadmaps.catrep.html).
- (a) For recent reviews see: R. A. Sheldon, I. W. C. E. Arend and A. Dijksman, Catal. Today, 2000, 57, 157 Search PubMed; (b) B. Z. Zhan and A. Thompson, Tetrahedron, 2004, 60, 2917 CrossRef CAS.
- (a) R. A. Sheldon, Green Chem., 2000, 2, G1 RSC; (b) P. T. Anastas, L. B. Bartlett, M. M. Kirchhoff and T. C. Williamson, Catal. Today, 2000, 55, 11 CrossRef CAS.
- (a) K. Kaneda, Y. Fujii and K. Morioka, J. Org. Chem., 1996, 61, 4502 CrossRef CAS; (b) K. Kaneda, Y. Fujii and K. Ebitani, Tetrahedron Lett., 1997, 38, 9023 CrossRef CAS; (c) K. P. Peterson and R. C. Larock, J. Org. Chem., 1998, 62, 3185 CrossRef CAS; (d) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, Tetrahedron Lett., 1998, 39, 6011 CrossRef CAS; (e) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750 CrossRef CAS; (f) G.-J. T. Brink, I. W. C. E. Arends and R. A. Sheldon, Science, 2000, 287, 1636 CrossRef; (g) K. Hallman and C. Moberg, Adv. Synth. Catal., 2001, 343, 260 CrossRef CAS; (h) M. J. Schultz, C. C. Park and M. S. Sigman, Chem. Commun., 2002, 3034 RSC; (i) D. R. Jensen, M. J. Schultz, J. A. Mueller and M. S. Sigman, Angew. Chem. Int. Ed., 2003, 42, 3810 CrossRef CAS; (j) G.-J. T. Brink, I. W. C. E. Arends and R. A. Sheldon, Adv. Synth. Catal., 2002, 344, 355 CrossRef CAS; (k) T. Nishimura and S. Uemura, Synlett, 2004, 201 CAS; (l) S. Paavola, K. Zetterberg, T. Privalov, I. Csöregh and C. Moberg, Adv. Synth. Catal., 2004, 346, 237 CrossRef CAS; (m) T. Iwasawa, M. Tokunaga, T. Obora and Y. Tsuji, J. Am. Chem. Soc., 2004, 126, 6554 CrossRef CAS; (n) M. J. Schultz, S. S. Hamilton, D. R. Jensen and M. S. Sigman, J. Org. Chem., 2005, 70, 3343 CrossRef CAS; (o) T. Iwasawa, M. Tokunaga, Y. Obora and Y. Tsuji, J. Am. Chem. Soc., 2004, 126, 6554 CrossRef CAS; (p) For a recent review on palladium catalyzed oxidation of alcohols see: J. Muzart, Tetrahedron, 2003, 59, 5789 Search PubMed; (q) For recent interesting reviews on palladium catalyzed aerobic oxidations of organic chemicals see: S. S. Stahl, Angew. Chem. Int. Ed., 2004, 43, 3400 Search PubMed; (r) T. Nishimura and S. Uemura, Catal. Surv. Jpn., 2000, 4, 135 CrossRef CAS; (s) B. A. Steinhoff, A. E. King and S. S. Stahl, J. Org. Chem., 2006, 71, 1861 CrossRef CAS.
- (a) T. Mallat and A. Baiker, Catal. Today, 1994, 19, 274; (b) K. Ebitani, Y. Fujie and K. Kaneda, Langmuir, 1999, 15, 1907; (c) T. Nishimura, N. Kakiuchi, M. Inoue and S. Uemura, Chem. Commun., 2000, 1245 RSC; (d) N. Kakiuchi, Y. Maeda, T. Nishimura and S. Uemura, J. Org. Chem., 2001, 66, 6620 CrossRef CAS; (e) N. Kakiuchi, M. Nishimura, M. Inoue and S. Uemura, Bull. Chem. Soc. Jpn., 2001, 74, 165 CrossRef CAS; (f) K. Moroi, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2002, 124, 11572 CrossRef CAS; (g) K.-M. Choi, T. Akita, T. Mizugaki, K. Ebitani and K. Kaneda, New. J. Chem., 2003, 27, 324 RSC; (h) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657 CrossRef CAS; (i) U. R. Pillai and E. Sahle-Demessie, Green Chem., 2004, 6, 161 RSC.
- (a) Y. Uozumi and R. Nakao, Angew. Chem. Int. Ed., 2003, 42, 3810 CrossRef CAS; (b) B. Corain, K. Jerabek and P. Centomo, Angew. Chem. Int. Ed., 2004, 43, 959 CrossRef CAS; (c) Z. Hou, N. Theyssen, A. Brinkmann and W. Leitner, Angew. Chem.Int. Ed., 2005, 44, 1346 CrossRef CAS; (d) M. S. Kwon, N. Kim, C. M. Park, J. S. Lee, K. Y. Kang and J. Park, Org. Lett., 2005, 7, 1077 CrossRef CAS; (e) Z. Hou, N. Theyssen and W. Leitner, Green Chem., 2007, 9, 127 RSC; (f) K. Hara, S. Tayama, H. Kano, T. Masuda, S. Takakusagi, T. Kondo, K. Uosaki and M. Sawamura, Chem. Commun., 2007, 4280 RSC; (g) For an excellent recent study of aerobic oxidation of alcohols in supercritical carbon dioxide using supported palladium nanoparticles on hybrid mesoporous silica see: Z. Hou, N. Theyssen, A. Bronkmann, K. V. Klementiev, W. Grünert, M. Bühl, W. Schmidt, B. Spliethoff, B. Tesche, C. Weidenthaler and W. Leitner, J. Catal., 2008, 258, 315 Search PubMed.
- (a) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657 CrossRef CAS; (b) A. Abad, P. Concepción, A. Corma and H. García, Angew. Chem. Int. Ed., 2005, 44, 4066 CrossRef CAS; (c) D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espiriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Huchings, Science, 2006, 311, 362 CrossRef CAS; (d) Y. M. A. Yamada, T. Arakawa, H. Hocke and Y. Uozumi, Angew. Chem. Int. Ed., 2007, 46, 704 CrossRef; (e) H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem. Int. Ed., 2007, 46, 4151 CrossRef CAS.
- B. Karimi, S. Abedi, J. H. Clark and V. Budarin, Angew. Chem. Int. Ed., 2006, 45, 4776 CrossRef CAS.
- P. Panster and S. Wieland, Applied Homogeneous Catalysis with Organometallic Compounds Vol. 2 (Eds. B. Cornils, W. A. Hermann, VCH, Weinheim, 1996, pp. 605-623 Search PubMed.
- (a) A. Corma and H. Garcia, Chem. Rev., 2002, 102, 3879 CrossRef CAS; (b) Z. L. Lu, E. Lindner and H. A. Mayer, Chem. Rev., 2002, 102, 3543 CrossRef CAS; (c) D. E. De Vos, M. Dams, B. F. Sels and P. A. Jacobs, Chem. Rev., 2002, 102, 3615 CrossRef CAS; (d) A. P. Wight and M. E. Davis, Chem. Rev., 2002, 102, 3589 CrossRef CAS; (e) J. H. Clark and D. J. Macquarrie, Chem. Commun., 1998, 853 RSC.
- B. Karimi, A. Zamani and J. H. Clark, Organometallics, 2005, 24, 4695 CrossRef CAS.
- S. Paul and J. H. Clark, Green Chem., 2003, 5, 635 RSC.
- (a) D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS; (b) D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- (a) A. T. Bell, Science, 2003, 299, 1688 CrossRef CAS; (b) D. R. Rolison, Science, 2003, 299, 1698 CrossRef CAS; (c) M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef CAS.
- (a) R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340 CrossRef CAS; (b) Y. Li, E. Boone and M. A. El-Sayed, Langmuir, 2002, 18, 4921 CrossRef CAS; (c) J.-L. Bars, U. Specht, J. S. Bradley and D. G. Blackmond, Langmuir, 1999, 15, 7621 CrossRef.
- (a) A. Howard, C. E. J. Mitchell and R. G. Egdell, Surf. Sci., 2002, 515, L504 CrossRef CAS; (b) A. Imre, D. L. Beke, E. Contier-Moya, I. A. Szabo and E. Gillet, Appl. Phys. A, 2000, 71, 19 CAS.
- (a) For recent examples of the preparation and the use of metal nanoparticles deposited on ordered porous solids see: C. Yang, P. Liu, Y. Ho, K. Chiu and C. Chao, Chem. Mater., 2003, 15, 275 Search PubMed; (b) J. Zhu, Z. Konya, V. F. Puntes, I. Kiricsi, C. X. Miao, J. W. Ager, A. P. Alivisatos and G. A. Somorjai, Langmuir, 2003, 19, 4396 CrossRef CAS; (c) J. He, T. Kunitake and A. Nakao, Chem. Mater, 2003, 15, 4401 CrossRef CAS; (d) T. F. Baumann and J. H. Satcher, Chem. Mater., 2003, 15, 3745 CrossRef CAS; (e) Y. Guari, C. Thieuleux, D. Mehdi, C. Reye, R. J. P. Corriu, S. Gomez-Gallardo, K. Philippot and B. Chaudret, Chem. Mater., 2003, 15, 2017 CrossRef CAS; (f) S. D. Jackson, G. D. McLellan, G. Webb, L. Conyers, B. T. Keegan, S. Matter, S. Simpson, P. B. Wells, D. A. Whan and R. Whyman, J. Catal., 1996, 162, 10 CrossRef CAS; (g) G. Jacobs, F. Ghadiali, A. Pisanu, A. Borgna, W. Alvarez and D. E. Resasco, Appl. Catal. A, 1999, 188, 79 CrossRef CAS; (h) S. Mandal, D. Roy, R. V. Chaudhari and M. Sastry, Chem. Mater., 2004, 16, 3714 CrossRef CAS; (i) C. P. Mehnert, D. W. Weaver and J. Y. Ying, J. Am. Chem. Soc., 1998, 120, 12289 CrossRef CAS.
- B. Karimi and D. Enders, Org. Lett., 2006, 8, 1237 CrossRef CAS.
- (a) For some examples of metal colloids as reservoirs for homogeneous metal species, see: S. Tasler and B. H. Lipshutz, J. Org. Chem., 2002, 64, 1190 Search PubMed; (b) I. W. Davis, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139 CrossRef CAS.
- (a) Andrew J. Butterworth, James H. Clark, Paul H. Walton and Simon J. Barlow, Chem. Commun., 1996, 1859 RSC; (b) Jacob A. Elings, Rachida Ait-Meddour, James H. Clark and Duncan J. Macquarrie, Chem. Commun., 1998, 2707 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: experimental procedures; characterization of the catalysts; schemes and figures. See DOI: 10.1039/b805824e |
| This journal is © The Royal Society of Chemistry 2009 |

