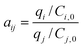Nanoporous molecular basket sorbent for NO2 and SO2 capture based on a polyethylene glycol-loaded mesoporous molecular sieve
Xiaoxing
Wang
,
Xiaoliang
Ma
,
Shuqi
Zhao
,
Bei
Wang
and
Chunshan
Song
*
Clean Fuels and Catalysis Program, EMS Energy Institute, Department of Energy & Mineral Engineering, The Pennsylvania State University, 209 Academic Projects Building, University Park, PA 16802, USA. E-mail: csong@psu.edu.; Fax: +1 814 865 3573; Tel: +1 814 863 4466
First published on 7th May 2009
Abstract
A novel type of nanoporous molecular basket sorbent (MBS) has been designed for NO2 and SO2 capture and separation from gas streams at room temperature based on polyethylene glycol (PEG)-loaded mesoporous molecular sieve SBA-15. The MBS material prepared, PEG(50)/SBA-15, is a nanoporous composite of 50 wt% PEG and SBA-15. The sorption capacity and selectivity of PEG(50)/SBA-15 have been evaluated for NO2 and SO2 removal from the simulated gas streams in a fixed-bed flow sorption system at room temperature under atmospheric pressure. It was found that the PEG(50)/SBA-15 sorbent is capable of efficiently removing >99.6% of NO2 and SO2 in gas mixtures, i.e., from 2000 to below 5 ppmv NO2 in N2 and from 500 to below 2 ppmv SO2 in N2, respectively, at ambient conditions with high capacity and selectivity. In addition, the spent sorbent can be regenerated easily and completely by heating to 100 °C. With this new approach, the present study may open a new window for developing high-performance sorbents for SO2 and NO2 removal from various gas streams.
Broader contextRemoval of environmentally harmful NO2 and SO2 from various gas streams is important for the protection of human health and the environment, for CO2 capture and sequestration (CCS) and for energy-efficient fuel cell applications. Although there are commercial technologies such as flue gas desulphurization (FGD) for SO2 removal and selective catalytic reduction (SCR) for NO2 removal, alternative and more environmentally benign methods based on solid sorbents with better energy efficiency and easier handling are highly desirable. The present work aims at a new approach based on a polymer-based solid sorbent for removing toxic NO2 and SO2 at room temperature under atmospheric pressure without using catalyst and without involving chemical reactions. We have developed a novel type of nano-porous molecular basket sorbent (MBS) based on polyethylene glycol (PEG)-loaded mesoporous molecular sieve SBA-15 for removing more than 99% of NO2 and SO2 from gas streams at room temperature and atmospheric pressure. The spent sorbent can be regenerated easily and completely by heating to 100 °C. With this new approach, the present study may open a new window for developing environmentally-friendly high-performance solid sorbents for SO2 and NO2 removal from various gas streams. |
Introduction
It is important to remove sulfuric and nitric oxides (SOx and NOx) in exhaust gases from combustion of fossil fuels, which are well known to have detrimental impacts on human health and the environment.1,2 Recently, great attention has been paid to the capture and sequestration of CO2 from flue gas, which is considered as one of the key options for greenhouse gas (GHG) control.3–7 However, many CO2 sorbents being used in commercial or emerging processes suffer from significant degradation due to SOx and NOx.7 Thus, NOx and SOx in flue gas need to be removed before CO2 sorbents are applied for CO2 capture. Even for oxy-combustion in which the CO2 separation process is not necessary, the remvoal of NOx (<50 ppm) and SO2 (<10 ppm) gases may be required before CO2 can be pressurized and passed into a pipeline for transportation and storage.8 In addition, the presence of trace NOx and SOx contaminants in air may significantly degrade the performance of the cathode catalysts in fuel cells9–12 and they must be removed from the cathode air. Consequently, the removal of NOx and SOx from various gas streams is crucial and has attracted a great deal of attention.Many flue gas desulfurization (FGD) technologies have been developed and reviewed in the literature.1,2,13 Among them, wet FGD using limestone has been applied widely.14 However, a large amount of water and further treatment of resultant wastewater are required and the process produces a large quantity of waste sludge that requires landfill disposal. For the reduction of NOx emissions, many power plants utilize selective catalytic reduction process (SCR) with ammonia or urea injection, which is considered as the most effective current technology. Within this process, re-heating of the flue gas is needed on catalysts such as vanadium–titanium. Although these mature technologies have been widely applied, alternative and more environmentally benign methods with better energy efficiency and easier handling are still highly desirable.
A SO2 scrubbing system has been recently developed by Cansolv Technologies Inc. using a regenerable amine solvent.15 The advantages of the process are the ability to remove 99% of SO2 in the gas stream, low water consumption, no solid waste and legacy landfill obligations. However, the absorption capacity of the solvent can not be recovered completely after the regeneration, which is an energy-intensive process. Therefore, developing a selective and regenerable solid sorbent with high capacity for more efficient and environmentally-friendly removal of SO2 and NO2 from various gas streams is more preferable. High-surface-area polystyrenic sorbents (350–1400 m2 g−1) for SO2 removal from flue gas has been reported by Kikkinides and Yang,16 which can remove 90% of SO2 from flue gas and showed the best relative-selectivity of ca. 20 for SO2 to CO2. Various carbon materials working both as the adsorbent for the removal of SO2 and NO2 from flue gas, and as the catalyst for the consequent conversion of the adsorbed SO2 and NO2 to the corresponding acids have been reported by Mochida and coworkers,2 where water is required in most cases.
The present work involves a new approach for developing solid sorbent for NO2 and SO2 capture based on a new concept of “molecular basket” sorbent (MBS) that has been developed in our laboratory for separation of CO2 and H2S from various gas streams (e.g., flue gas, natural gas, biogas, coal gasification gas, reformate, etc.) with high capacity and high selectivity.17–20 The MBS materials are nano-porous composites of organic polymers and inorganic matrix with pore channels in nano-meter range. Although the MBS consisting of polyethylenimine (PEI)-loaded MCM-41 can also sorb SOx and NOx from flue gas,21 it degrades due to the formation of heat-stable amine salts between amine groups in PEI and stronger acidic gases, SOx and NOx. In order to overcome this disadvantage, we recently developed a new type of MBS by selecting a different polymer, polyethylene glycol (PEG), for NO2 and SO2 removal from various gas streams at ambient conditions, and the new findings with this new approach are reported in the present paper.
Experimental
The nanoporous composite sorbent was prepared by loading 50 wt% polyethylene glycol (PEG, Mn, ca. 400, Aldrich) into the mesoporous molecular sieve SBA-15, which is denoted as PEG(50)/SBA-15. SBA-15 was synthesized using a modified procedure19,22 based on the original reports by Stucky et al.23,24 Typically, 4.0 g of PEG was dissolved in 32 g of methanol under stirring for ∼15 min, followed by adding 4.0 g of calcined SBA-15 into the above solution with continuously stirring at room temperature for 8 h. The slurry was then dried with stirring at room temperature. XRD confirmed the ordered mesoporous structure of SBA-15 before and after PEG loading, while the N2-sorption experiments showed a decrease of the surface area and pore volume with the loading of PEG. The BET surface area, pore volume, and pore diameter were 950 m2 g−1, 1.31 cm3 g−1, and 6.6 nm for SBA-15 and were 109 m2 g−1, 0.25 cm3 g−1, and 5.3 nm for PEG(50)/SBA-15, respectively, which were obtained from the physisorption of N2 at −196 °C on the Micromeritics ASAP 2020 surface area and porosity analyzer. The characterization results indicated that PEG was mainly loaded into the channels of SBA-15, rather than existing on the external surface of the molecular sieve particles, as observed over the PEI/SBA-15 samples.19,20A fixed-bed flow sorption system with an electrical furnace was assembled to evaluate the sorbent. About 1.6 g of PEG(50)/SBA-15 sorbent was filled in the sorption tube (stainless steel with O.D. of ca. 9.5 mm and I.D. of ca. 7.8 mm). Before sorption, the sorbent bed was heated to 100 °C in helium at 50 ml min−1 and held overnight to ‘clean’ the sorbent. After the bed was cooled to room temperature, a model gas containing 2000 ppmv NO2 in N2 or 500 ppmv SO2 in N2 (both supplied by GT&S Inc.) was introduced into the sorbent bed at a flow rate of 60 ml min−1 under atmospheric pressure. The pressure drop across the sorbent was about 2–5 psig, which was measured by a pressure sensor. After the sorbent was saturated, i.e., the outlet NO2 or SO2 concentration reached the initial feed concentration, the gas line was switched back to helium at a flow rate of 100 ml min−1 and the bed temperature was increased to 100 °C and held at this temperature to perform the desorption. The outlet NO2/SO2 concentration in the effluent was monitored on-line by a total nitrogen/sulfur analyzer (ANTEK 9000NS). The detection limit of the analyzer for nitrogen and sulfur was ca. 5 and 2 ppmv, respectively.
Results and discussion
Fig. 1(A) shows a typical NO2 breakthrough curve over PEG(50)/SBA-15 for a model gas containing 2000 ppmv NO2 in N2 at ambient temperature and pressure. The fresh PEG(50)/SBA-15 sorbent can effectively sorb NO2 from the gas mixture. In the first 6.8 L g−1 of treated gas, the outlet NO2 concentration was below 5 ppmv, which was the limitation for nitrogen analysis of the current analytic instrument. It means that more than 99.7% of NO2 has been removed from the gas stream at current conditions by using PEG(50)/SBA-15 sorbent. After about 6.8 L g−1 of feed gas being treated, NO2 broke through and then the sorbent was saturated quickly. The calculated sorption breakthrough and saturation capacity were 13 and 17 mg NO2 g−1, respectively, which is comparable to other adsorbents. | ||
| Fig. 1 (A) Breakthrough curve and (B) desorption curve of NO2 over the PEG(50)/SBA-15 sorbent. Conditions: (A) Sorption: W, 1.6 g; T, 25 °C; F, 60 ml min−1; gas composition, 2000 ppmv NO2 in N2; (B) Desorption: T, 100 °C; purge gas, He; F, 100 ml min−1. | ||
The desorption of the spent PEG(50)/SBA-15 after NO2 sorption was carried out at 100 °C by flowing 100 ml min−1 of He as the purge gas. The result is shown in Fig. 1(B). It clearly shows that the sorbed NO2 can be easily and quickly desorbed at 100 °C. The majority of sorbed NO2 can be removed within the first 30 min. The desorbed amount of NO2 was about 13 mg NO2 g−1, which was calculated via an integration of the desorption curve. In comparison with the saturation capacity of the fresh sorbent (17 mg NO2 g−1), the result indicates that almost all sorbed NO2 can be removed in the present desorption conditions within the experiment error.
In order to examine the regenerability of the PEG(50)/SBA-15 sorbent for NO2 removal, the NO2 sorption over the regenerated sorbent was conducted and the result is also presented in Fig. 1(A). The NO2 breakthrough curve over the regenerated sorbent was similar to the fresh one. The breakthrough and saturation capacities for the regenerated one were 15 and 18 mg NO2 g−1, respectively, slightly higher than those of the fresh one (13 and 17 mg NO2 g−1, respectively). These results indicate that the spent sorbent can be easily regenerated and the capacity can be completely recovered at mild conditions.
The sorption performance of PEG(50)/SBA-15 for SO2 removal was also evaluated. Fig. 2(A) shows the outlet SO2 concentration as a function of the treated gas volume over PEG(50)/SBA-15 using a model gas stream containing 500 ppmv SO2 at room temperature and atmospheric pressure. The fresh PEG(50)/SBA-15 can effectively sorb SO2 from the gas mixture, although the sorption temperature was much lower than those for other reported sorbents (e.g., 150–300 °C for CuO/Al2O325). Within the first 4 L g−1 of treated gas volume, the outlet SO2 concentration was below 2 ppmv, which was the detection limitation for sulfur analysis, better than the Cansolv SO2 process (down to ∼15–30 ppmv).15 It indicates that at least 99.6% of SO2 can be removed. After that, SO2 broke through and then the sorbent was saturated quickly, as shown in Fig. 2(A). The calculated SO2 sorption breakthrough and saturation capacities were about 5.4 and 7.3 mg SO2 g−1, respectively. The capacity of PEG(50)/SBA-15 is significantly higher than that of the best polystyrenic sorbent (NO-treated XAD-16) reported in the literature, which had a saturation capacity around 3 mg SO2 g−1 at 26 °C and an SO2 equilibrium concentration of 1000 ppmv in He.16
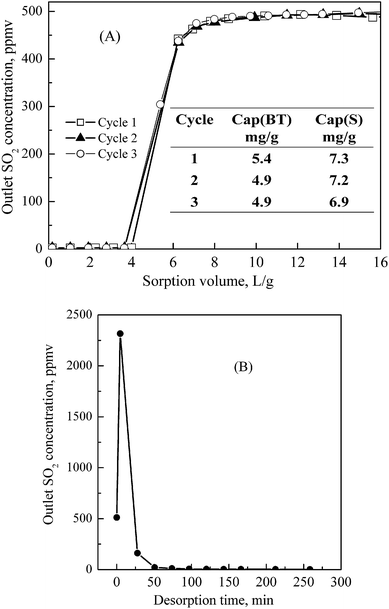 | ||
| Fig. 2 (A) Breakthrough curve and (B) desorption curve of SO2 over the PEG(50)/SBA-15 sorbent. Conditions: (A) Sorption: W, 1.6 g; T, 25 °C; F, 60 ml min−1; gas composition, 500 ppmv SO2 in N2; (B) Desorption: T, 100 °C; purge gas, He; F, 100 ml min−1. | ||
Similarly, desorption of the spent PEG(50)/SBA-15 after SO2 sorption was also conducted at 100 °C by purging 100 ml min−1 of He and the result is presented in Fig. 2(B). As expected, the sorbed SO2 can be easily and quickly desorbed. The desorbed amount of SO2 was about 6.3 mg SO2 g−1. In comparison with the saturation capacity of the fresh sorbent (7.3 mg SO2 g−1), the result indicates that almost all sorbed SO2 can be removed in the present desorption conditions within the experiment error range. In order to examine the regenerability and stability of PEG(50)/SBA-15, three sorption–desorption cycles were conducted. As shown in Fig. 2(A), these three breakthrough curves almost completely overlapped. The calculated saturation capacity for these three cycles were 7.3, 7.2 and 6.9 mg SO2 g−1, respectively. Fig. 3 shows the measured sorption capacity of PEG(50)/SBA-15 for SO2 as a function of the number of the sorption–desorption cycles. During the 6 cycles, the SO2 sorption capacity was kept at around 7.7 mg SO2 g−1 and no significant change in the SO2 sorption capacity was observed. The results indicate that the PEG(50)/SBA-15 sorbent has good regenerability and stability for SO2 removal at the conditions employed.
 | ||
| Fig. 3 The SO2 sorption capacity versus the number of the sorption–desorption cycles over PEG(50)/SBA-15 sorbent. | ||
The above results show that in addition to the high sorption capacity of PEG/SBA-15 for both NO2 and SO2, the operating temperatures and regeneration temperature of the spent PEG/SBA-15 sorbent are much lower comparing to those for many metal–oxides-based sorbents,25–29 which allow the NO2 and SO2 removal to be conducted more energy- and cost-efficiently.
Besides the high sorption capacity, high selectivity is critical for real application since CO2 co-exists with SO2 and NO2 in most cases. In order to compare the selectivities of PEG(50)/SBA-15 for SO2, NO2 and CO2, the sorption of CO2 over PEG(50)/SBA-15 sorbent was also conducted at room temperature using a model gas with 2.0 vol% CO2. Table 1 summarizes the sorption performance of PEG(50)/SBA-15 for CO2, SO2 and NO2 with different concentration levels at room temperature. It can be seen that PEG(50)/SBA-15 exhibited poor performance for CO2 capture from the gas stream with 2 vol% CO2. The saturation capacity for CO2 was only 0.05 mmol g−1, much lower than those for SO2 and NO2 (0.113 and 0.370 mmol g−1 for 0.05 vol% SO2 and 0.2 vol% NO2, respectively).
On the basis of the sorption results, the relative-selectivity αij can be calculated from the following equation:
The relative-selectivity for SO2 and NO2 to CO2 over PEG(50)/SBA-15 was as high as 90 and 74, respectively. The relative-selectivity reported for NOx to CO2 over PEI/MCM-41 was only about 2.21 Over the polystyrenic sorbent, the relative-selectivity reported for SO2 to CO2 was about 17–20.16 Accordingly, the newly developed PEG(50)/SBA-15 not only possesses better capacity and regenerability for SO2 and NO2 removal, but also has much higher selectivity for SO2 and NO2 to CO2.
It should be mentioned that the relative-selectivity of the commercial SELEXOL solvent for SO2 to CO2, which is used for gas clean-up in industry, is about 93.30SELEXOL solvent is a mixture of dimethyl polyethylene glycol ethers with average molecular weight of ca. 280. The molecular formula of dimethyl polyethylene glycol ethers and polyethylene glycol (PEG) are presented in Scheme 1. Apparently, due to similar molecular structure, PEG has similar physical and chemical properties to those of the dimethyl polyethylene glycol ethers. The very similar relative-selectivity of SELEXOL solvent and PEG(50)/SBA-15 for SO2 to CO2 suggests that the sorption of SO2 and NO2 over PEG(50)/SBA-15 is probably due to the dissolution of SO2 and NO2 in PEG which is located inside mesopore channels of SBA-15. At such high PEG loading, the mesopores of SBA-15 may work as a nano-sized container for PEG, where PEG exists as nano-sized isolated liquid droplets inside the mesopores of SBA-15. It also can explain why the PEG(50)/SBA-15 sorbent has such good regenerability for SO2 and NO2 removal. However, more detailed investigations are required to clarify the mechanism, which are underway in our lab via both experiment and molecular simulations. Interestingly, we also found that the presence of moisture has a positive effect on the removal of both SO2 and NO2 over the PEG(50)/SBA-15 in the ongoing study; these results along with the study on the SO2/NO2 sorption mechanism will be reported in the future.
 | ||
| Scheme 1 Molecular formula of polyethylene glycol (PEG) and dimethyl polyethylene glycol ethers. | ||
Conclusions
A new type of regenerable SO2/NO2 sorbent has been designed and prepared based on the molecular basket sorbent concept by loading polyethylene glycol (PEG) into mesoporous molecular sieve SBA-15. The nanoporous composite sorbent can successfully and effectively remove more than 99% of NO2 and SO2 from simulated gases at ambient conditions.The saturation capacity is ca. 17 mg NO2 g−1 at an equilibrium NO2 concentration of 2000 ppmv, and ca. 7 mg SO2 g−1 at an equilibrium SO2 concentration of 500 ppmv under atmospheric pressure with high relative-selectivity of 74 and 90, respectively for NO2 and SO2 to CO2. The spent PEG(50)/SBA-15 can be regenerated easily and quickly at 100 °C.
The present approach may provide a new way to develop high-performance, energy-efficient sorbents for SO2 and NO2 removal from various gas streams at ambient conditions.
Acknowledgements
This work is supported in part by the US Office of Naval Research (ONR) through the Grant #N00014-08-1-0123, and by the US Department of Energy, National Energy Technology Laboratory through DOE Grant DE-FC26-08NT0004396. We wish to thank Donald Hoffman and John Heinzel of US ONR and Wayne Surdoval and Travis Schultz of US DOE for their support and encouragement.References
- R. K. Srivastava, W. Jozewicz and C. Singer, Environ. Prog., 2001, 20, 219 CrossRef CAS.
- I. Mochida, Y. Korai, M. Shirahamaa, S. Kawano, T. Hada, Y. Seo, M. Yoshikawa and A. Yasutake, Carbon, 2000, 38, 227 CrossRef CAS.
- C. Azar and H. Rodhe, Science, 1997, 276, 1818 CrossRef CAS.
- E. A. Parson and D. W. Keith, Science, 1998, 282, 1053 CrossRef CAS.
- A. B. Rao and E. S. Rubin, Environ. Sci. Technol., 2002, 36, 4467 CrossRef CAS.
- IPCC, Special Report on Carbon Dioxide Capture and Storage. Intergovernmental Panel on Clime Change: Geneva, Switzerland, 2005 Search PubMed.
- DOE, Carbon Sequestration Technology Roadmap and Program Plan, 2007 Search PubMed.
- G. Pipitone and O. Bolland, Environ. Prog. Sustain. Energy, 2008, 28, 20 Search PubMed.
- R. Mohtadi, W.-K. Lee and J. W. Van Zee, J. Power Sources, 2004, 138, 216 CrossRef CAS.
- D. J. Yang, J. X. Ma, L. Xu, M. Z. Wu and H. J. Wang, Electrochim. Acta, 2006, 51, 4039 CrossRef CAS.
- F. Jing, M. Hou, W. Shi, J. Fu, H. Yu, P. Ming and B. Yi, J. Power Sources, 2007, 166, 172 CrossRef CAS.
- Y. Nagahara, S. Sugawara and K. Shinohara, J. Power Sources, 2008, 182, 422 CrossRef CAS.
- R. K. Srivastava and W. Jozewicz, J. Air Waste Manage. Assoc., 2001, 51, 1676 CAS.
- X. Ma, T. Kaneko, T. Tashimo, T. Yoshida and K. Kato, Chem. Eng. Sci., 2000, 49, 4643 CrossRef.
- R. Birnbaum, A Novel SO2 Scrubbing Process for Industrial SO2 Emission Control. Alberta NOx and SOx Control Technologies Symposium, Edmonton, Alberta, April 9, 2008 Search PubMed.
- E. S. Kikkinides and R. T. Yang, Ind. Eng. Chem. Res., 1993, 32, 2365 CrossRef CAS.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Microporous Mesoporous Mater., 2003, 62, 29 CrossRef CAS.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Energy Fuels, 2002, 16, 1463 CrossRef CAS.
- X. X. Wang, X. L. Ma, L. Sun and C. S. Song, Green Chem., 2007, 9, 695 RSC.
- X. X. Wang, X. L. Ma, X. C. Xu, L. Sun and C. S. Song, Top. Catal., 2008, 49, 108 CrossRef CAS.
- X. C. Xu, C. S. Song, B. G. Miller and A. W. Scaroni, Fuel Process. Technol., 2005, 86, 1457 CrossRef CAS.
- X. X. Wang, Q. H. Zhang, S. F. Yang and Y. Wang, J. Phys. Chem. B, 2005, 109, 23500 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- L. Li and D. L. King, Ind. Eng. Chem. Res., 2005, 44, 168 CrossRef CAS.
- X. Xing, Z. Liu and J. Yang, Fuel, 2008, 87, 1705 CrossRef CAS.
- L. Limousy, H. Mahzoul, J. F. Brilhac, P. Gilot, F. Garin and G. Marie, Appl. Catal., B, 2003, 42, 237 CrossRef CAS.
- P. Zhang, H. Wanko and J. Ulrich, Chem. Eng. Technol., 2007, 30, 635 CrossRef CAS.
- G. Centi and S. Perathoner, Appl. Catal., B, 2007, 70, 172 CrossRef CAS.
- D. J. Kubek, E. Polla, F. P. Wilcher, Purification and Recovery Options for Gasification, UOP Report, UOP2715,200PPS1K, Des Plaines, IL, 2000 Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |