New liquid absorbents for the removal of CO2 from gas mixtures†
Dulce M.
Muñoz
*a,
Ana Filipa
Portugal
b,
Angel E.
Lozano
a,
José G.
de la Campa
a and
Javier
de Abajo
a
aInstituto de Ciencia y Tecnología de Polímeros, C.S.I.C, Juan de la Cierva 3, E28006, Madrid, Spain. E-mail: dulcem@ictp.csic.es; Fax: +34-915644853; Tel: +34-915622900
bLEPAE, Departamento de Engenharia Química, Faculdade de Engenharia, Universidade do Porto, Rua Roberto Frias, 4200-465, Porto, Portugal
First published on 14th May 2009
Abstract
New liquid absorbents (LA) consisting of water solutions of CO2-complexing agents have been developed and tested in an experimental lab-scale to be used in CO2 separation from gas mixtures. The results here presented concern the preparation of these amino acid based new liquid absorbents and the studies carried out to determine their performance as CO2 scrubbers. The novel systems show good performance in terms of sorption and stability compared to the standard alkanolamines. Moreover, the experimental results have shown that among the studied carriers, arginine and ornithine are the natural amino acids with greater affinity towards CO2 and that some of the here synthesized amino acids show outstanding absorption capacities, superior to MEA or any other amino acid tested. The developed materials have a direct use as mobile carrier membranes for facilitated transport and present a great potential for application in gas separation processes. Computational (DFT) and spectroscopic (1H and 13C-NMR) methods have been applied to make clear the mechanism of carbamate formation from the amine group of amino acids and CO2.
Broader contextEnvironmental concerns, such as global climate change, are now one of the most important and challenging environmental issues facing the world community and have motivated intensive research on CO2 capture and sequestration. Carbon dioxide is currently responsible for over 60% of the enhanced greenhouse effect. A wide range of technologies currently exist for separation and capture of CO2 from gas streams. Absorption with amine-based absorbents (monoethanolamine, MEA, or diethanolamine, DEA) is the most common technology for CO2 removal today. It is a process with considerable inherent problems. The processes require large investment costs and high energy consumption, and the absorbents in use today are not stable and form degradation products that need to be handled. Novel technologies based on membrane absorbents contactors which allow to selectively remove CO2 from gas streams have recently gained a major interest. Membrane contactors consist of special hollow fiber modules where the gas flows on one side of the membrane and a liquid absorbent flows on the other without phase dispersion, thus offering numerous advantages such as operational flexibility, high volumetric mass transfer rates and easy linear scale up. The new developed liquid absorbents based on amino acids here presented represent an interesting alternative to conventional methods and fulfil the requirements of cyclic capacity and high reaction/absorption rate for CO2, as well as high chemical stability, low vapour pressure and bio-compatibility. |
Introduction
Capture of carbon dioxide (CO2) by various techniques has received great interest in recent years.1,2 The main CO2 separation technologies from gas mixtures are currently: adsorption,3,4 absorption,5,6 cryogenic methods7,8 and separation with membranes.9,10 The first three methods are traditional and present some limitations such as elevated energy consumption and therefore high cost.11Nevertheless, there have recently appeared some novel technologies based on membrane absorbents contactors12,13 which allow to selectively remove CO2 from gas streams. Membrane contactors consist of special hollow fiber modules where the gas flows on one side of the membrane and a liquid absorbent (LA) flows on the other without phase dispersion, thus, offering numerous advantages such as operational flexibility, high volumetric mass transfer rates and easy linear scale up. The membrane itself does not perform the separation, but the components in the solution.
The commonly used absorbents for CO2 removal from gas mixtures in industry are aqueous solutions of alkanolamines such as monoethanolamine (MEA) and diethanolamine (DEA). The gas is contacted with the amine solution, which preferentially absorbs the CO2. The amine solution is then heated and almost pure CO2 is released from the stripper. However, the use of amines has some disadvantages,14 for example MEA and DEA are not very stable and under the process conditions some of these amines suffer decomposition, what results in a lowering of the scrubbing efficiency, increase of viscosity and excessive foaming. Also, during its handling and the scrubbing process, they can be released to the atmosphere as contaminants. Besides, the processes are very energy-intensive and too expensive for most applications today. Consequently, there is a need to find a process which fits the market demands, what involves finding an absorbent that not only presents low vapour pressure, high thermal stability and high absorption rates, but which is not susceptible to degrade under industrial processes’ conditions.
Also, CO2 is continuously released in hermetically closed travel crafts (submarines, spacecraft, etc.) by its occupants. The technology nowadays used in these systems15–17 is very similar to the one used in anaesthesia for removing carbon dioxide. This gas is continuously generated by the patient and is traditionally removed from the anaesthetic systems using soda lime packed in a canister.18 This procedure presents some problems, namely: (i) soda lime reacts with some anaesthetic additives, the halogenated anaesthetics, producing by-products that are potentially harmful for the patients; (ii) the canisters are hospital solid waste, dangerous and expensive to treat and (iii) the soda lime–CO2 reaction produces water (which condenses in the tubing) and heat (contributing to the rising of the system's temperature above the desirable patient-comfort values). Presently, there is no on-line system available for continuous removal of carbon dioxide and nitrogen from anaesthetic circuits, therefore, such technology would be a significant step for the development of anaesthesia. Membrane contactors present also an alternative to this problem and can serve to remove carbon dioxide from such anaesthetics closed systems, where energy-efficiency, bio-compatibility and environmental safety are essential. The absorbents have to be highly selective, posses a high absorption capacity, present as low cost as possible and not be harmful to the environment.
Standard absorption technologies for trapping of CO2 and acid gases in general using primary amines and amino alcohols as efficient sorption media are limited for this purpose because they do not fit the requirements of regeneration, bio-compatibility and sterilization temperature imposed by the intended applications. Thus, a more complex system has to be developed. Ionic liquids (low-temperature molten salts) have been shown to be good CO2 scrubbing agents; they can facilitate the sequestration of gases without loss of the capture agent or solvent into the gas stream. The lack of vapour pressure due to the Coulombic attraction between the ions of these liquids makes them useful for gas processing.19,20 Moreover, some amino acid salts solutions are considered a promising alternative to the conventional absorbents mentioned above, where a positive balance between water solubility, intrinsic basicity, thermal stability, gas recovery, selectivity, and availability (processing and price) is sought. Over recent years, only a few examples using amino acids21–23 as CO2 scrubbers have appeared. In this regard, absorption liquids based on mixtures of amino acids and amino acid salts, CORAL®,24 have been developed by TNO for CO2 recovery. Thus, due to the growing concern on this area, there is an interest to extend this application and to find alternative absorbents, particularly based on amino acids.25
The aim of this work has been to find an amine replacement with an improved CO2 absorption capacity. In our case, an aqueous solution of an amino acid salt will act as the carrier solution in the membrane contactor system. Herein, we report a preliminary study on the performance of new liquid absorbents based on natural and new synthetic amino acids of diverse structures (Table 1) and the results of their performances, in an experimental lab-scale, as CO2 absorbents.
Also, we describe here the full synthesis of these new non-natural amino acids through various synthetic methods.
Experimental
Materials
Amino acids and other chemicals used in the preparation of absorbents were of analytical grade, purchased form Aldrich Chemical Co. and Lancaster, and were used without further purification.Synthesis of amino acid absorbents
The novel amino acids were prepared following various methods of synthesis (a–f, Scheme S1).†Spectroscopic data for the three amino acids are as follows:
1a:1H-NMR (D2O, 300 MHz, ppm): 8.05 (d, 2H, J = 4.9 Hz, 2H-6); 6.50 (t, 1H, J = 4.9 Hz, H-7); 3.66 (s, 2H, 2H-2).
13C-NMR (D2O, 75 MHz, ppm): 178.4 (C-1); 161.3 (C-4), 158.5 (C-7), 111.0 (C-6), 45.0 (C-2).
MS (ES): 154.1 (MH+, 100).
1b:1H-NMR (D2O, 300 MHz, ppm): 8.18 (d, 2H, J = 5.0 Hz, 2H-7); 6.62 (t, 1H, J = 5.0 Hz, H-8); 3.44 (t, 2H, J = 6.9 Hz, 2H-3), 2.43 (t, 2H, J = 6.9 Hz, 2H-2).
13C-NMR (D2O, 75 MHz, ppm): 181.2 (C-1); 161.2 (C-5), 158.6 (C-7), 110.9 (C-6), 38.5 (C-2), 37.1 (C-3).
1c:1H-NMR (D2O, 300 MHz, ppm): 8.22 (d, 2H, J = 5.0 Hz, 2H-8); 6.64 (t, 1H, J = 5.0 Hz, H-9); 3.26 (t, 2H, J = 7.0 Hz, 2H-4); 2.21 (t, 2H, J = 7.6 Hz, 2H-2); 1.80 (m, 2H, 2H-3).
13C-NMR (D2O, 75 MHz, ppm): 183.3 (C-1); 161.5 (C-6), 158.7 (C-8), 110.7 (C-9), 41.1 (C-4), 35.2 (C-2), 25.7 (C-3).
MS (ES): 182.1 (MH+, 100).
1H-NMR (D2O, 300 MHz, ppm): 3.84 (dd, 1H, J = 3.8 and 11.1 Hz, 1H-2); 3.70 (dd, 1H, J = 3.8 and 13.8 Hz, 1H-3), 3.57–3.43 (m, 2H), 3.31–3.13 (m, 3H).
13C-NMR (D2O, 75 MHz, ppm): 171.0 (C-1); 55.7 (C-2), 43.9, 40.9 and 40.8 (C-3, C-5 and C-6).
1H-NMR (D2O, 300 MHz, ppm): 2.96 (s, 2H, 2H-2); 2.77 (t, 4H, J = 5.1 Hz, 4H-4); 2.47 (m, 4H, 4H-5).
13C-NMR (D2O, 75 MHz, ppm): 177.8 (C-1); 62.1 (C-2), 52.8 (C-4), 44.1 (C-5).
MS (ES): 145.0 (MH+, 100).
1H-NMR (DMSO, 300 MHz, ppm): 7.50 (brs, 1H, CO2H); 3.29 (q, 1H, J = 7.1 Hz, 1H-2); 3.05 (m, 2H, 2H-5), 2.79 (m, 2H, 2H-4), 1.17 (d, 3H, J = 7.1 Hz, CH3).
13C-NMR (DMSO, 75 MHz, ppm): 173.3 (C-1); 61.2 (C-2), 45.3, 43.2 and 42.3 (C-4 and C-5), 14.1 (CH3).
MS (ES): 231.1 (25), 159.1 (MH+, 100).
1H-NMR (D2O, 300 MHz, ppm): 2.99 (t, 1H, J = 6.9 Hz); 2.84 (s, 2H, 2H-2); 2.72 (t, 1H, J = 7.2 Hz); 2.39–2.30 (m, 10H).
13C-NMR (D2O, 75 MHz, ppm): 177.5 (C-1), 61.6 (C-2), 57.4 (C-11), 51.9 (C-5), 38.0 (C-3), 36.7 (C-2).
MS (ES): 188.1 (MH+, 100).
1H-NMR (D2O, 300 MHz, ppm): 3.84–346 (m, 5H, 1H-8, 1H-6, 1H-9 and 2H-3); 3.26 (dd, 1H, J = 6.6 and 20 Hz, 1H-5); 3.24 (dd, 1H, J = 12.9 and 20 Hz, 1H-5); 3.07 (dt, 1H, J = 12.9 and 1.7 Hz); 1.36 (d, 3H, J = 6.3 Hz, 3H-10 or 3H-11); 1.26 (d, 3H, J = 6.3 Hz, 3H-10 or 3H-11).
13C-NMR (D2O, 75 MHz, ppm): 173.9 (C-1), 56.7 (C-5), 53.3 (C-9), 48.9 (C-6), 48.7 (C-8), 45.6 (C-3), 28.9 (C-2), 15.1 (C-10 or C-11), 13.7 (C-10 or C-11).
MS (ES): 259.1 (100), 187.1 (MH+, 82).
Preparation of liquid absorbents (LA)
The liquid absorbents were prepared in 1 M concentration dissolving the natural and synthetic amino acids in deionized water and using a 1 : 1 alkali salt : amino acid ratio. Potassium hydroxide (KOH) was used as an alkali. Aqueous MEA solutions used for comparison were prepared by dissolving pure amine in deionized water.Absorption experiments
Absorption experiments were performed at 20 °C in a set-up basically composed of two connected tanks with well defined volumes (one tank available only for the gas and the other one containing the absorbent solution). Both tanks were submerged in a thermostatic bath (Fig. S1).† Initially, vacuum was applied to the system in order to remove all the gases present in the solution. The pressure recorded at this initial moment is the vapour pressure of the solution (Pvapor). After that, the valve between the two tanks was closed and the gas tank (tank A, Fig. S1)† was filled with carbon dioxide up to a certain pressure (PA). The experiment was started by opening the valve that separates the two tanks and leaving the system to reach the equilibrium. During the whole experiment, the absorbent solution was vigorously stirred with a magnetic bar. Finally, when the pressure stabilized, the value was recorded (Pfinal). The amount of gas absorbed (nabs) was calculated applying the ideal gases law to the system, as follows: | (1.1) |
 | (1.2) |
| nabs = nA − nfinal | (1.3) |
Absorption was also monitored by spectroscopic methods. 13C-NMR spectra were recorded at 20 °C on a Varian XL spectrometer at 75 MHz. D2O was used as solvent. The assays were performed in a glass tube (1 cm × 10 cm) having a porous plate for a better gas diffusion and containing a 1 M solution of the absorbents in D2O in both the absence and presence of equimolecular quantities of base (KOH). Tubes containing the absorbent solution were flushed with CO2 gas at 2 L min−1 flow and the spectra recorded at different times of gas exposure, to compare with the corresponding CO2 free samples.
Theoretical calculations
Semiempirical calculations were performed using the original parameters of the program AM1 based on the restricted Hartree–Fock (RHF) method, included in MOPAC version 6.0,26 using as graphics interface and data analysis the Cerius2 program.27 These semiempirical methods are commonly accepted to allow a good description of the lone-pair/lone-pair repulsion in several compounds. The MOPAC program ran on a Silicon Graphics Origin 300 workstation.Density functional theory (DFT) calculations were carried out with the Gaussian 03 package28 using as data and graphical interface the Cerius2 program. Through the Z-matrix input data from an AM1 calculation, the geometry and the total electronic energy were calculated by choosing the RHF method and the B3LYP/6-31G* basis set. Geometries were optimized in internal coordinates. For AM1 calculations, the optimization was stopped when Herbert or Peter tests were satisfied in the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method29 after placing the CO2 molecule. The PRECISE option was applied during the optimization process with the gradient norm set to 0.01. The calculations were carried out with full geometry optimization (bond lengths, bond angles and dihedral angles) without any assumption of symmetry. The binding energies have been corrected for the basis set superposition error (BSSE) by using the Counterpoise method.30 This method estimates the BSSE as the difference between the energies of the isolated monomers and the energies of the monomers with the total basis of the aggregate.
Results and discussion
The selection of the amino acids object of study was based on their chemical and physical properties (aqueous solubility, basicity and stability) and, thus, natural amino acids bearing a variety of functional groups in their structure (aliphatic, polar and charged groups) were studied. An important factor to be also considered was the basic character of the amino acid; so that the pKa values of the amino groups present in the amino acids (Table 2) were taken into account.In addition, absorbents based on hindered amines or cycloalkylamines, as well as amine blends, have been used for the recovery of acidic gases. In the present study, we decided to prepare a series of synthetic amino acids by chemical modification of natural amino acids and piperazine derivatives. Some advantages of this type of reactants are: an adequate basicity for a fast reaction with CO2, high N/C ratio, a high concentration of amino groups, and low volatility.
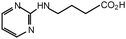
The novel compounds based on chemical modification of glycine, β-aminopropionic acid (BAPA), and γ-aminobutyric acid (GABA) were prepared by direct coupling of these natural amino acids with 2-chloropyrimidine (a, Scheme S1),† giving rise to the structures 1a–c (Table 1).
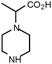
Also, the synthetic derivatives of piperazine were prepared either by direct reduction of the commercially available pyrazine-2-carboxylic acid giving rise to 2 (b, Scheme S1),† or by coupling of piperazine with 2-bromoacetic acid (c, Scheme S1),† and 2-bromopropionic acid (d, Scheme S1),† to give compounds 3 and 4, respectively. Other amino acids containing piperazine (5 and 6) were prepared by direct reaction of 2-bromoacetic acid and 1-(2-aminoethyl)piperazine (e, Scheme S1),† or by reaction of 2,5-dimethylpiperazine and methyl acrylate, followed by saponification to yield the free acid 6 (f, Scheme S1).†
The performance of each liquid absorbent containing the above synthetic compounds was investigated and compared to those containing natural amino acids.
Amino acids dissolved in water exist preferentially as a zwitterionic form, which means that the amino group is protonated and hence not able to react with CO2. Therefore, an equimolecular amount of base was added to free the amino group in the aqueous solution.
After the absorption equilibrium was reached, desorption of CO2 was initially performed at 20 °C just applying vacuum to the system. However, total CO2 removal was not complete. Thus, the solution was heated to 85 °C, whilst a nitrogen stream passed through the solution, and cooled again to 20 °C and the absorption equilibrium measured once more. After this treatment, it could be verified that the second absorption capacity was highly improved, even if it did not achieve the initial values; which indicated that applying the thermal treatment, the absorbent regeneration was more efficient (over 76% in average). When the regeneration at 85 °C was repeated and another equilibrium absorption point at 20 °C was determined, the same absorption capacity value was achieved. The absorbent loses capacity after the first absorption but it remains constant after the second one. Table 3 shows the molar ratio of absorbed CO2 per mol of amino acid at the equilibrium.
| Absorbent | First cycle | Second cycle |
|---|---|---|
| a All amino acid solutions (1.0M) were treated with an equimolar amount of KOH. b Ornithine HCl solution was treated with a 2.0 M KOH solution. | ||
| GABA | 0.78 | 0.43 |
| BAPA | 0.86 | 0.45 |
| Proline | 0.89 | 0.45 |
| Glycine | 0.70 | 0.50 |
| Taurine | 0.78 | 0.52 |
| Threonine | 0.73 | 0.58 |
| Serine | 0.70 | 0.65 |
| Histidine | 1.00 | 0.77 |
| Arginine | 1.70 | 0.80 |
| Ornithine HClb | 1.65 | 0.90 |
| MEA | 0.88 | 0.60 |
The combination of CO2 with the absorbents gives rise to a carbamate following the sequence described in Scheme 1. Addition of a base abstracts a proton from the zwitterionic form of the amino acid (II-A), releasing the free amino group (III-A). This amino group interacts with CO2 to form a complex (IV-A) by an equilibrium reaction. Afterwards, a basic group is needed to remove a proton to give the final carbamate (V-A), this later step being the key one. Thus, if a base is not used in this step, an amino group of the amino acid has to take this role to give a carbamate of ammonium derivative. This is the case for typical amino acids containing aliphatic groups when just one mol of base (used to release the proton of the compound II-A) is added; and hence the theoretical CO2 load is 0.5 mol CO2 per mol amine. Accordingly, the absorption values obtained for the tested amino acids containing only aliphatic groups: glycine (0.5 mol CO2 per mol amino acid), proline (0.45 mol CO2 per mol amino acid), BAPA (0.45 mol CO2 per mol amino acid), GABA (0.43 mol CO2 per mol amino acid) and taurine (0.5 mol CO2 per mol amino acid) are equal or lower than 0.5 mol CO2 per mol amino acid. From the results obtained with the amino acids tested, which contain different aliphatic chemical structure, it seems that there is no direct influence of the amino acids chain length on the absorption capacity.
 | ||
| Scheme 1 | ||
Furthermore, the absorption values listed in Table 3 fairly agree with data previously reported.32–34
Screening studies of natural occurring amino acids as potential CO2 absorbents showed the high affinity to CO2 of amino acids containing basic groups in their side chain [amine (ornithine), guanidinium (arginine) and imidazonium (histidine) groups].
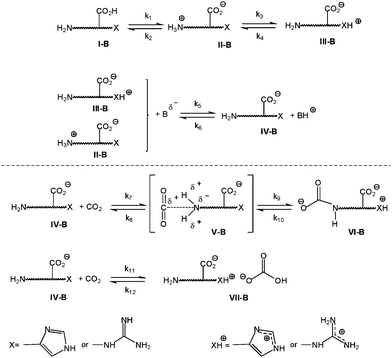
So, ornithine and arginine showed the best absorption capacities (0.9 and 0.8 mol CO2 per mol amino acid in the second cycle, respectively). For arginine and ornithine (Scheme 2), their lateral chains have strong basic character as revealed by their pKa values (12.48 and 10.53, respectively, Table 2) and, therefore, the abstraction of the hydrogen from the CO2–amino acid complex (V-B) to give the carbamate moiety (VI-B) is easily accomplished by the lateral guanidinium or amine group. Among the many organic bases known to have some promoting CO2 fixation ability, amidines and guanidines are the ones with superior effectiveness.35
 | ||
| Scheme 2 | ||
Depending on the pKa value of the lateral chain groups, the equilibrium constant rate (k3/k4), and the pH of the system, there will be a relationship between the II-B and III-B species determined by the Henderson–Hasselbalch equation:
 | (2.1) |
Thus, when the value of pKa is very high, as happens with the guanidinium group in arginine (12.48), III will be the predominant species at the solution working pH. Moreover, the above-mentioned guanidinium group is able to efficiently remove the proton of the CO2–amino acid complex (IV-B) to give the carbamate (V-B) in high yield. When the pKa value is not so high (10.53 for ornithine), III-B will also be the predominant species. However, k7 will be lower and the carbamate formation will be accomplished in a lower yield.
Other amino acids containing a lateral chain with basic character (imidazonium) is histidine (pKaR = 6.20), which showed very good absorption value (0.77 mol CO2 per mol amino acid). Because the imidazole group has a pKa lower than that of the amino group, the initial system at pH > 8 is predominantly composed of II-B species (Scheme 2). Therefore, the proton abstraction, necessary to form the carbamate, is not especially favored and, consequently, the k9 kinetic constant will be lower than in arginine and ornithine. However, the experimental CO2 load values of arginine are close to that of histidine (Table 3).
A second possible mechanism for the absorption process when a basic unit is part of the absorbent is the proposed by Jessop and co-workers.35–39 They consider that on exposure to CO2, amidines and guanidines mixed with water will form amidinium and guanidinium bicarbonate salts. Therefore, compound IV-B will interact with CO2, with the consequent formation of the bicarbonate salt [AAH+][HCO3−] (VI-B).
Since basicity alone does not explain the behavior of histidine, it could be explained by the nucleophilic character of the heterocyclic moiety, which is able to interact with a Lewis acid to give complexes type VI-B (this result is similar to that obtained for MEA, as it will be commented below). The combination of these two processes does determine the high load obtained for histidine. Obviously, the kinetic process has to be very different from arginine and ornithine, and a deeper study of apparent constant rates is being carried out.
It should be noted that not only the amino groups can interact with CO2 but that other groups present in the system are also capable. So, as the pH of the solution is higher than 7, hydroxide ions react with CO2 to give hydrogen carbonate ions eqn (3.1) which can react again with hydroxide ions as follows eqn (3.2):
| CO2 + OH− ⇆ HCO3− | (3.1) |
| HCO3− + OH− ⇆ CO32− + H2O | (3.2) |
| CO2 + CO32− + H2O ⇆ 2HCO3− | (3.3) |
Thus, two hydroxide ions react very fast with one CO2 molecule to give a system where only carbonate ions are theoretically present at the end of the process. On the other hand, carbonate ions react with CO2 molecules (eqn (3.3)) to give hydrogen carbonate species with a much lower reaction rate.
All this can be tested by measuring the pH of the solutions before and after the absorption process (Table 4). In all cases, the initial pH values were basic, and after the first absorption and regeneration they diminished to similar values.
| Amino acid solution | Before 1st absorption | Before 2nd absorption | Final |
|---|---|---|---|
| a All amino acid solutions (1.0 M) were treated with an equimolar amount of KOH. b Ornithine HCl solution was treated with an extra equivalent of KOH. | |||
| Glycine | 10.96 | 10.54 | 8.25 |
| Taurine | 12.77 | 10.57 | 7.81 |
| Proline | 12.43 | 10.80 | 8.48 |
| BAPA | 11.85 | 10.32 | 7.85 |
| Ornithine HClb | 12.20 | 10.77 | 7.82 |
| GABA | 12.03 | 10.73 | 7.94 |
| Arginine | 13.77 | 10.43 | 7.84 |
| Serine | — | 10.52 | 7.57 |
| Threonine | 10.41 | 10.19 | 7.41 |
| Histidine | — | 10.44 | 7.65 |
Consequently, in the first absorption, CO2 is transformed into carbamate (by interaction with –NH2) and into carbonate and hydrogen carbonate ions (because of the high pH of the system), being the ratio CO32−/HCO3− a function of the pH. After the first desorption, the carbamate formation is reversed but some of the carbonate and hydrogen carbonate ions remain in the system and, consequently, the CO2 removal is not completely achieved. Therefore, in the second absorption, only the CO2 that can become a carbamate or that can react with HCO3− (eqn (3.3)) is absorbed. This means that second and subsequent absorptions are lower than the first one.
In an attempt to establish the new absorbents competence, the absorption capacity of MEA was also tested under the same conditions and included here for comparison. This widely used scrubber agent performed slightly less efficiently (0.60 mol CO2 per mol MEA) than many of the LA here studied (ornithine, arginine, histidine and serine). The value is higher than that of taurine (0.5 mol CO2 per mol), indicating that the hydroxyl group of MEA should be acting as a weak base or, more plausibly, interacting with the CO2 molecules and forming complexes comparable to the V-A and VI-B species (Scheme 1 and 2). This behaviour is similar to that obtained for histidine, which has been commented above.
With the purpose of enhancing the absorption capacity through the design and preparation of synthetic amino acids, we have also explored the absorption capacity of synthetic amino acids prepared by us as described previously. On one side, the multifunctional compounds prepared were based on natural amino acids chemically modified with a pyrimidine ring so that secondary amines of different chain length (1a–c) were obtained. As shown in Table 5, the chemical modification of BAPA and GABA generated compounds with higher absorption capacities, while modification of glycine yielded a less productive absorbent.
| Absorbent | First cycle | Second cycle |
|---|---|---|
| a All amino acid solutions (1.0 M) were treated with an equimolar amount of KOH. | ||
| 1a | 1.08 | 0.26 |
| 1b | 0.91 | 0.53 |
| 1c | 0.80 | 0.59 |
| 2 | 1.20 | 0.75 |
| 3 | 0.82 | 0.60 |
| 4 | 0.41 | 0.23 |
| 5 | 1.86 | 1.11 |
| 6 | 2.23 | 1.13 |
| MEA | 0.88 | 0.60 |
Besides, new absorbents based on piperazine (2, 3, 4, 5 and 6) and containing different architectures have been prepared. The choice was based on the fact that piperazine has been previously used for this application and it is known that secondary amines, and also hindered amines with substituents next to the amino group, can easily form the carbamate species and also release the CO2 upon heating. Their absorption measurements are detailed in Table 5.
Although most of these new absorbents presented similar absorption capacities to those showed by the natural amino acids (0.23–0.75 mol CO2 per mol amino acid), two absorbents (5 and 6) showed exceptional performances, with absorption capacities (1.11 and 1.13 mol CO2 per mol amino acid) much higher than any of the other absorbents tested. These molecules have several amino groups as well as a carboxylic acid in their structure.
In conclusion, the LA based on arginine and ornithine solutions can absorb CO2 from simulated flue gas effectively with a high absorption capacity. The molar uptake of CO2 per mole of these amino acids approaches the value of 1 mol of CO2 per mol of amino acid. This CO2 uptake is superior to that of a standard sequestering amine such as MEA. Interestingly, synthetic amino acids based on piperazine containing an alkyl chain presented molar uptakes superior to 1 mol of CO2 per mol of amino acid. These findings make these amino acids ideal materials to be used in LA for CO2 recovery.
The process of CO2 uptake is reversible, being the CO2 released from the LA solution upon heating (85 °C). Thus, the recovered liquid absorbent was repeatedly recycled for CO2 uptake with no significant loss of efficiency.
Spectroscopic analysis of the carbamate formation
The sequestration of CO2 by an amino group via its fixation as a carbamate has been borne out by comparison of the 13C-NMR spectra of the gas-free and gas treated liquid absorbent. Initially, a 1 M solution of arginine in D2O was thoroughly bubbled with a vigorous nitrogen flux and the spectra were recorded. Afterwards, the solution was flushed for 30 min with CO2 in the absence of base, at room temperature, and new spectra were recorded. For the 13C-NMR spectra of the untreated and CO2-treated arginine see (a) and (b) in Fig. S2.†Prior to CO2 exposure, carbon signals of arginine appeared at 24.5, 31.9, 41.2, 55.7, 157.0 and 183.3 ppm. As previously reported for similar systems;19 after CO2 bubbling, signals are “doubled” and new signals (24.8, 30.1, 41.1, 55.7, 164.0 and 181.5 ppm) appeared in addition to the previous ones, corresponding to one half of the amine becoming a carbamate. Also, two additional signals corresponding to the fluxed CO2 and carbamate carbonyl carbon are observed at 160.6 and 164.0 ppm, respectively. Arginine was also CO2-treated in the presence of base, but no differences in the spectra were observed when compared with those obtained without base. This indicated that the chemical interaction of arginine and CO2 occurs in the absence of base due to the intrinsic basicity of the guanidinium group, which possesses a pKaR value of 12.48.
In conclusion, NMR spectroscopy is a suitable tool to make a first evaluation of the ability of the selected LA to bind CO2.
Theoretical calculations
A theoretical study of reactivity was performed using methods of computational chemistry to get information of the affinity towards CO2 of the different functional groups present in the tested natural amino acids.First, the molecules with amine and hydroxy groups were modelled by the AM1 semiempirical method. A CO2 molecule was placed at 1.9 Å, perpendicular to the nitrogen–carbon or oxygen–carbon bond, as depicted in Fig. 1a.
 | ||
| Fig. 1 AM1 semiempirical modelling before (a) and after complex formation (b). | ||
Then, the system was minimized, obtaining geometries (Fig. 1b) that were the starting geometries for DFT calculations. The main geometrical parameters obtained for these systems than can account for the interaction between carbon dioxide and amines are the distance C(CO2)–N and the angle O–C–O of carbon dioxide. This angle ranges from 178° to 172°, depending on the amine, in DFT calculations and takes values around 150° for AM1 calculations, denoting an electronic transfer from the sp3 lone pair nitrogen orbital to the electrophile carbon dioxide, much larger in the low-level method. The interatomic distances between the nitrogen of the amine and the carbon atom of CO2 obtained by AM1 were always shorter than those obtained from DFT calculations. For sake of simplicity and because DFT calculations are very CPU-time consuming, we have only studied in the majority of cases the individual interaction of each amino group with a single CO2 molecule.
The most important parameter, which gives a clear indication of complex formation, is the interaction energy. This energy, formation enthalpy (ΔH), in semiempirical methodology and electronic energy, ΔEelc, in DFT calculations, was calculated as the difference between total energy of complex CO2–amino acid and the sum of the individual energies of CO2 and the amino acid (Fig. 2). The electronic energy values obtained by DFT, were calculated for each amino (1 to 4) and hydroxy group in the amino acid, and are shown in Table S1.† These values ranged from 0.26 to 4.72 Kcal mol−1 (values similar to medium-strong interactions, such as hydrogen bonds are) depending on the considered amine or hydroxyl. These results showed that arginine (−4.72 Kcal mol−1) together with GABA (−4.68 Kcal mol−1), proline (−4.23 Kcal mol−1) and ornithine (−4.00 Kcal mol−1) form the most stable complexes with carbon dioxide and, therefore, seem to be the ones with higher affinity for the molecules of gas.
 | ||
| Fig. 2 Electronic energy by DFT calculations. | ||
However, ornithine presents the advantage of having two binding sites, which eventually enhances the carbon dioxide uptake per mol. All of them present energy values higher than MEA (−3.93 Kcal mol−1). The other studied molecules present lower values of energy and, therefore, lower affinity to the gas. It is interesting to note out the increase of the energy values when the amino acid is modelled as the free anion, with values that go from 5.05 Kcal mol−1 to 17.02 Kcal mol−1 (Table S1).†
Conclusions
New LA containing CO2-complexing agents have been tested in an experimental lab-scale against CO2. The LA here studied can absorb CO2 gas effectively with a high absorption capacity, superior in some cases to the frequently used MEA. The experimental data pointed out that from the natural amino acids, arginine and ornithine showed the greatest affinities to CO2. We synthesized via several efficient methods new amino acids containing different chemical structures. Among the synthetic amino acids, 5 and 6 showed outstanding absorption capacities, superior to MEA or any other amino acid tested in this study. From the trends found in this study, it can stated that LA based on new synthetic amino acids prepared and based on a piperazine ring are efficient systems for CO2 absorption.The absorbed CO2 can be reversibly desorbed by heating and the LA reused. This method has potential applications such as removal of CO2 pollutants or the separation of gas mixtures when used in combination with a polymeric membrane. This matter is currently under study and will be published in a near future.
The experimental data are supported by 13C-NMR studies in addition to theoretical calculations. These NMR results show that the chemical shifts of the positions next to the amine group of the amino acid can be used as a powerful parameter for predicting CO2 loading capacity without any previous measurement. The theoretical calculations allow getting a previous idea of the affinity of the molecules selected to the gas, prior to the study at a laboratory scale, and in most cases are in concordance with the experimental data.
Acknowledgements
Financial support from the Spanish Ministerio de Ciencia e Innovacion (MAT2007-62392) is gratefully acknowledged.References
- J. W. Dijkstra and D. Jansen, Energy, 2004, 29, 1249–1257 CrossRef CAS.
- T. Kosugi, A. Hayashi, T. Matsumoto, K. Akimoto, K. Tokimatsu, H. Yoshida, T. Tomoda and Y. Kaya, Energy, 2004, 29, 1297–1308 CrossRef CAS.
- A. Meisen and X. S. Shuai, Energy Convers. Manag., 1997, 38, S37–S42 CrossRef CAS Suppl. S.
- S. I. Plasynski and Z.-Y. Chen, 220th ACS National Meeting, Fuel Chemistry Division Preprints, 2000, 41 Search PubMed.
- L. Dragos, O. Nada, C. Fluerau and N. Scarlat, Energy Convers. Manag., 1996, 37, 923–928 CrossRef CAS.
- P. Riemer, Energy Convers. Manag., 1996, 37, 665–670 CrossRef.
- US Department of Energy, DOE/ER30194 Giugno, 1993.
- J. Yao and R. K. Goel, Cryogenic carbon dioxide fractionation, an energy workshop, Houston, Texas, September, 1984 Search PubMed.
- M. L. Srivastava, N. K. Shukla, S. K. Sungh and M. R. Jaiswal, J. Membr. Sci., 1996, 117, 39–44 CrossRef CAS.
- C. Moreno and M. Valiente, J. Membr. Sci., 1999, 155, 155–162 CrossRef CAS.
- B. D. Bhide, A. Voskericya and S. A. Stern, J. Membr. Sci., 1998, 140, 27–49 CrossRef CAS.
- P. S. Kumar, J. A. Hogendoorn and P. H. M. Feron, Chem. Eng. Sci., 2002, 57, 1639–1651 CrossRef CAS.
- P. H. M. Feron and A. E. Jansen, Sep. Purif. Technol., 2002, 27, 231–242 CrossRef CAS.
- G. T. Rochelle, S. Bishnoi, S. Chi, H. Dang and J. Santos, DE-AF26–99FT01029; U.S. Department of Energy-Federal Energy Technology Center: Pittsburgh, PA, 2001.
- H. A. Zinnen, A. R. Oroskar and C.-H. Chang, US Pat., 4810266, 1989.
- P. J. Birbara and T. A. Nalette, US Pat., 5492683, 1996.
- P. J. Birbara, T. P. Filburn and T. A. Nalette, US Pat., 5876488, 1999.
- J. M. Murray and A. Bedi, Scientific Principles of Anaesthesia, Newsletters, 2000, 50, 287–289 Search PubMed.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, Jr., J. Am. Chem. Soc., 2002, 124(6), 926–927 CrossRef CAS.
- J. Zhang, S. Zhang, K. Dong, Y. Zhang, Y. Shen and X. Lv, Chem. Eur. J., 2006, 12, 4021–4026 CrossRef CAS.
- G. Sartori and W. A. Thaler, US Pat., 4405579, 1983.
- G. Sartori and W. A. Thaler, US Pat., 4405586, 1983.
- L. J. Shulik, G. Sartori, W. W. S. Ho, W. Thaler and G. E. Milliman, US Pat., 4759866, 1988.
- A. E. Jansen and P. H. M. Feron, US Pat., 5749941, 1998.
- A. E. Lozano, J. G. de la Campa, J. de Abajo, D. M. Muñoz, T. Wolf and A. F. Portugal, ES200602545 applied for 2006-10-06.
- J. P. P. Stewart, MOPAC: Quantum Chemistry Program Exchange revision 6.0, 1990 Search PubMed.
- Cerius2 version 4.8, Molecular Modelling Package. Accelrys: San Diego, CA, USA, 2005 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 03, Revision C.02, Gaussian, Inc.: Wallingford CT, 2004 Search PubMed.
- E. Anders, R. Koch and P. Freunscht, J. Comput. Chem., 1993, 14, 1301–1312 CrossRef CAS.
- (a) S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566; (b) S. Simon, M. Duran and J. J. Dannenberg, J. Chem. Phys., 1996, 105, 11024–11031 CrossRef CAS.
- R. M. C. Dawson, D. C. Elliott, W. H. Elliott, K. M. Jones, Data for Biochemical Research, Oxford, Clarendon Press, 1959 Search PubMed.
- J. L. Lee, F. D. Otto and A. E. Mather, J. Appl. Chem. Biotechnol., 1976, 26, 541–549 CAS.
- P. S. Kumar, J. A. Hogendoorn, P. H. M. Feron and G. F. Versteeg, Ind. Eng. Chem. Res., 2003, 42, 2832–2840 CrossRef CAS.
- P. Singh, J. P. M. Niederer and G. F. Versteeg, International Journal of Greenhouse Gas Control, 2007, 1, 5–10 Search PubMed.
- D. J. Heldebrant, P. G. Jessop, C. A. Thomas, C. A. Eckert and C. L. Liotta, J. Org. Chem., 2005, 70, 5335–5338 CrossRef CAS.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS.
- Y. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958–960 CrossRef CAS.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Energy Environ. Sci., 2008, 1, 487–493 RSC.
- L. Phan and P. G. Jessop, Green Chem., 2009, 11, 307–308 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Scheme S1, Fig. S1, Fig. S2 and Table S1. See DOI: 10.1039/b901307e |
| This journal is © The Royal Society of Chemistry 2009 |