On-chip direct methanol fuel cells of a monolithic design: consideration on validity of active-type system
Satoshi
Tominaka
,
Hiroyuki
Obata†
and
Tetsuya
Osaka
*
Graduate School of Advanced Science and Engineering, Waseda University, Okubo 3-4-1, Shinjuku, Tokyo 169-8555, Japan. E-mail: osakatets@waseda.jp
First published on 13th May 2009
Abstract
The performance of an on-chip direct methanol fuel cell of monolithic design with an active-type system was improved more than 6-fold up to >100 µW by using hydrogen peroxide as an oxidant solution instead of dissolved oxygen, but the energy-loss caused by strong capillary forces required a redesign of both microchannel size and the system.
Broader contextRecent developments in micro-devices cause an increasing demand for miniature power sources, e.g., micro-batteries, micro-solar cells and micro-fuel cells. Of these power sources, micro-fuel cells are attractive in terms of their possible operation as long as fuel is supplied. Hence, we have developed a prototype on-chip fuel cell with an active-type system having pumps and microchannels to supply both fuel and oxidant. So far, our research work has demonstrated that the performance strongly depends on the oxidant flow rate: performance improved with higher flow rate. But, we were concerned about the energy-loss caused by a pressure-loss associated with liquid flow through the microchannels. In view of this, this paper discusses the validity of the cell system in terms of its energy balance, based on the estimation of energy balance of the prototype cell. The power generated from the cell was increased more than 6-fold (up to >100 µW) using a concentrated oxidant solution, i.e., a hydrogen peroxide aqueous solution; however, the energy-loss was found to be larger than the power thus generated. Consequently, we concluded that a practical solution to the energy-loss reduction would be to double the diameter of the microchannels. |
Recent developments in micro-devices (e.g., micro-sensors1,2) have resulted in increasing demand for miniature power sources, e.g., micro-batteries,3,4 micro-solar cells5 and micro-fuel cells.6–8 Among the power sources, micro-fuel cells are attractive in terms of their possible operation as long as fuel is supplied. Smyrl et al. for the first time fabricated a miniature of conventional polymer electrolyte fuel cells using microfabrication techniques.7 Later, numerous research works on the development of micro-fuel cells have been reported.9–14 Since optimum size and design of micro-fuel cells are considered to be dependent on the application of choice, we have focused on a monolithic design consisting of microchannels of submilimeter size to develop fuel cells applicable to integration on a chip. Fuel cells of monolithic design, in which the anode and the cathode are fabricated on a single substrate,15 are attractive for miniaturizing fuel cells in terms of: (i) the elimination of a troublesome alignment process of the small electrodes, (ii) the possibility for controlling ohmic loss of membrane resistance by adjusting the distance between the electrodes, and (iii) the ability to design different sizes of anode and cathode.
Our laboratory group designed and fabricated micro-direct methanol fuel cells (µ-DMFCs) of a monolithic design, which were composed of catalyzed microchannels small enough (width: 50–200 µm) for application as on-chip power sources (Fig. 1).6 The cell operated on a fuel solution and an oxidant solution supplied with pumps, and the performance was found to be dominated by the oxidant flow rate.16 This probably originated from the choice of oxidant, i.e., dissolved oxygen in aqueous solution, which was not sufficient for large current operation due to its low concentration (ca. 1.4 mM at room temperature) and low diffusion coefficient (2 × 10−5 cm2 s−1).17,18 Though a decent performance can be obtained by using high flow rate of 80 µL min−1 (i.e., 41 cm min−1),16,19 some concerns remained about the energy-loss caused by reactant flow through the microchannels.20
 | ||
| Fig. 1 Schematic diagrams illustrating the concept of an on-chip direct methanol fuel cell of a monolithic design with an active-type system. (a) Birds-eye view of the cell consisting of two parallel microchannels covered with a polymer electrolyte membrane. (b) Cross-sectional view of the cell. Catalysts were deposited on the microchannels. | ||
Discussion of the validity of the cell in terms of its energy balance is of essential importance for the development of quite small fuel cells. In view of this, this paper discusses the direction of development of on-chip fuel cells by estimating the energy balance of our prototype cell. First, cell performance was improved by using a concentrated oxidant solution. Hydrogen peroxide (H2O2) was reported to be a promising liquid oxidant alternative to oxygen,21,22 thus we used H2O2 aqueous solutions. Second, to evaluate the energy balance of this system, energy-loss caused by pressure-loss associated with liquid flow through microchannels was estimated.
The cell was fabricated on a Si substrate through a series of microfabrication procedures.6,16 First, two parallel trapezoidal microchannels (top width: 100 µm; depth: 50 µm; length: 15.2 mm (6 mm for electrodes); distance between the channels: 50 µm) were fabricated on a Si(100) substrate (n-type, 1–10 Ω cm, 200 ± 20 µm thick) by anisotropic etching with KOH after lithographic patterning of a photoresist (OFPR, spin-coating) and etching of SiO2 with a buffered hydrogen fluoride. Second, 200 nm thick Au current collectors with 30 nm thick Ti adhesion layers were deposited after lithographic patterning of a photoresist (AZ P4620, spray-coating). The area of each Au current collector in the channel was 0.0091 cm2. Third, the current collectors were catalyzed by electrodeposition of Pt for the cathode and of Pt–Ru for the anode, following our previously published methods.19,23 Finally, the microchannels were covered with a Nafion membrane pretreated at 60 °C in 3% H2O2, in pure water and then in 0.5 M H2SO4.
The cell performance of the whole cell was evaluated in terms of current–voltage characteristics and polarization characteristics of each individual electrode. Current–voltage (I–V) curves were measured at room temperature with supplying reactant solutions at 80 µL min−1 using micro-syringe pumps. The flow rate was optimized in the case of use of dissolved-oxygen as the oxidant solution. The fuel solution contained 0.5 M H2SO4 and 2 M methanol. The oxidant solution contained 0.5 M H2SO4 and (i) saturated-dissolved oxygen or (ii) hydrogen peroxide (H2O2). The polarization curves of each electrode were measured during the I–V measurements by using a reference electrode, silver/silver chloride (Ag/AgCl), set in the oxidant solution source.
The cell performance of the µ-DMFC operating on MeOH and O2 is shown in Fig. 2. The current–power (I–P) curve shows that the maximum power (Pmax) was ca. 12 µW at 60 µA. This power is relatively-high for on-chip fuel cells,24,25 but net power is expected to be lower due to the energy-loss caused by the pumps. This point is discussed later in this paper. The I–V curve (inset Fig. 2) shows that the voltage was drastically decreased in the current range of >60 µA, this occurred at the cathode side, considering the cathode potential decrease in the same current range. Thus, it was confirmed that the performance was limited by cathode potential. This potential decrease is attributable to a lack of oxidant supply, because the concentration of dissolved oxygen is much lower (ca. 1.4 mM) than that of methanol (2 M). Thus, we investigated the use of a concentrated oxidant, i.e., H2O2 solutions, and evaluated the effects on cell performance.
 | ||
| Fig. 2 Current–power curve of an active-type on-chip fuel cell operating on oxygen-saturated solution (cathode) and 2 M methanol solution (anode) at room temperature. Flow rate: 80 µL min−1. Inset shows a current–voltage curve (●) and current–potential curves for the anode (△) and the cathode (▲). | ||
The cell performance was drastically increased by the use of H2O2 as shown in Fig. 3. The Pmax of the cell operating on 30 mM H2O2 was ca. 77 µW, i.e., 6 times as high as the cell operating on O2. The Pmax value was dependent on H2O2 concentration and increased up to >100 µW around 0.9 M (inset Fig. 3a). The significant increase in performance was considered to occur in cathode mass-transfer, as judged by the increase in cathode potential (Fig. 3b). Thus, we confirmed that the use of a concentrated oxidant solution successfully increased the cell performance of this micro-fuel cell. However, the Pmax value was decreased by the use of an excessively concentrated solution. This decrease in performance can be attributed to H2O2 crossover to the anode channel, as evidenced by the anode potential of H2O2 use which was more positive than that of O2 use, around open-circuit potential (Fig. 3b). In addition, the voltage of the cell operating on a concentrated H2O2 solution (>30 mM) fluctuated, probably caused by the formation of bubbles, which were observed in the outlet of the oxidant solution and could result from chemical decomposition of H2O2.21
 | ||
| Fig. 3 Comparison of the performance of an active-type on-chip fuel cell operating on a different oxidant solution: (●○) 30 mM H2O2 solution and (▲△) oxygen-saturated solution. The fuel was a 2 M methanol solution. Flow rate was 80 µL min−1. (a) Current–voltage curves (open) and current–power curves (filled). Inset shows the maximum power at different H2O2 concentration (triangles: maximum power using O2). (b) Current–potential curves for the anode (open) and the cathode (filled). | ||
In view of the high performance for an on-chip fuel cell,24 this cell could be a promising candidate for on-chip power sources. Nonetheless, one concern is the energy-loss caused by strong capillary forces within the microchannels. For this reason, to evaluate the energy balance of this active-type system, such an energy-loss was estimated as follows. In the microchannels, laminar flow dominates the fluidic phenomena because of the low Reynolds number, Re (–), of 21 at 80 µL min−1, and thus the energy-loss caused by liquid flow can be calculated based on hydrodynamics of laminar flow. Theoretical energy-loss, ΔP (W), was calculated from the theoretical values of pressure gradient, Pg (Pa m−1), in trapezoidal microchannels as reported by Weilin et al.26 using the following equations.
| ΔP = PglQ | (1) |
| Pg = kRe | (2) |
| Re = ρQdh/(Acµ) | (3) |
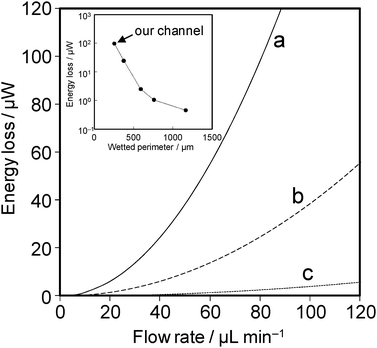 | ||
| Fig. 4 Theoretical relationship between energy-loss and flow rate for a single microchannel with a different hydraulic diameter (a) 51 µm, our channel; (b) 63 µm, (c) 119 µm. The inset shows the energy-loss at 80 µL min−1, which drastically decreases with increasing in the wetted perimeter. | ||
The energy-loss drastically increased by increasing the flow rate or by decreasing the channel size. The energy-loss of our channels (dh = 51.1 µm) was found to be 98 µW per single channel (at 80 µL min−1), i.e., the cell had to generate at least 196 µW to supply power to external devices. This value is quite high in view of the fact that the maximum Pmax value obtained in this work was lower than this energy-loss. These results suggested that this µ-DMFC with an active-type system should be redesigned to improve its energy balance, i.e., to reduce energy-loss without performance degradation. One possible approach is to use larger channels with little energy-loss. The size can be estimated based on wetted perimeters, because the energy-loss is dominated by Ac/dh ratio (equal to a quarter of wetted perimeter). As shown in the inset of Fig. 4, the wetted perimeter should be preferably more than 500 µm, i.e., more than twice as large as that of our current channels. Although some concerns remain over reactant efficiency in the case of large channels, we consider the size is still suitable for on-chip application. Another possible approach is to reduce the flow rate by using (i) a concentrated oxidant solution free of bubble formation and crossover, e.g., vanadium redox species,27,28 or (ii) an oxidant with high diffusivity, e.g., gaseous oxygen. Based on the latter, we recently focused on the development of on-chip fuel cells of an air-breathing design, which can use gaseous O2 in air.24
In summary, the cell performance was significantly increased up to >100 µW using a concentrated oxidant solution, i.e., H2O2 solution; however, energy-loss in microchannels was found to be higher than the energy generated from the cell. Thus, we will continue to optimize the channel size, e.g., by doubling the wetted perimeter, or the system, e.g., by using another oxidant. Our research is ongoing with the aim of developing on-chip fuel cells that are practicable as power sources for on-chip devices.
Acknowledgements
This work was partly supported by the Grant-in-Aid for Specially Promoted Research “Establishment of Electrochemical Device Engineering” and by the Global COE program “Center for Practical Chemical Wisdom” from MEXT, Japan.Notes and references
- R. Popovtzer, T. Neufeld, N. Biran, E. Z. Ron, J. Rishpon and Y. Shacham-Diamand, Nano Lett., 2005, 5, 1023–1027 CrossRef CAS.
- D. Niwa, K. Omichi, N. Motohashi, T. Homma and T. Osaka, Sens. Actuators, B, 2005, 108, 721–726 CrossRef.
- H. Nakano, K. Dokko, J. I. Sugaya, T. Yasukawa, T. Matsue and K. Kanamura, Electrochem. Commun., 2007, 9, 2013–2017 CrossRef CAS.
- D. Golodnitsky, V. Yufit, M. Nathan, I. Shechtman, T. Ripenbein, E. Strauss, S. Menkin and E. Peled, J. Power Sources, 2006, 153, 281–287 CrossRef CAS.
- J. B. Lee, Z. Z. Chen, M. G. Allen, A. Rohatgi and R. Arya, J. Microelectromech. Syst., 1995, 4, 102–108 CrossRef CAS.
- S. Motokawa, M. Mohamedi, T. Momma, S. Shoji and T. Osaka, Electrochem. Commun., 2004, 6, 562–565 CrossRef CAS.
- S. C. Kelley, G. A. Deluga and W. H. Smyrl, Electrochem. Solid-State Lett., 2000, 3, 407–409 CAS.
- S. C. Kelley, G. A. Deluga and W. H. Smyrl, AIChE J., 2002, 48, 1071–1082 CrossRef CAS.
- M. Hayase, T. Kawase and T. Hatsuzawa, Electrochem. Solid-State Lett., 2004, 7, A231–A234 CrossRef CAS.
- T. Ito, K. Kimura and M. Kunimatsu, Electrochem. Commun., 2006, 8, 973–976 CrossRef CAS.
- T. Ito and M. Kunimatsu, Electrochem. Commun., 2006, 8, 91–94 CrossRef CAS.
- S. J. Lee, A. Chang-Chien, S. W. Cha, R. O'Hayre, Y. I. Park, Y. Saito and F. B. Prinz, J. Power Sources, 2002, 112, 410–418 CrossRef CAS.
- N. T. Nguyen and S. H. Chan, J. Micromech. Microeng., 2006, 16, R1–R12 CrossRef CAS.
- Y. Yamazaki, Electrochim. Acta, 2004, 50, 663–666 CrossRef CAS.
- H. L. Maynard and J. P. Meyers, J. Vac. Sci. Technol., B, 2002, 20, 1287–1297 CrossRef CAS.
- S. Motokawa, H. Obata, M. Mohamedi, T. Momma, S. Shoji and T. Osaka, Electrochemistry (Tokyo, Jpn.), 2005, 73, 352–355 CAS.
- R. S. Jayashree, L. Gancs, E. R. Choban, A. Primak, D. Natarajan, L. J. Markoski and P. J. A. Kenis, J. Am. Chem. Soc., 2005, 127, 16758–16759 CrossRef CAS.
- E. Narita, F. Lawson and K. N. Han, Hydrometallurgy, 1983, 10, 21–37 CrossRef CAS.
- S. Tominaka, T. Momma and T. Osaka, Electrochim. Acta, 2008, 53, 4679–4686 CrossRef CAS.
- S. W. Cha, R. O'Hayre, Y. Saito and F. B. Prinz, J. Power Sources, 2004, 134, 57–71 CrossRef CAS.
- E. Kjeang, A. G. Brolo, D. A. Harrington, N. Djilali and D. Sinton, J. Electrochem. Soc., 2007, 154, B1220–B1226 CrossRef CAS.
- N. Luo, G. H. Miley, K. J. Kim, R. Burton and X. Y. Huang, J. Power Sources, 2008, 185, 685–690 CrossRef CAS.
- S. Tominaka, S. Ohta, T. Momma and T. Osaka, ECS Trans., 2007, 11, 1369–1377 Search PubMed.
- S. Tominaka, S. Ohta, H. Obata, T. Momma and T. Osaka, J. Am. Chem. Soc., 2008, 130, 10456–10457 CrossRef CAS.
- G. Erdler, M. Frank, M. Lehmann, H. Reinecke and C. Muller, Sens. Actuators, A, 2006, 132, 331–336 CrossRef.
- Q. Weilin, G. M. Mala and L. Dongqing, Int. J. Heat Mass Transfer, 2000, 43, 353–364 CrossRef.
- R. Ferrigno, A. D. Stroock, T. D. Clark, M. Mayer and G. M. Whitesides, J. Am. Chem. Soc., 2002, 124, 12930–12931 CrossRef CAS.
- E. Kjeang, R. Michel, D. A. Harrington, N. Djilali and D. Sinton, J. Am. Chem. Soc., 2008, 130, 4000–4006 CrossRef CAS.
Footnote |
| † Current affiliation: Toyota Motor Corporation. |
| This journal is © The Royal Society of Chemistry 2009 |
