Nanochemistry aspects of titania in dye-sensitized solar cells
Mario
Pagliaro
*a,
Giovanni
Palmisano
ab,
Rosaria
Ciriminna
a and
Vittorio
Loddo
b
aIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146, Palermo, PA, Italy. E-mail: mario.pagliaro@ismn.cnr.it; Tel: +39 091 680 093 70
b“Schiavello-Grillone” Photocatalysis Group, Dipartimento di Ingegneria Chimica dei Processi e dei Materiali, Università degli Studi di Palermo, viale delle Scienze, 90128, Palermo, PA, Italy
First published on 27th April 2009
Abstract
We analyze the main nanochemistry factors affecting photovoltaic performance in TiO2 employed as wide bandgap semiconductor in dye-sensitized solar cells (DSCs). What is the best morphology of the oxide? Which processes yield the required structures? Finally, putting the discussion in the context of the rapid evolution of photovoltaic technologies, we argue that new titania nanostructures will form the basic component of second-generation solar modules based on dye solar cells.
 Mario Pagliaro Mario Pagliaro | Mario Pagliaro is a research chemist and management educator based at Palermo's CNR, where he leads Sicily's Photovoltaics Research Pole and a research group collaborating with researchers in 10 countries. Mario's research and educational interests at the interface of materials science, chemistry, biology and cultural studies are reflected in seven books, 80 research papers, five patents, and several book chapters. |
 Giovanni Palmisano Giovanni Palmisano | Giovanni Palmisano has a doctorate in chemical engineering and is currently a research fellow at the University of Rome “Tor Vergata”, under the tutorage of Aldo Di Carlo and in close collaboration with Vincenzo Augugliaro at Palermo University and Mario Pagliaro at CNR. He also works as a private consultant for the industrial development of photovoltaic products whereas his research currently focuses on TiO2-based new functional materials for DSC cells and catalytic applications. Giovanni has co-authored some 30 research papers. |
 Rosaria Ciriminna Rosaria Ciriminna | Rosaria Ciriminna is a research chemist at CNR Italy, based at the Institute of Nanostructured Materials in Palermo. Originally working in the field of organic chemistry, her research interests now include sol–gel materials for a variety of applications, including catalysis, sensing, functional coating and photovoltaics. Rosaria has co-authored three books, 60 research papers and four patents. |
 Vittorio Loddo Vittorio Loddo | Vittorio Loddo obtained a PhD in chemical engineering at the University Federico II of Naples in 1998. He is currently researcher in the Department of Chemical Engineering at the University of Palermo. His research focuses mainly on reactor modelling for photocatalytic reactions by using supported TiO2 and he is author of many publications on this topic. In the course of his scientific activity, Vittorio has contributed to the following fields: chemical kinetics of heterogeneous photocatalytic systems, modelling of heterogeneous photoreactors, radiation field modelling in absorbing–reacting media, advanced oxidation processes for environment remediation and green synthesis, TiO2 films for dye-sensitized solar cells. |
Broader contextAbundant and cheap electricity from solar radiation is the single most important technology achievement needed to face the global sustainability crisis caused by prolonged burning of fossil fuels to generate electrical power. Global warming, air and water pollution and low energy security are all due to our continuing dependence on oil, coal and natural gas. Manufactured as coloured, large area glasses and deposited by low cost printing techniques, dye-sensitized solar cells (DSC) will be massively used in off-grid applications in developing countries and for architecturally-integrated solutions because of their low cost and unsurpassed elegance. Along with the sensitizing dye, these solar cells make use of a nanostructured layer of titania. The nanochemistry approach is emerging as a powerful tool to improve the efficiency and the stability of this 20-year old photovoltaic technology, which is finally being commercialized. |
1 Introduction
Cost and efficiency are the most important factors in the success of any solar-based technology aiming to produce electricity from the sun's irradiation. To become widely adopted, photovoltaic (PV) solar cells must generate electricity at a lower cost than what is now spent on fossil fuels. In fact, a number of new PV technologies are emerging to replace traditional cells based on costly silicon.1 Among these, dye-sensitized solar cells (DSC) are ideally suited for off-the-grid applications in developing countries, and for building integrated photovoltaics (BIPV).2 Current DSC-based modules in fact have 5% energy conversion efficiency with good performance under any atmospheric condition and low irradiance.3Low price is due to the inexpensive materials (glass, titania, dye, electrolyte and carbon powders) used to manufacture the cells by screen printing equipment rather than by costly vacuum systems. Further lowering costs, the raw materials do not need the extreme purity of silicon employed in conventional PV cells. In the second grand field of forthcoming applications, namely BIPV, semi-transparent DSC offer multi-color range possibilities (by changing the dye) which along with stable performance at high temperature, and non standard irradiation and solar incidence angle, open the route to power-producing windows and façades of elegant design and thus to a high-value segment of the real estate market.4
A clear demonstration of these assumptions recently emerged from experimentation in Germany where the ColorSol consortium manufactured and installed solar PV glass based on DSC and compared them to commercial PV glass using amorphous silicon (a-Si) solar cells. Results showed that the cost of solar electricity generated by the DSCs is lower (4 €/Wpvs 6 €/Wp) than their a-Si analogues, whereas the elegance of the coloured glassy surface is well illustrated in Fig. 1.5
 | ||
| Fig. 1 The DSC-based PV façade of the Capricorn Haus in Germany. (Reproduced from Gattermann und Schossig Architekten, with permission). | ||
DSCs were first described in 1977,6 but the first breakthrough 7% efficiency was reached in 1991 only when scientists used a nanoscopic TiO2 particle layer and a polypyridyl ruthenium complex as a light absorber.7 The dye is adsorbed throughout the whole TiO2 surface at the interface of TiO2 and a hole-transport material, and the TiO2nanostructure enhances the area that is used for collecting photons by a factor 100–1000. So efficient was the outcome that, as put by Hupp and co-workers, the most efficient DSCs have had essentially the same configuration for the last 17 years, namely nanoparticle TiO2 sensitized with [Ru(4,4′-dicarboxy-2,2′-bipyridine)2(NCS)2] in contact with I3−/I−.8
The working mechanism of these photoelectrochemical cells is analogous to photosynthesis where a dye absorbs the photons, and this concept has been studied in depth.9 Light creates an excitation in the dye that consists of a highly energetic electron, which is rapidly injected to the TiO2 particles. Nanoparticulate TiO2 functions as the transporter of light-induced electrons towards the external contact, generally a transparent conductor that lies at the basis of the TiO2 film. The oxidized dye molecule is very rapidly regenerated by the hole transporting material, usually the redox I3−/I− electrolyte. Careful design of the dye minimizes loss mechanisms and improves light harvesting.10
Under the non realistic standard illumination conditions of STC (1000 Wm−2, 25 °C and Air Mass 1.5) used to compare different solar cells, efficiency for small DSC had reached 11.1% (in 2006), whereas current module highest reported efficiency (by Australia's Dyesol) is around 5%.11 Mendes et al. recently provided arguments supporting as realistic an efficiency target of 15% within the next few years.2 In order to realize the full promise of DSCs as high efficiency energy-conversion devices, it is necessary to alter at least two of the three major components—the dye, redox shuttle and photoanode—simultaneously.8 To achieve this goal, along with higher light-harvesting efficiency (augmented by dyes with an enhanced near infrared response) the other main efficiency boost is expected to come from the development of nanostructured TiO2 to improve electron transport and collection efficiency in the electrode. Here, thus, we focus on the photoanode: a 15–20 µm thick layer of mesoporous layer of crystalline titania nanoparticles (10–20 nm diameter), usually deposited by screen printing from a sol–gel precursor solution which undergoes calcination under air.
Defined by Ozin as the utilization of synthetic chemistry to make nanoscale building blocks of different size and shape, composition and surface structure that can be useful in their own right or in a self-assembled structure,12 nanochemistry deals in particular with an approach to materials chemistry in which “size and shape are as important as structure and composition”; namely, the approach that is actually being used to synthesize new sol–gel titania nanostructures for DSCs. Commenting on three major configurations of TiO2, i.e., nanocrystalline titania, core–shell photoanode, and TiO2nanostructures, in this article we investigate the nanochemistry aspects of TiO2 in DSCs aiming to elucidate principles and emphasize open questions for further research.13
2 Mesoporous, nanocrystalline titania
Anatase, a crystalline form of TiO2, is the preferred semiconductor in DSCs because it has a high bandgap energy (3.2 eV) and absorbs only below 388 nm making it invisible to most of the solar spectrum, thus reducing the recombination rate of photoinjected electrons. Additionally, it has good thermal stability, is chemically inert, non-toxic and relatively cheap. TiO2 however is a non stoichiometric oxide whose functional properties are strictly related to its actual composition,14| TiO2 | a,b,c,d,e,f,g,h | (1) |
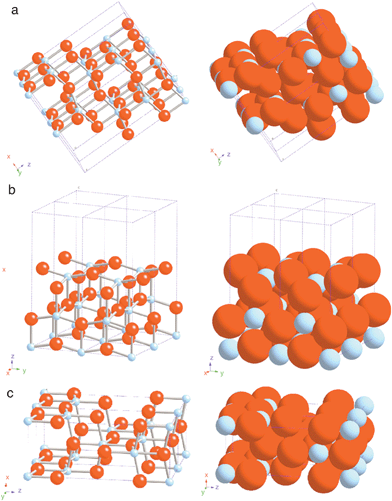 | ||
| Fig. 2 Schematic representations of selected low-index faces of anatase: (a) (101); (b) (100); and (c) (001). (Reproduced from ref. 15, with permission). | ||
In order to increase light-harvesting, and thus PV efficiency, the ideal titania layer must have a very high surface area, allowing monolayer absorption of dye molecules. This is because of the low absorbance of dye monolayers and the low efficiency of dye multilayers.16
At the same time, the titania layer must be structured in order to maximize electron conductivity to the surface of the electrode. As a result, dye-sensitized solar cells are typically constructed from thick films of TiO2nanoparticles that are sintered into a mesoporous “spongy” network with a large internal surface area. Normally, the photoanode is sol–gel fabricated obtaining randomly oriented anatase nanocrystals packed in mesoporous films of ca. 20 micron thickness.
In other words, TiO2 film morphology is a major variability factor in DSC performance17 mainly because of:
(i) its influence in the electron recombination rate through the electron diffusion coefficient; and,
(ii) easy accessibility to the dye and to the electrolyte containing the redox couple needed to close the circuit.
Recombination indeed occurs close to the substrate (glass coated with a transparent conducting oxide) and not throughout the whole titania matrix as one would perhaps first think.18 For this reason, researchers use a compact19 or nanocrystalline20 TiO2 blocking layer, particularly effective when using organic dyes as sensitizers.21
Showing the relevance of enhanced accessibility of the sol–gel cages by the dye and the electrolyte, sol–gel templated mesoporous TiO2 thin films grown via a layer-by-layer dip-coating procedure show enhanced solar energy conversion efficiency by about 50% compared to that of traditional films of the same thickness made from randomly oriented anatase nanocrystals (Fig. 3). The 1 µm thick mesoporous film, made by the superposition of three layers, was prepared as described.22
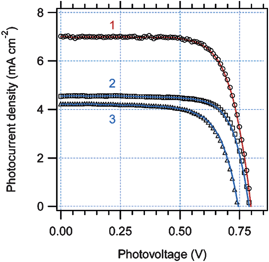 | ||
| Fig. 3 Photocurrent–voltage characteristics of a solar cell, based on TiO2 films sensitized by N945. Pluronic-templated three-layer film, 1.0 µm thick (1); nonorganized anatase treated by TiCl4, 0.95 µm thick (2); nonorganized anatase nontreated by TiCl4, 0.95 µm thick (3). (Reproduced from ref. 21, with permission). | ||
Aqueous HCl (9.7 g, 37%) was added to 12.7 g of titanium ethoxide under vigorous stirring. Separately, 4.0 g of block copolymer Pluronic P123 was dissolved in 36.3 g of 1-butanol and added to the HCl/Ti(EtO)4 solution. This solution was aged by stirring at ambient temperature for at least 3 h. The films were deposited by dip coating (withdrawal rate of 0.8 mm s−1) onto 7.5 × 2.5 cm2 sized glass slides or F-doped SnO2. The layer was aged at 75% relative humidity at a temperature of 24–25 °C for 30 h and then calcined in air at 350 °C for 2 h (heating rate: 1 °C min−1). For the preparation of thicker films consisting of two or three layers, the procedure was repeated once or twice. Finally, the film was calcined at 450 °C for 30 min.
The older synthetic protocol optimized for DSCs application afforded films capable of 10.4% conversion efficiency, albeit top performance was only achievable on 18 µm thick films. Accordingly, the resulting standard nonorganized nanocrystalline TiO2 film was grown via a sol–gel route using two TiO2 colloids (acidic and basic) subsequently impregnated with TiCl.23 The improvement observed with templated films results from a notable enhancement of the short circuit photocurrent (Fig. 3) which, in its turn, implies the formation of a huge surface area highly accessible to both the dye and the electrolyte.
Indeed, the roughness factor (RF) of ca. 460 for the 1µm thick film is dramatically larger, by a factor of 5 or 50, compared to the RF value of a film of randomly oriented 12 nm-sized TiO2 particles. The TEM images (Fig. 4) confirm that the morphologies of the one-layer film and three-layer film are similar, showing the expected mesopore size around 7 nm, due to shrinking of the inorganic framework during calcination, resulting in almost intact underneath films by subsequent layer deposition.
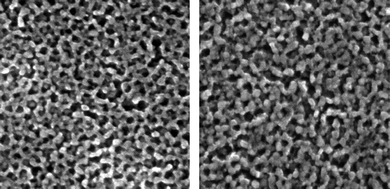 | ||
| Fig. 4 The morphologies of the one-layer film and three-layer film are similar, showing the expected mesopore size around 7 nm. (Reproduced from ref. 21, with permission). | ||
The second assumption, namely the importance of order for enhanced conductivity, is revealed by photocurrent measurements in aqueous electrolyte from layers of porous TiO2 fabricated by electrophoretic deposition at different temperatures, with subsequent sintering in air. Results clearly indicated a dramatic increase of the effective diffusion coefficient ascribed to a higher degree of ordering in the nanoporous TiO2 layer.24
Characterization, in terms of average degree of preferred orientation, shows that low deposition temperature results in optimal orientation of the nanocrystals forming the porous film (Fig. 5) with the diffusion coefficient going from 1.6 × 10−5 to 1.4 × 10−4 cm2 s−1, strongly dependent on the solution temperature during the TiO2 layer deposition.
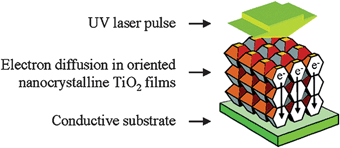 | ||
| Fig. 5 Electron conductivity is greatly facilitated in ordered titania arrays. (Reproduced from ref. 23, with permission). | ||
3 The core–shell photoanode
Another recent advancement particularly relevant from a practical viewpoint has been the design of the core–shell electrode.25 The new electrode is based on a conductive nanoporous core rather than a semiconducting one. Thus, the electrode consists of a conductive nanoporous matrix (for example, made of TCO) that is coated with standard wide bandgap titania. In principle, now the conducting core extends the current collector into the nanoporous network and consequently the distance between the injection spot and the current collector decreases from several micrometers in the standard electrode, to several nanometers throughout the nanoporous electrode (Fig. 6).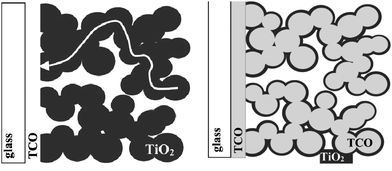 | ||
| Fig. 6 Schematic view of (left) of the charge transport in the volume of a standard nanoporous electrode during the DSC operation, and (right) of the new collector-shell electrode consisting of a conductive nanoporous matrix coated TiO2. (Reproduced from ref. 24, with permission). | ||
In other words, all electrons injected into the electrode, including those that are generated several micrometers away from the substrate, have to travel a very short distance before reaching the current collector. Zaban and coworkers24 discovered that only compact TiO2 films whose thickness is thicker than 6 nm show reasonably high open circuit photovoltage (Fig. 7).
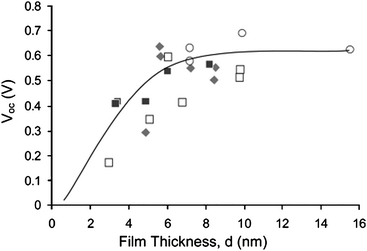 | ||
| Fig. 7 Correlation between thickness of the TiO2 layer and the open circuit photovoltage in a DSC. The symbols represent sets of electrodes that were made by different preparation methods of the compact TiO2. (Reproduced from ref. 24, with permission). | ||
In contrast, layers that are thinner impose significant decrease of the VOC. As the thickness of the TiO2 layer increases and crosses 6 nm, the difference between the electron lifetime τ(V) curves, which is a function of the cell's voltage, becomes small resulting in slower recombination rates per a given voltage. Such significant thickness dependence of the electron lifetime is mainly associated with thin electrodes; pointing to a change in recombination rate with the thickness of the TiO2 layer.
In general, a recent elegant combined experimental and theoretical investigation26 into the charge transport and recombination in dye-sensitized mesoporous TiO2 has shown (Fig. 8) that the charge recombination is mainly governed by the recombination reaction rate constant; and that the charge density dependence is mainly a result of the bimolecular nature of the recombination process.
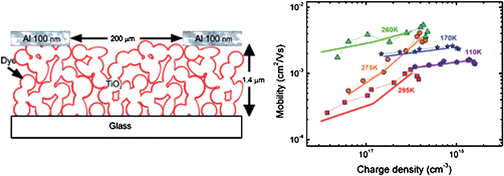 | ||
| Fig. 8 Correlation between charge density and electron mobility in dye-sensitized mesoporous TiO2 illustrates that charge recombination is mainly governed by the recombination reaction rate constant and that charge density dependence is mainly a result of the bimolecular nature of the recombination process. (Reproduced from ref. 26, with permission). | ||
The implication to future material design for DSC is that if the mobility can be enhanced without increasing the charge density in the film, then this will greatly increase the charge carrier diffusion lengths in dye-sensitized solar cells.
4 TiO2nanostructures
The use of nanostructures such as nanotubes and nanowires applied to DSC electrodes in place of the semiconducting mesoporous layer is rapidly emerging as a promising means to enhance performance either in terms of efficiency or stability. In general, nanostructures are particularly favorable in solvent-free electrolytes based on ionic liquids,27 which are the ideal candidates to replace volatile acetonitrile normally employed as a solvent in the electrolyte system.28Like in other well established sol–gel self-assembly processes, titania molecular precursors spontaneously self-organize into assemblages such as nanowires or nanotubes due to molecular forces that operate at length scales beyond the molecular, forming a particular architecture with a structural design which is determined by size and shape of the individual templating agent; and by the map of bonding forces between the resulting nanocomponents. In one remarkable example, the sol–gel polycondensation of precursor tetraisopropylorthotitanate (TIPT) was carried out in the presence of surfactant laurylamine hydrochloride, using acetylacetone to coordinate titanium centers and thus moderate reactivity in the hydroloytic polycondensation.29
A yellow solution of TIPT is added to a 0.1 M laurylamine hydrochloride (LAHC) aqueous solution (pH 4–4.5) at a molar ratio of TIPT to LAHC equal to 4. Precipitation occurs immediately and after stirring for several days at 313 K in order to dissolve the precipitate, with an extra 3 days at 353 K, a white gel is formed and the nanostructured titania is separated by filtration.
Despite the resulting TiO2nanowires having a constrained surface area, the electrode efficiency is now increased because the nanowires provide a direct passageway for photoexcited electrons to get to the conducting substrate; this smart compromise between electronic conductivity and specific surface area available for dye adsorption has the potential to boost performance. Indeed, free electrons in the nanostructured titania show non-ideal thermodynamic behaviour so that the collection efficiency for photoinjected electrons in the cells made of TiO2nanotubes (NTs) is close to 100% (under short circuit conditions), even for a 20 micron thick nanotube array, because the electron diffusion length in such titania nanotube cells is in the order of 100 micron.30
Titania nanotubes can also be prepared at room temperature by anode oxidation of titanium foils in an organic electrolyte, by applying a high potential (such as 120 V) for times ranging from tens of minutes to some hours. In this manner, layer thickness of 1, 5, 10, and 20 µm can be easily achieved. Subsequent annealing yields an active photoanode that is further assembled into a DSC; the best version of, with oriented anatase nanowires, reached a remarkable light-to-electricity conversion yield of 9.3% (Fig. 9).31
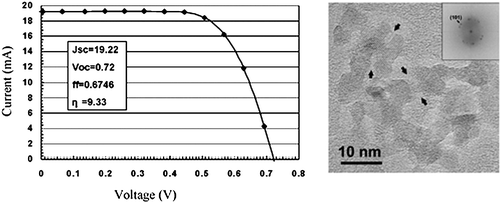 | ||
| Fig. 9 Arrays of closely packed and oriented titania (35 µm long NTs) afford 9.33% efficient DSC. (Reproduced from ref. 31, with permission). | ||
In another example, a network of anatase/TiO2nanowires almost perfectly aligned with each other is formed by surfactant-assisted self-assembling processes at room temperature (Fig. 10).32
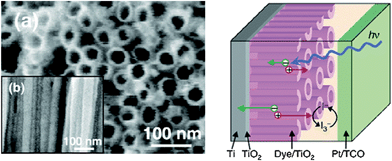 | ||
| Fig. 10 Along with enhanced conductivity, arrays of titania NTs with typical intertube spacings of 8–10 nm and pore diameters of about 30 nm show enhanced light-harvesting efficiencies owing to stronger internal light-scattering effects. (Reproduced from ref. 32, with permission). | ||
Alignment here is due to the oriented attachment mechanism, resulting in the high rate of electron transfer through the anatase nanonetwork. The direction of crystal growth of oriented attachment was controlled by changing the acetylacetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti molar ratio, thereby regulating both the adsorption of surfactant molecules via control of the reaction rate, and the surface energy.
Ti molar ratio, thereby regulating both the adsorption of surfactant molecules via control of the reaction rate, and the surface energy.
A single-crystalline anatase exposing mainly the {101} plane was thus prepared, which adsorbed an amount of ruthenium dye more than 4 times higher when compared to a P-25 titania commercial sample. Recombination, furthermore, is much slower in NT compared to nanoparticle (NP) films, indicating that the NT-based DSCs have significantly higher charge-collection efficiencies than their NP-based counterparts, whereas both morphologies display comparable transport times. The same study also showed that dye molecules cover both the interior and exterior walls of the NTs, with enhanced light-harvesting efficiencies (compared to DSCs incorporating NPs) owing to stronger internal light-scattering effects.
Finally, using supercritical CO2 as drying technique to produce bundle- and crack-free NT films, resulted in further enhancement of solar conversion efficiency and photocurrent density owing to optimal light-harvesting efficiency. This indicates that (i) bundling creates additional pathways via intertube contacts and, that (ii) reducing intertube contacts increases the internal surface area of the films accessible to dye molecules (Fig. 11).33
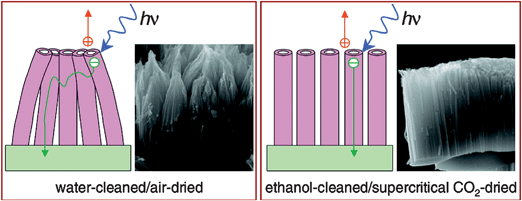 | ||
| Fig. 11 Well aligned titania NTs in DSC show enhanced light-harvesting efficiency owing to higher dye adsorption and to shorter and more direct pathways for electron conductivity. (Reproduced from ref. 33, with permission). | ||
Removing contacts alters the transport mechanism from a combination of one- and three-dimensions to the expected one-dimension, and shortens the electron-transport pathway in the more aligned NT arrays, providing a result of general validity for devices consisting of nominally one-dimensional architectures.
Showing the practical relevance of this nanochemistry approach scientists in South Korea have reported a simple growth-detachment-and-transfer preparation protocol of highly-ordered TiO2nanotube arrays affording individual free-standing TiO2nanotube arrays that, incorporated into DSC as a 35 micron layer of photoactive material, afforded efficiency of 7.6%.34
5. Outlook and conclusions
Three major configurations of TiO2, i.e., nanocrystalline titania, core–shell photoanode, and TiO2nanostructures, are emerging as the main structures employed in the manufacture of DSCs. For practical applications, these elegant nanostructures will have to show the prolonged physico-chemical stability that is required to produce robust solar modules. Nevertheless, they do show optimal electron conductivity, while the tailored design of the structures obtained using different nanochemistry protocols clearly points to further enhancements both in photocurrent and in light harvesting, and thus in overall efficiency.Being the first developed and best known material for DSCs, nanocrystalline titania is currently generally employed in the manufacture of real modules by companies such as Dyesol in Australia or the consortium ColorSol in Germany. The optimal configuration uses a ∼12 µm thick layer of mesoporous crystalline titania nanoparticles (10–20 nm diameter) covered by a ∼4 µm thick film of much larger (∼400 nm diameter) particles that scatters photons back into the transparent film.8
Finally, the core–shell photoanode approach has the potential to provide the advantages of both latter methods, namely long-term stability and enhanced photoelectron conductivity. Developed by Zaban at Bar Ilan University24, this methodology is being applied by the Israeli company 3GSolar with the aim of manufacturing large solar panels with enhanced lifetime, size and efficiency. The modules will use cells with an area of 15 × 15 cm2 featuring a sponge-like array of titania nanodots.
Mass application of DSCs has so far been limited by manufacturing complexity and the long-term stability problems associated with the liquid redox electrolyte used in the most efficient cells. Both these problems are now being rapidly addressed. In this context of rapid innovation, long-awaited commercial dye PV modules will be used to power the basic electricity needs of a large number of people who currently live without electricity in developing countries; and will also find application for elegant BIPV architectural solutions in industrialized countries. In this and related forthcoming practical applications the development of new titania nanostructures has been and will be instrumental. The immense versatility of the sol–gel process producing materials with the right physical and chemical properties is the key through which affordable photovoltaic electricity will become a reality. By describing some of the most notable advancements and emphasizing open research issues this paper aims to contribute to such progress.
Acknowledgements
This paper is dedicated to Umberto Arcara, great physiotherapist and invaluable friend to M.P. We are grateful to Professor Aldo Di Carlo, director of the Polo Solare Organico della Regione Lazio (CHOSE), for a grant to G.P.References
- M. Pagliaro, G. Palmisano, R. Ciriminna, Flexible Solar Cells, Wiley-VCH, Weinheim, 2008 Search PubMed.
- L. Moreira Gonçalves, V. de Zea Bermudez, H. Aguilar Ribeiroa and A. M. Mendes, Energy Environ. Sci., 2008, 1, 655 RSC.
- G. Tulloch, J. Photochem. Photobiol., A, 2004, 164, 209 CrossRef CAS.
- I. B. Hagemann, New Perspectives in BIPV with Dye Solar Cells (DSC), 2nd DSC Industrialisation Conference, St Gallen (Switzerland), 11–13 Septepmber, 2007 Search PubMed.
- A. Hinsch and et al., Sol. Energy Mater. Sol. Cells, 2009 DOI:10.1016/j.solmat.2008.09.049.
- T. Skotheim, US Pat. 4,190,950 - Dye-sensitized solar cells, 1980 Search PubMed.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- T. W. Hamann, R. A. Jensen, A. B. F. Martinson, H. Van Ryswyk and J. T. Hupp, Energy Environ. Sci., 2008, 1, 66 RSC.
- J. Bisquert, D. Cahen, G. Hodes, S. Rühle and A. Zaban, J. Phys. Chem. B, 2004, 108, 8106 CrossRef CAS.
- N. Robertson, Angew. Chem., Int. Ed., 2006, 45, 2338 CrossRef CAS.
- J. Kroon, N. Bakker, H. Smit, P. Liska, K. Thampi, P. Wang, S. Zakeeruddin, M. Grätzel, A. Hinsch, S. Hore, U. Würfel, R. Sastrawan, J. Durrant, E. Palomares, H. Pettersson, T. Gruszecki, J. Walter, K. Skupien and G. Tulloch, Prog. Photovoltaics. Res. Appl., 2007, 15, 1 Search PubMed.
- G. A Ozin, A. C. Arsenaultand L. Cademartiri, Nanochemistry. A Chemical Approach to Nanomaterials, RSC Publishing, Cambridge, UK, 2008 Search PubMed.
- One area of research that is not covered here is that of core-shell electrodes in which titania is coated with other agents, such as alumina, zirconia, magnesia, indium oxide, etc. Interested readers are referred to the thorough review in Ref. 2.
- J. Nowotny, Energy Environ. Sci., 2008, 1, 565 RSC.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- M. Gratzel, Prog. Photovoltaics. Res. Appl., 2000, 8, 171 Search PubMed.
- Z. Wang, H. Kawauchi, T. Kashima and H. Arakawa, Coord. Chem. Rev., 2004, 248, 1381 CrossRef CAS.
- K. Zhu, E. Schiff, N. Park, J. Lagemaat and A. Frank, Appl. Phys. Lett., 2002, 80, 685 CrossRef CAS.
- J. Hart, D. Menzies, Y. Cheng, G. Simon and L. Spiccia, C. R. Chim., 2006, 9, 622–626 CrossRef CAS.
- S. Ito, K. Ishikawa, C. Wen, S. Yoshida and T. Watanabe, Bull. Chem. Soc. Jpn., 2000, 73, 2609 CrossRef CAS.
- A. Burke, S. Ito, H. Snaith, U. Bach, J. Kwiatkowski and M. Grätzel, Nano Lett., 2008, 8, 977 CrossRef CAS.
- M. Zukalová, A. Zukal, L. Kavan, M. K. Nazeeruddin, P. Liska and M. Grätzel, Nano Lett., 2005, 5, 1789 CrossRef CAS.
- M. K. Nazeeruddin, P. Pechy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613 CrossRef CAS.
- S. Tirosh, T. Dittrich, A. Ofir, L. Grinis and A. Zaban, J. Phys. Chem. B, 2006, 110, 16165 CrossRef CAS.
- S. Chappel, L. Grinis, A. Ofir and A. Zaban, J. Phys. Chem. B, 2005, 109, 1643 CrossRef CAS.
- A. Petrozza, C. Groves and H. J. Snaith, J. Am. Chem. Soc., 2008, 130, 12912 CrossRef CAS.
- M. Law, L. Greene, J. Johnson, R. Saykally and P. Yang, Nat. Mater., 2005, 4, 455 CrossRef CAS.
- Y. Bai and et al., Nat. Mater., 2008, 7, 626 CrossRef CAS.
- M. Adachi, Y. Murata, J. Takao, J. Jiu, M. Sakamoto and F. Wang, J. Am. Chem. Soc., 2004, 126, 14943 CrossRef CAS; J. R. Jennings, A. Ghicov, L. M. Peter, P. Schmuki and A. B. Walker, J. Am. Chem. Soc., 2008, 130, 13364 CrossRef CAS.
- J. R. Jennings, A. Ghicov, L. M. Peter, P. Schmuki and A. B. Walker, J. Am. Chem. Soc., 2008, 130, 13364 CrossRef CAS.
- M. Adachi, Y. Murata, J. Takao, J. Jiu, M. Sakamoto and F. Wang, J. Am. Chem. Soc., 2004, 126, 14943 CrossRef CAS.
- K. Zhu, N. R. Neale, A. Miedaner and A. J. Frank, Nano Lett., 2007, 7, 69 CrossRef CAS.
- K. Zhu, T. B. Vinzant, N. R. Neale and A. J. Frank, Nano Lett., 2007, 7, 3739 CrossRef CAS.
- J. Park, T. Lee and M. Kang, Chem. Commun., 2008, 2867 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
