High molar extinction coefficient amphiphilic ruthenium sensitizers for efficient and stable mesoscopic dye-sensitized solar cells†
Lingamallu
Giribabu
*a,
Challuri
Vijay Kumar
a,
Chikkam Srinivasa
Rao
a,
Veeranagari Gopal
Reddy
a,
Paidi Yella
Reddy
a,
Malapaka
Chandrasekharam
a and
Yarasi
Soujanya
b
aNanomaterials Laboratory, Inorganic & Physical Chemistry Division, Indian Institute of Chemical Technology, Hyderabad, 500007, India. E-mail: giribabu@iict.res.in; Fax: +91 40 27160921; Tel: +91 40 27193186
bMolecular Modelling Group, Indian Institute of Chemical Technology, Hyderabad, 500007, India
First published on 23rd April 2009
Abstract
Two efficient heteroleptic ruthenium (II) complexes, cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-bis(2-(3,5-di tert-butylphenyl)ethenyl)-2,2′-bipyridine) ruthenium (II) (HRD-1) and cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-bis(2-(2,3,5-trimethylphenyl)ethenyl)-2,2′-bipyridine) ruthenium (II) (HRD-2) were synthesized and characterized, which when anchored onto nanocrystalline TiO2 shows efficiency of 5.77 and 4.87% respectively, using durable redox electrolytes.
Broader contextAs fossil fuel resources are depleting rapidly, the development of clean renewable energy sources are of paramount importance. In this regard, dye-sensitized solar cells are a cleaner and low cost alternative technology to existing conventional solid-state photovoltaic devices. In these devices, the sensitizer and electrolyte are key components responsible for conversion of solar radiation into electric current. We describe the development of two new heteroleptic ruthenium (II) complexes based on an extended-π conjugation concept having amphiphilic ligands such as 3,5-di-tert-butyl–phenyl (HRD-1) and 2,4,6-trimethyl–phenyl (HRD-2) groups. We have used either high boiling point solvent or solvent free ionic liquid redox electrolytes in the fabrication of test cell devices and found efficiencies of 5.77 and 4.87% with HRD-1 and HRD-2 complexes, respectively. Both the complexes were found to have excellent stability under thermal and light soaking experiments and therefore are promising for large-scale outdoor applications. |
The forecast for the next 50 years predicts that human energy needs are likely to double while fossil energy reserves shrink. At the same time, on earth, per year, plants and bacteria capture and convert light into a quantity of biological material greater than 100 times the food needed to sustain mankind. One solution to meet future energy demand, would be to mimic plant or bacterial mechanisms to develop environmental friendly energy production systems at low cost with easy manufacturing technology. In this regard, mesoscopic dye-sensitized solar cells (DSSC) have attracted considerable attention for the last one and half decades because of their high photon-to-electricity conversion efficiency.1 In these cells, the sensitizer is one of the key components for high power conversion efficiency, and the most efficient and successful sensitizers so far known are ruthenium (II) polypyridyl complexes, which have yielded power conversion efficiency of up to 8–11%.2 To increase photovoltaic performance (efficiency and durability) of a device, much effort has been made towards the development of sensitizer, electrolyte and photoanode material.
Though ruthenium (II) polypyridyl complexes are known to exhibit the best performance overall, other sensitizers also have been used including metal-free organic dyes, porphyrins and phthaloycanines.3 Much attention has been paid to a number of details in the development of ruthenium polypyridyl complexes in order to improve the photoelectric conversion efficiency and stability of test cell devices. One of the ways to improve the efficiency of a test cell device is to increase the molar extinction coefficient through the introduction of amphiphilic groups in ruthenium sensitizers. Grätzel and co-workers introduced an extended π-conjugation concept and reported several heteroleptic (either ion coordinating or hydrophobic group) ruthenium complexes, which improved considerably the efficiency and durability of the test cell device.4 The hydrophobicity parameters in dyes is revealed to be crucial in DSSC for improving long term stability and performance. Ion coordinating groups on bipyridyl groups of ruthenium complexes influence JSC and VOC of a test cell device. Herein, we report two new ruthenium sensitizers i.e., cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-bis(2-(3,5-di tert-butyl–phenyl)ethenyl)-2,2′-bipyridine) ruthenium (II) (HRD-1) and cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-bis(2-(2,3,5-trimethyl–phenyl)ethenyl)-2,2′-bipyridine) ruthenium (II) (HRD-2) based on the extended-π conjugation concept. Both the sensitizers have amphiphilic alkyl groups on bipyridyl ligands and their test cell performances are compared with the less hydrophobic sensitizer cis-di(thiocyanato)(4,4′-dicarboxylic acid-2,2′-bipyridine)(4,4′-bis(2-(4-tert-butyloxy–phenyl)ethenyl)-2,2′bipyridine ruthenium (II) (K77).5 The new ruthenium sensitizers are compatible with ionic redox electrolytes.
The structures of HRD-1 and HRD-2, which incorporate 3,5-di-tert-butylphenyl and 2,3,5-trimethylphenyl-substituted bipyridine, respectively, as ancillary ligands are shown in Fig. 1. The synthetic details and structural characterization are given in ESI.†
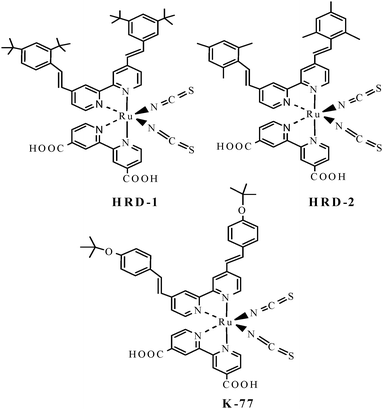 | ||
| Fig. 1 Molecular structures of ruthenium sensitizers. | ||
The electronic absorption spectra of the dye molecules measured in ethanol (see ESI †) display a lower energy MLCT band for HRD-1 centred at 543 nm with a molar extinction coefficient of 19.3 × 103 M−1 cm−1. This value is higher than those of the dyes HRD-2 (16.1 × 103 M−1 cm−1 at 532 nm) and K-77 (19.0 × 103 M−1 cm−1 at 546 nm). Both the sensitizers display similar spectral features in solution as well as when adsorbed on 2 µm transparent TiO2 films, except for a slight red shift of the absorption maxima due to interaction of anchoring groups to the surface. The emission spectra of HRD-1 and HRD-2 were measured in ethanol solvent and the corresponding emission maxima located at 760 and 758 nm, respectively. Based on both the absorption and emission spectra, the E0-0 transition energies of HRD-1 and HRD-2 were estimated to be 1.90 and 1.93 eV, respectively.2g
The highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of HRD-1 and HRD-2 were performed with DFT calculations using Gaussian-03 programme. The orbital profiles for HOMO and LUMO of each dye are presented in Fig. 2. The calculation illustrates that HOMO is delocalized on the ruthenium metal and NCS ligands. LUMO is localized on dcbpy ligands facilitating electron injection from the excited state Ru complex to the conduction band of TiO2. These results are in agreement with the other ruthenium polypyridyl complexes reported in the literature.6
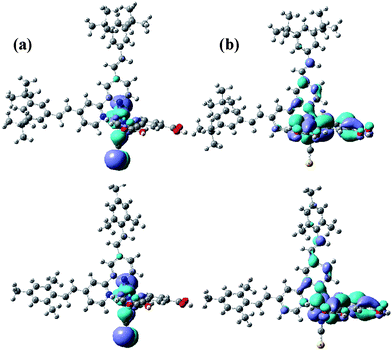 | ||
| Fig. 2 Frontier molecular orbitals of HOMO and LUMO calculated with DFT on Gaussian-03 of (a) HRD-1 and (b) HRD-2. | ||
Electrochemical properties of HRD-1 and HRD-2 were scrutinized by cyclic voltammetry in acetonitrile solution using 0.1 M tetra-butyl ammonium perchlorate and ferrocene as an internal standard at 0.42 V vs. SCE. The oxidation and reduction potentials of HRD-1 were obtained at 0.75 and −0.94 V while those of HRD-2 were obtained at 0.80 and −0.94 V vs. SCE, respectively. The oxidation potential is sufficiently more positive than the redox potential of an electrolyte leading to efficient dye regenaration. The excited state oxidation potentials of HRD-1 and HRD-2 were −1.15 V and −1.13 V vs. SCE, which are sufficiently more negative than the TiO2 conduction band.
The photovoltaic performance of HRD-1 and HRD-2 was tested using two different redox electrolytes composed of 1.0 M LiI, 0.05 M I2 in γ-butyrolactone solvent (Ei-301) and for the other ionic liquid electrolyte, 0.2 M I2, 0.5 M guanidinium thiocyanate (GuSCN) and 0.5 M N-methyl benzimidazole (NMB) in a 65 : 35 v/v% mixture of 1-propyl-3-methylimidazolium iodide / 1-ethyl-3-methyl-imidazolium tetracyanoborate [PMII / EMIB(CN)4] (Z580). The test cell data for HRD-1 and HRD-2 were compared with K77 under similar test cell conditions. Mesoporous titania films were employed for fabrication, assembly of complete, hot-melt sealed cells and have been described previously.7 A 8 µm thick film of 19 nm sized TiO2 particles was first screen-printed on a fluorine-doped SnO2 (FTO) conducting glass electrode and a 4 µm thick second layer of 400 nm-sized light scattering anatase particles were subsequently coated onto the first layer. The TiO2electrodes were gradually heated under an air flow at 500 °C for 30 min followed by treatment with TiCl4 and sintered at 500 °C for 20 min. The TiO2electrodes were then immersed into the dye solutions (0.3 mM in ethanol) and soaked at room temperature for 16 h. Platinized FTO glass was used as the counter electrode. The active area of the test cell device was 0.74 cm2.
The photocurrent action spectra of test cell devices based on new ruthenium sensitizers with Ei-301 and Z580 redox electrolytes are shown in Fig. 3, where photon-to-current conversion efficiency (IPCE) is plotted as a function of wavelength. The photocurrent action spectra resemble the absorption spectra except for a slight red shift by ca. 5 nm. The photoresponse of thin films extends up to 800 nm. We observed an IPCE of 85% in HRD-1 and HRD-2 with Ei301 redox electrolyte, while in the case of Z580 redox electrolyte, the IPCE was 60 and 64% with HRD-1 and HRD-2, respectively. Under similar test cell conditions, we have observed an IPCE of 80 and 55% with Ei-301 and Z580 redox electrolytes, respectively for K-77.
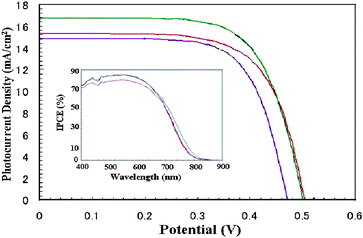 | ||
Fig. 3 Photocurrent–voltage characteristics and incident photon-to-current conversion efficiency plotted as a function of excitation wavelength (insert) of ( ) HRD-1, ( ) HRD-1, ( ) HRD-2 and ( ) HRD-2 and ( ) K-77 sensitized solar cells with Ei-301 redox electrolyte under AM 1.5 full sunlight (100 mW cm−2). ) K-77 sensitized solar cells with Ei-301 redox electrolyte under AM 1.5 full sunlight (100 mW cm−2). | ||
Fig 3 shows the photocurrent density–voltage curves for HRD-1 and HRD-2 with Ei-301 and Z580 redox electrolytes under AM 1.5 simulated sunlight at a light intensity of 100 mV cm−2 and the corresponding data are presented in Table 1. In both the redox electrolytes Ei-301 and Z580, HRD-1 showed high power conversion efficiency of 5.8 and 4.8%, respectively. The advantage of γ-butyrolactone in Ei-301 redox electrolyte instead of acetonitrile and valeronitrile solvent mixture, is that it is non-volatile, has high viscosity and a high boiling point which could increase the durability of a test cell device. The high concentration of LiI in Ei-301 redox electrolyte decreases VOC due to a positive shift of band edge but increases electron transport property and acts as the driving force for electron injection.8 It is known from the literature that the presence of guanidinium thiocyanate in redox electrolyte improves VOC by reducing the dark current.9 We assume that in this case guanidinium thiocyanate in Z580 redox electrolyte is also responsible for the improvement in VOC. In both ruthenium sensitizers the VOC increased with Z580 when compared with the Ei-301 redox electrolyte.
| Sensitizer | Electrolyte | V OC/mV | J SC/mA cm−2 | FF | η (%) |
|---|---|---|---|---|---|
| HRD-1 | Ei-301 | 500 | 16.98 | 0.66 | 5.77 |
| HRD-1 | Z580 | 590 | 10.90 | 0.78 | 4.93 |
| HRD-2 | Ei-301 | 470 | 15.00 | 0.69 | 4.87 |
| HRD-2 | Z580 | 600 | 10.50 | 0.78 | 4.91 |
| K-77 | Ei-301 | 500 | 15.40 | 0.67 | 5.16 |
| K-77 | Z580 | 565 | 7.95 | 0.78 | 3.50 |
In order to match specifications required for outdoor practical applications, light soaking and thermal stability tests of the DSSC devices were carried out in the laboratory for test cell devices fabricated using HRD-1 and HRD-2 sensitizers. Fig. 4 presents the variation in the photovoltaic parameters at 80 °C in the dark with ageing time for a test cell device based on HRD-1 sensitizer and Ei-301 redox electrolyte. The photocurrent decreased by 9% while VOC dropped by 20 mV, which was compensated by slight increase in the FF, during 1000 h of ageing at 80 °C. Thus, the device showed excellent stability by maintaining 91% of its initial photovoltaic performance. Similarly, the HRD-2 based test cell device also maintained 90% of its initial photovoltaic performance. In light soaking experiments also, both HRD-1 and HRD-2 retained more than 90% of their initial photovoltaic performance.
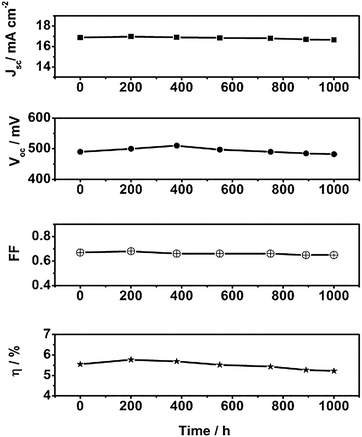 | ||
| Fig. 4 Variation in photovoltaic parameters (JSC, VOC, FF and η) with ageing time for the device based on HRD-1 with Ei-301 redox electrolyte during successive ageing at 80 °C in the dark. | ||
In conclusion, we have designed and synthesized two new high molar extinction coefficient heteroleptic ruthenium (II) complexes HRD-1 and HRD-2 which were tested in DSSC using durable redox electrolytes with an observed efficiency of 5.77%. Test cell devices exhibiting unprecedented long term stability (1000 h) under both thermal stressing and light soaking have been obtained using these new ruthenium (II) sensitizers.
Acknowledgements
We are thankful to the IICT – Aisin Cosmos collaborative project for financial support for this work. Ch. V is grateful to the Council of Scientific and Industrial Research (CSIR) for a research fellowship.References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS; R. F. Service, Science, 2003, 300, 1219 CrossRef CAS; M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS; F. Kong, S. Y. Dai and K. J. Wang, Adv. OptoElectron., 2007, 1 Search PubMed.
- (a) M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Valchopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS; (b) M. K. Nazeeruddin, P. Pechey, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613 CrossRef CAS; (c) M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS; (d) M. Grätzel, Inorg. Chem., 2005, 44, 6841 CrossRef; (e) K. J. Jiang, N. Masaki, J. B. Xia, S. Noda and S. Yanagida, Chem. Commun., 2006, 2460 RSC; (f) C. Y. Chen, S. J. Wu, J. Y. Li, C. G. Wu, J. G. Chen and K. C. Ho, Adv. Mater., 2007, 19, 3888 CrossRef CAS; (g) J. Hum, I. Jung, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2009, 2, 100 RSC.
- W. M. Campbell, A. K. Burrell, D. L. Officer and K. W. Jolley, Coord. Chem. Rev., 2004, 248, 1363 CrossRef CAS; L. Giribabu, Ch. Vijaykumar and P. Y. Reddy, J. Porphyrins Phthalocyanines, 2006, 10, 1017 CrossRef; P. Y. Reddy, L. Giribabu, Ch. Lyness, H. J. Snaith, Ch. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J. H. Yum, K. Kalyanasundaram, M. Grätzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2007, 46, 373 CrossRef CAS; D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2007, 46, 1949; S. Tatay, S. A. Haque, B. O'Regan, J. R. Durrant, W. J. H. Verhees, J. M. Kroon, A. Vidal-Ferran, P. Gavina and E. Palomares, J. Mater. Chem., 2007, 17, 3037 RSC; L. Giribabu, Ch. Vijaykumar, P. Y. Reddy, J. H. Yum, M. Grätzel and M. K. Nazeeruddin, J. Chem. Sci., 2009, 121, 75 CrossRef CAS.
- P. Wang, S. Zakeeruddin, J. E. Moser, R. Humphry-Baker, P. Comete, V. Aranyos, A. Hagfeldt, M. K. Nazeeruddin and M. Grätzel, Adv. Mater., 2004, 16, 1806 CrossRef CAS; P. Wang, C. Klein, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 808 CrossRef CAS.
- D. Kuang, C. Klein, S. Ito, J. E. Moser, R. Humphry-Baker, N. Evans, F. Duriaux, C. Gratzel, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2007, 19, 1133 CrossRef CAS; D. Kaung, C. Klein, Z. Zhang, S. Ito, J.-E. Moser, S. M. Zakeeruddin and M. Grätzel, Small, 2007, 3, 2094 CrossRef CAS.
- C.-Y. Chen, H.-C. Lu, C.-G. Wu, J.-G. Chen and K.-C. Ho, Adv. Funct. Mater., 2007, 17, 29 CrossRef CAS.
- L. Giribabu, Ch. Vijaykumar, V. G. Reddy, P. Y. Reddy, Ch. S. Rao, S.-R. Jang, J.-H. Hum, M. K. Nazeeruddin and M. Grätzel, Sol. Energy Mater. Sol. Cells, 2007, 91, 1611 CrossRef CAS; L. Giribabu, Ch. Vijaykumar, M. Raghavender, K. Somaiah, P. Y. Reddy and M. V. Rao, J. Chem. Sci., 2008, 120, 455 CrossRef CAS.
- A. Furube, R. Katoh, K. Hara, T. Sato, S. Murata, H. Arakawa and M. Tachia, J. Phys. Chem. B, 2005, 109, 16406 CrossRef CAS.
- N. Kopidakis, N. R. Neale and A. J. Frank, J. Phys. Chem. B, 2006, 110, 12485 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, synthesis and spectral data of HRD-1 and HRD-2. See DOI: 10.1039/b903967h |
| This journal is © The Royal Society of Chemistry 2009 |
